Abstract
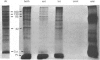
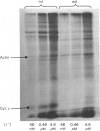
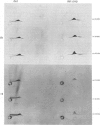
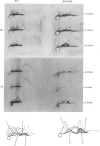
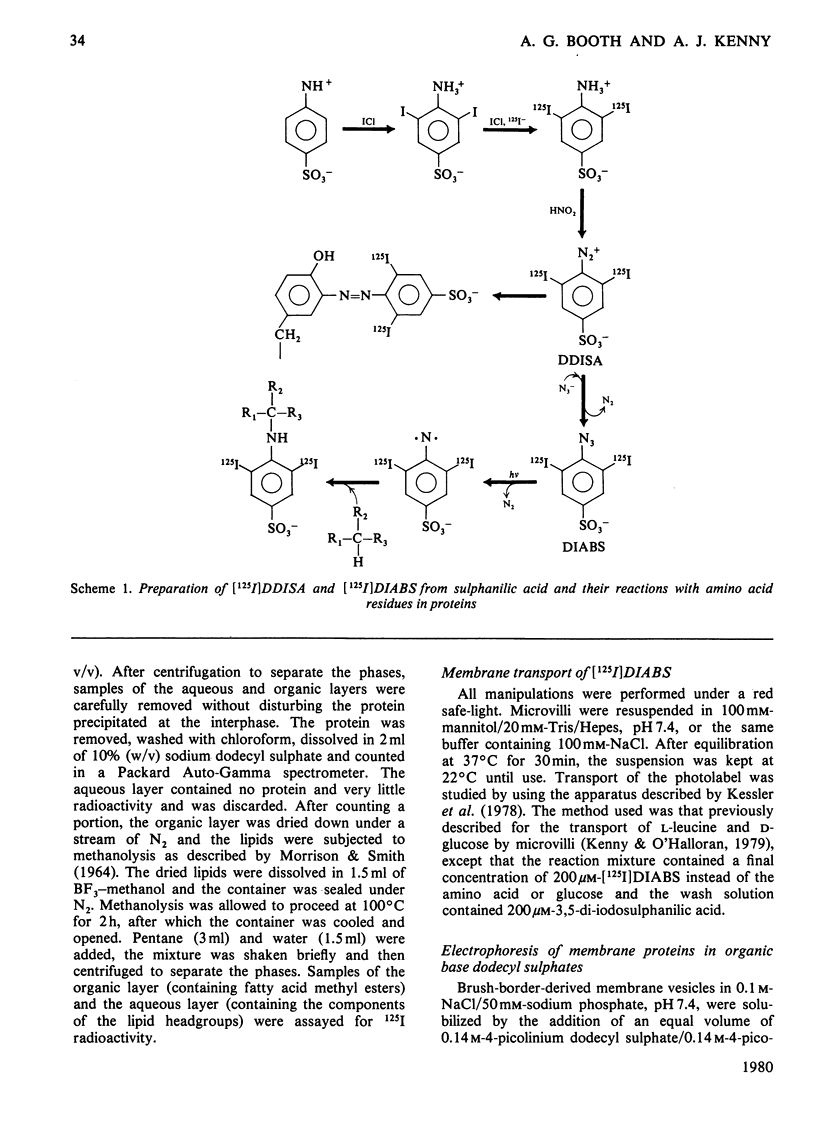
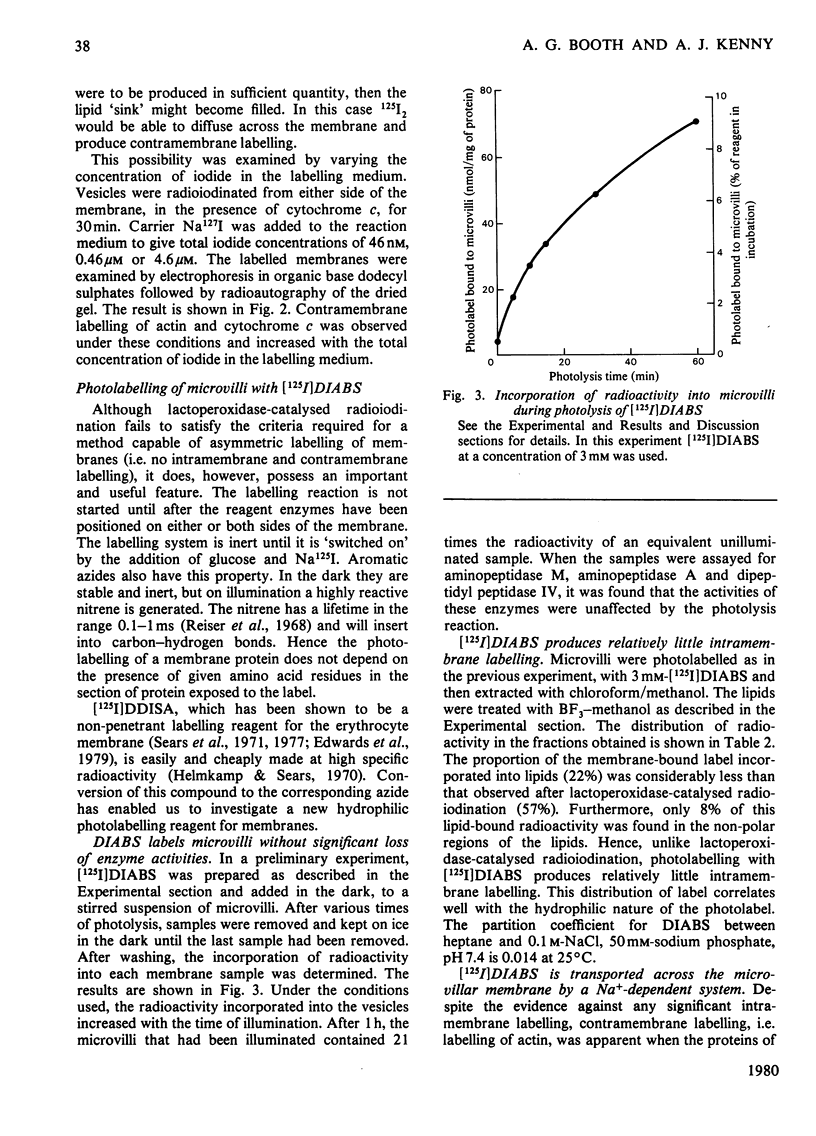
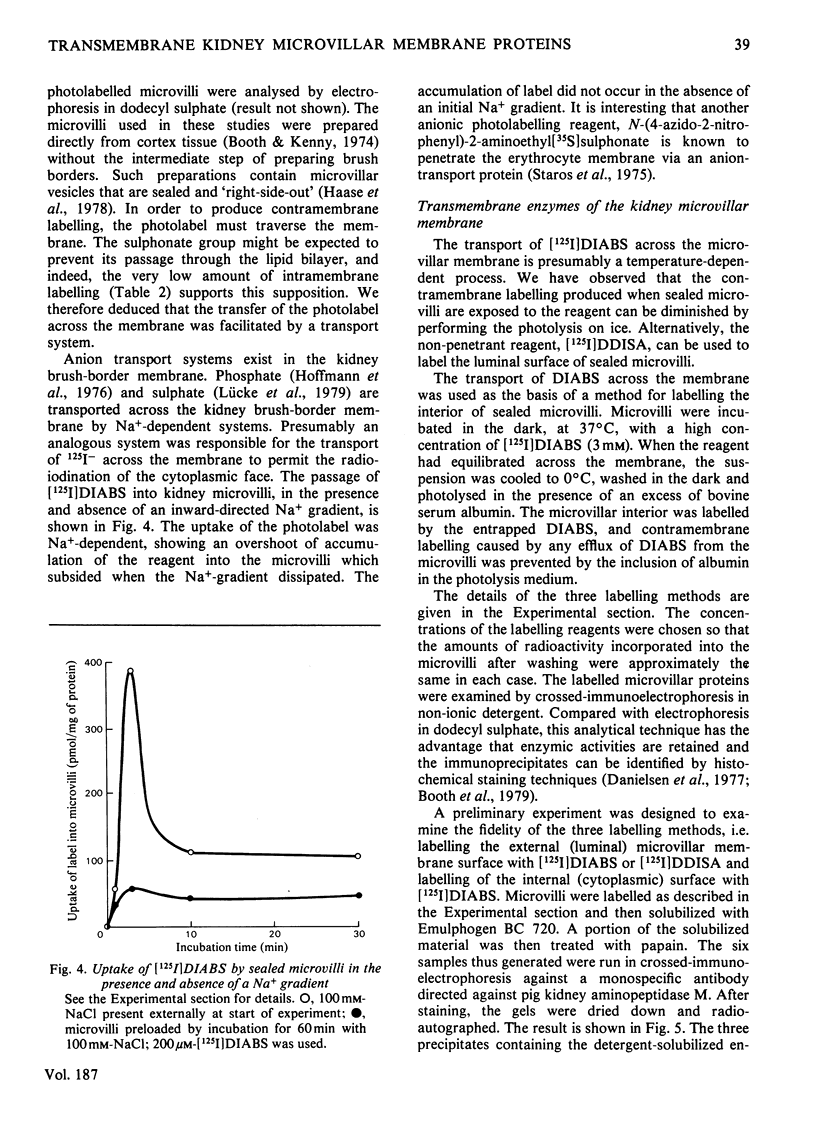
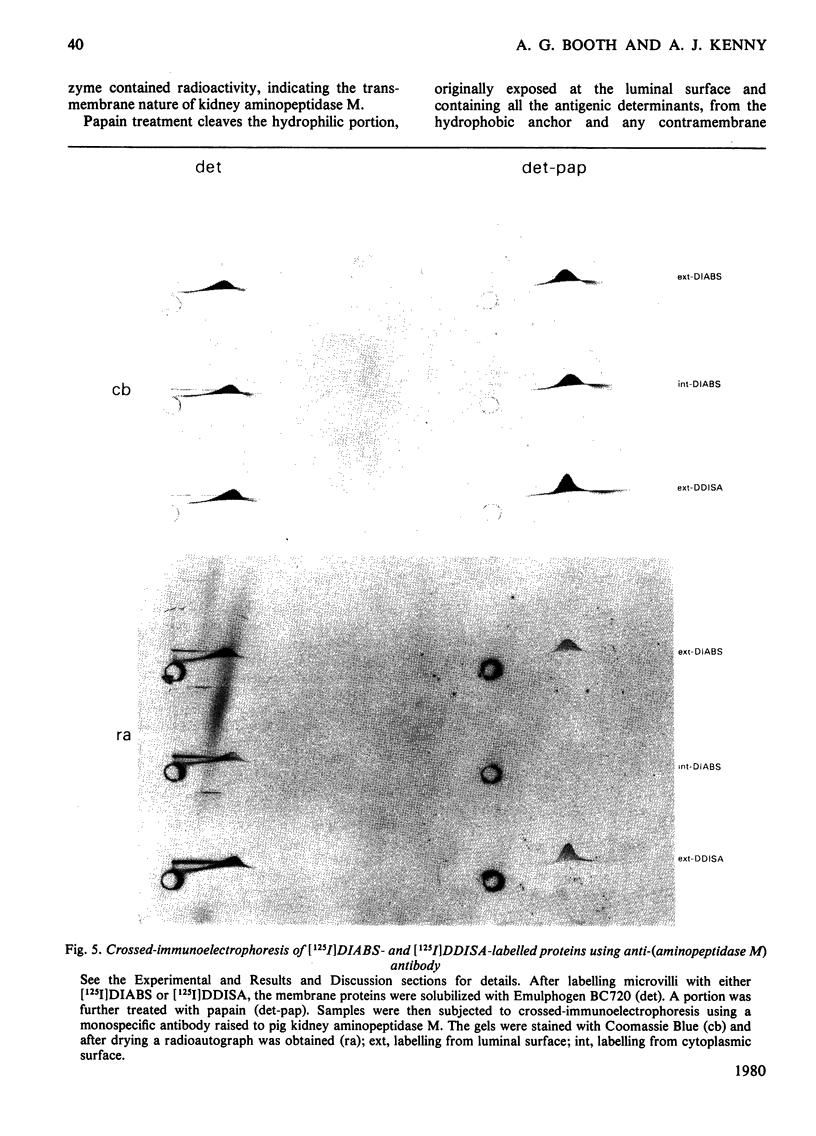
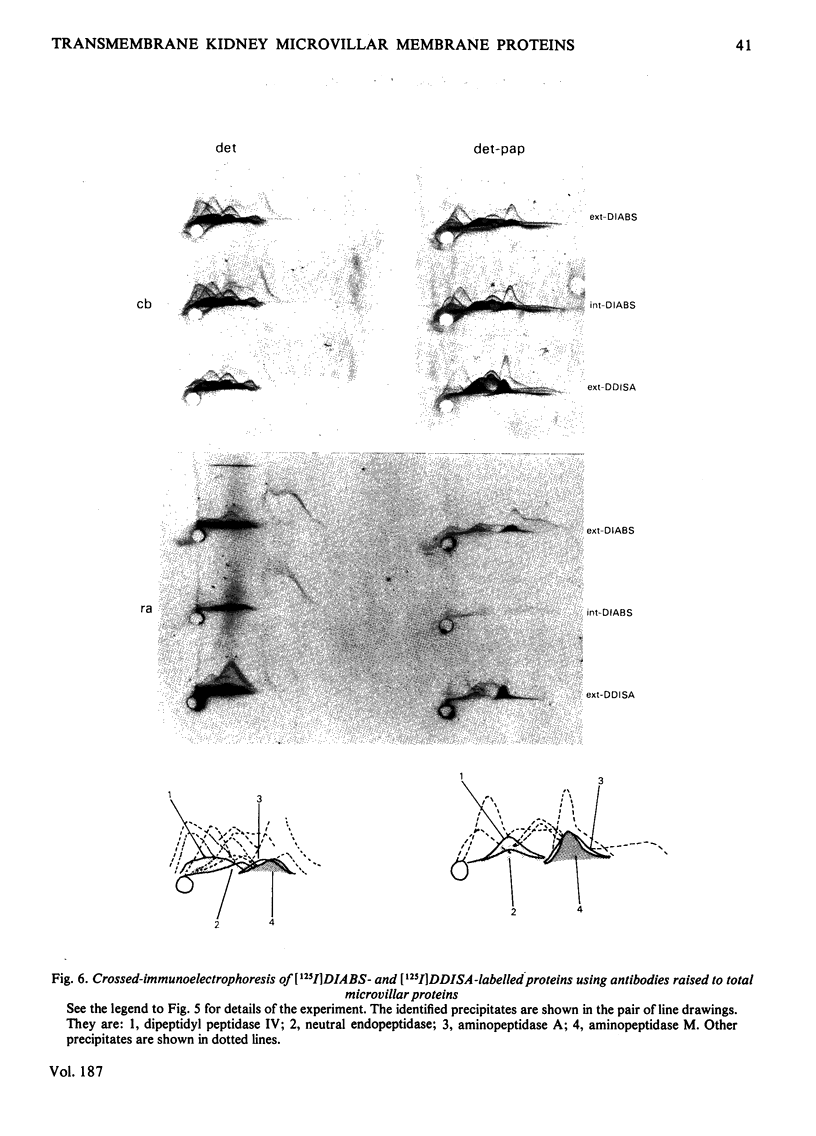
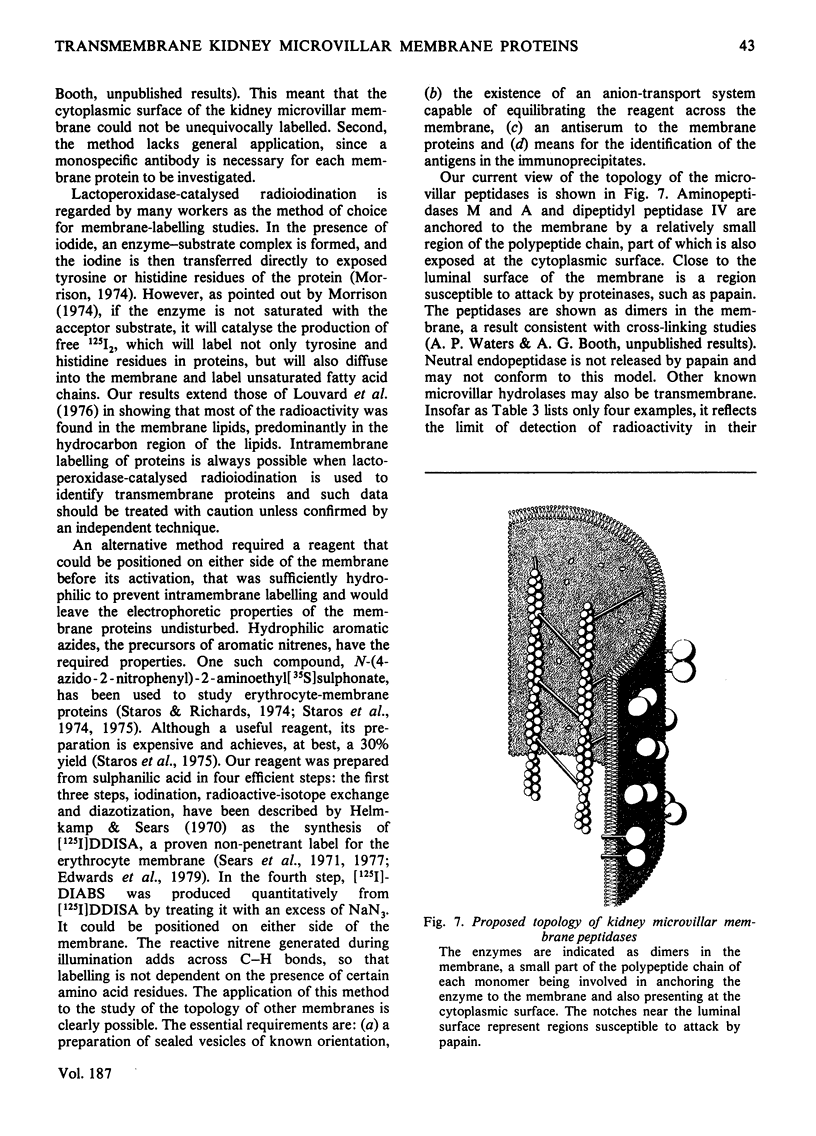
Two methods were used to label pig kidney microvillar membrane proteins from the luminal and cytoplasmic surfaces of closed membrane vesicles. The first method was lactoperoxidase-catalysed radioiodination. The enzyme reagents, lactoperoxidase and glucose oxidase, were positioned inside the vesicles before sealing or externally after sealing, iodination being initiated by the subsequent addition of glucose and 125I-. After resolution of the labelled proteins by electrophoresis in the presence of dodecyl sulphate, asymmetric labelling patterns on radioautographs were observed. However, the major disadvantage of this method is the high degree of intramembrane labelling of the fatty acid chains of membrane lipids, a reaction that undermines any conclusions about the location of the label in that region of the protein supposedly exposed at the surface of the membrane. The second method overcame this disadvantage. A new hydrophilic photoreagent, 3,5-di[125I]iodo-4-azidobenzesulphonate, was synthesized via the intermediate, diazotized 3,5-di[125I]iodosulphanilic acid. It was transported by a Na+-dependent system into microvillar vesicles, thus permitting labelling from either side of the membrane when the vesicles were photolysed. The labelling of membrane lipids was less than with the first method and was essentially confined to the polar headgroups. The activity of several microvillar peptidases survived the labelling reaction and they could be identified in the immunoprecipitates after resolution of the detergent-solubilized membrane proteins by crossed-immunoelectrophoresis. Treatment with papain converted the detergent-solubilized form of susceptible enzymes into the proteinase-solubilized form, which lacked the intramembrane domain and any portion exposed at the cytoplasmic surface. Radioautography established that aminopeptidases M and A, dipeptidyl peptidase IV and neutral endopeptidase were transmembrane proteins. This novel approach to the investigation of membrane topology may be applicable to other complex membranes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth A. G. A novel system for the two-dimensional electrophoresis of membrane proteins. Biochem J. 1977 Apr 1;163(1):165–168. doi: 10.1042/bj1630165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. G., Hubbard L. M., Kenny A. J. Proteins of the kidney microvillar membrane. Immunoelectrophoretic analysis of the membrane hydrolases: identification and resolution of the detergent- and proteinase-solubilized forms. Biochem J. 1979 May 1;179(2):397–405. doi: 10.1042/bj1790397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. Proteins of the kidney microvillus membrane. Identification of subunits after sodium dodecylsullphate/polyacrylamide-gel electrophoresis. Biochem J. 1976 Nov;159(2):395–407. doi: 10.1042/bj1590395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. G., Kenny J. A morphometric and biochemical investigation of the vesiculation of kidney microvilli. J Cell Sci. 1976 Aug;21(3):449–463. doi: 10.1242/jcs.21.3.449. [DOI] [PubMed] [Google Scholar]
- Boxer D. H., Jenkins R. E., Tanner M. J. The organization of the major protein of the human erythrocyte membrane. Biochem J. 1974 Mar;137(3):531–534. doi: 10.1042/bj1370531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S., Raff M. C. Mammalian plasma membranes. Nature. 1975 Nov 6;258(5530):43–49. doi: 10.1038/258043a0. [DOI] [PubMed] [Google Scholar]
- Brunner J., Hauser H., Braun H., Wilson K. J., Wacker H., O'Neill B., Semenza G. The mode of association of the enzyme complex sucrase.isomaltase with the intestinal brush border membrane. J Biol Chem. 1979 Mar 25;254(6):1821–1828. [PubMed] [Google Scholar]
- Cotmore S. F., Furthmayr H., Marchesi V. T. Immunochemical evidence for the transmembrane orientation of glycophorin A. Localization of ferritin-antibody conjugates in intact cells. J Mol Biol. 1977 Jul 5;113(3):539–553. doi: 10.1016/0022-2836(77)90237-6. [DOI] [PubMed] [Google Scholar]
- Danielsen E. M., Sjöström H., Norén O., Dabelsteen E. Immunoelectrophoretic studies on pig intestinal brush border proteins. Biochim Biophys Acta. 1977 Oct 26;494(2):332–342. doi: 10.1016/0005-2795(77)90163-5. [DOI] [PubMed] [Google Scholar]
- Edwards R. M., Kempson S. A., Carlson G. L., Dousa T. P. Diazontized (125I) diiodosulafanilic acid as a label for cell surface membranes. Studies on erythrocytes. Biochim Biophys Acta. 1979 May 3;553(1):54–65. doi: 10.1016/0005-2736(79)90030-0. [DOI] [PubMed] [Google Scholar]
- Haase W., Schäfer A., Murer H., Kinne R. Studies on the orientation of brush-border membrane vesicles. Biochem J. 1978 Apr 15;172(1):57–62. doi: 10.1042/bj1720057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmkamp R. W., Sears D. A. A label for the red cell membrane: diazotized diiodosulfanilic acid. Int J Appl Radiat Isot. 1970 Nov;21(11):683–685. doi: 10.1016/0020-708x(70)90127-4. [DOI] [PubMed] [Google Scholar]
- Hoffmann N., Thees M., Kinne R. Phosphate transport by isolated renal brush border vesicles. Pflugers Arch. 1976 Mar 30;362(2):147–156. doi: 10.1007/BF00583641. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. Externally disposed plasma membrane proteins. I. Enzymatic iodination of mouse L cells. J Cell Biol. 1975 Feb;64(2):438–460. doi: 10.1083/jcb.64.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny A. J., Booth A. G., Macnair R. D. Peptidases of the kidney microvillus membrane. Acta Biol Med Ger. 1977;36(11-12):1575–1585. [PubMed] [Google Scholar]
- Kenny A. J., Booth A. G. Microvilli: their ultrastructure, enzymology and molecular organization. Essays Biochem. 1978;14:1–44. [PubMed] [Google Scholar]
- Kenny A. J., O'Halloran D. M. Effect of chelating agents and phosphoramidon on the L-leucine transport system in microvillar vesicles from pig kidney. FEBS Lett. 1979 May 15;101(2):407–410. doi: 10.1016/0014-5793(79)81055-8. [DOI] [PubMed] [Google Scholar]
- Kessler M., Tannenbaum V., Tannenbaum C. A simple apparatus for performing short-time (1--2 seconds) uptake measurements in small volumes; its application to D-glucose transport studies in brush border vesicles from rabbit jejunum and ileum. Biochim Biophys Acta. 1978 May 18;509(2):348–359. doi: 10.1016/0005-2736(78)90053-6. [DOI] [PubMed] [Google Scholar]
- Louvard D., Semeriva M., Maroux S. The brush-border intestinal aminopeptidase, a transmembrane protein as probed by macromolecular photolabelling. J Mol Biol. 1976 Oct 5;106(4):1023–1035. doi: 10.1016/0022-2836(76)90350-8. [DOI] [PubMed] [Google Scholar]
- Lücke H., Stange G., Murer H. Sulphate-ion/sodium-ion co-transport by brush-border membrane vesicles isolated from rat kidney cortex. Biochem J. 1979 Jul 15;182(1):223–229. doi: 10.1042/bj1820223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Macnair D. C., Kenny A. J. Proteins of the kidney microvillar membrane. The amphipathic form of dipeptidyl peptidase IV. Biochem J. 1979 May 1;179(2):379–395. doi: 10.1042/bj1790379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroux S., Louvard D., Baratti J. The aminopeptidase from hog intestinal brush border. Biochim Biophys Acta. 1973 Sep 15;321(1):282–295. doi: 10.1016/0005-2744(73)90083-1. [DOI] [PubMed] [Google Scholar]
- Maroux S., Louvard D. On the hydrophobic part of aminopeptidase and maltases which bind the enzyme to the intestinal brush border membrane. Biochim Biophys Acta. 1976 Jan 21;419(2):189–195. doi: 10.1016/0005-2736(76)90345-x. [DOI] [PubMed] [Google Scholar]
- Morrison M. The determination of the exposed proteins on membranes by the use of lactoperoxidase. Methods Enzymol. 1974;32:103–109. doi: 10.1016/0076-6879(74)32013-7. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R., Mikkelsen R. B., Wallach D. F. Transmembrane disposition of the 55,000-dalton concanavalin A receptor protein of thymocyte plasma membranes. J Biol Chem. 1978 Oct 10;253(19):6973–6978. [PubMed] [Google Scholar]
- Sears D. A., Friedman J. M., George J. N. Topography of the external surface of the human red blood cell membrane studied with a nonpenetrating label, [125I]diazodiiodosulfanilic acid. J Biol Chem. 1977 Jan 25;252(2):712–720. [PubMed] [Google Scholar]
- Sears D. A., Reed C. F., Helmkamp R. W. A radioactive label for the erythrocyte membrane. Biochim Biophys Acta. 1971 Jun 1;233(3):716–719. doi: 10.1016/0005-2736(71)90170-2. [DOI] [PubMed] [Google Scholar]
- Staros J. V., Haley B. E., Richards F. M. Human erythrocytes and resealed ghosts. A comparison of membrane topology. J Biol Chem. 1974 Aug 10;249(15):5004–5007. [PubMed] [Google Scholar]
- Staros J. V., Richards F. M. Photochemical labeling of the cytoplasmic surface of the membranes of intact human erythrocytes. J Biol Chem. 1975 Oct 25;250(20):8174–8178. [PubMed] [Google Scholar]
- Staros J. V., Richards F. M. Photochemical labeling of the surface proteins of human erythrocytes. Biochemistry. 1974 Jun 18;13(13):2720–2726. doi: 10.1021/bi00710a010. [DOI] [PubMed] [Google Scholar]
- Vannier C., Louvard D., Maroux S., Desnuelle P. Structural and topological homology between porcine intestinal and renal brush border aminopeptidase. Biochim Biophys Acta. 1976 Nov 11;455(1):185–199. doi: 10.1016/0005-2736(76)90163-2. [DOI] [PubMed] [Google Scholar]
- Walsh F. S., Crumpton M. J. Orientation of cell-surface antigens in the lipid bilayer of lymphocyte plasma membrane. Nature. 1977 Sep 22;269(5626):307–311. doi: 10.1038/269307a0. [DOI] [PubMed] [Google Scholar]
- Wilfong R. F., Neville D. M., Jr The isolation of a brush border membrane fraction from rat kidney. J Biol Chem. 1970 Nov 25;245(22):6106–6112. [PubMed] [Google Scholar]