Abstract
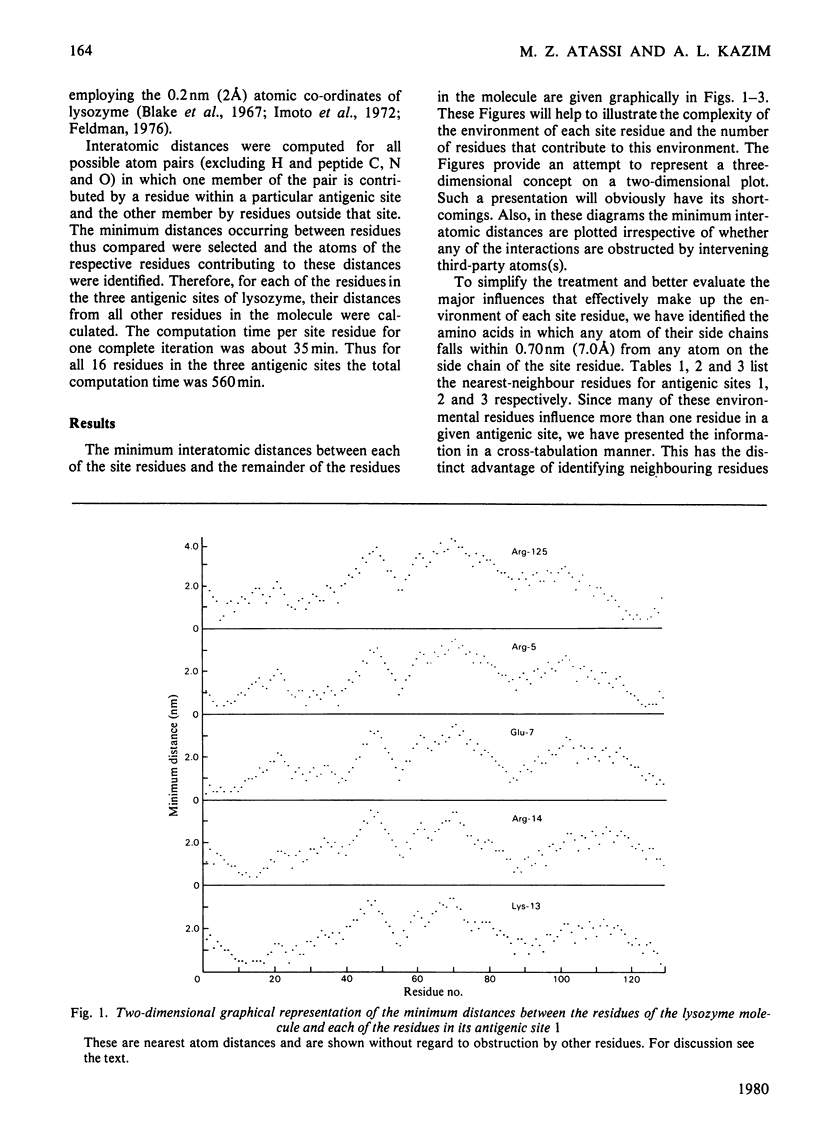
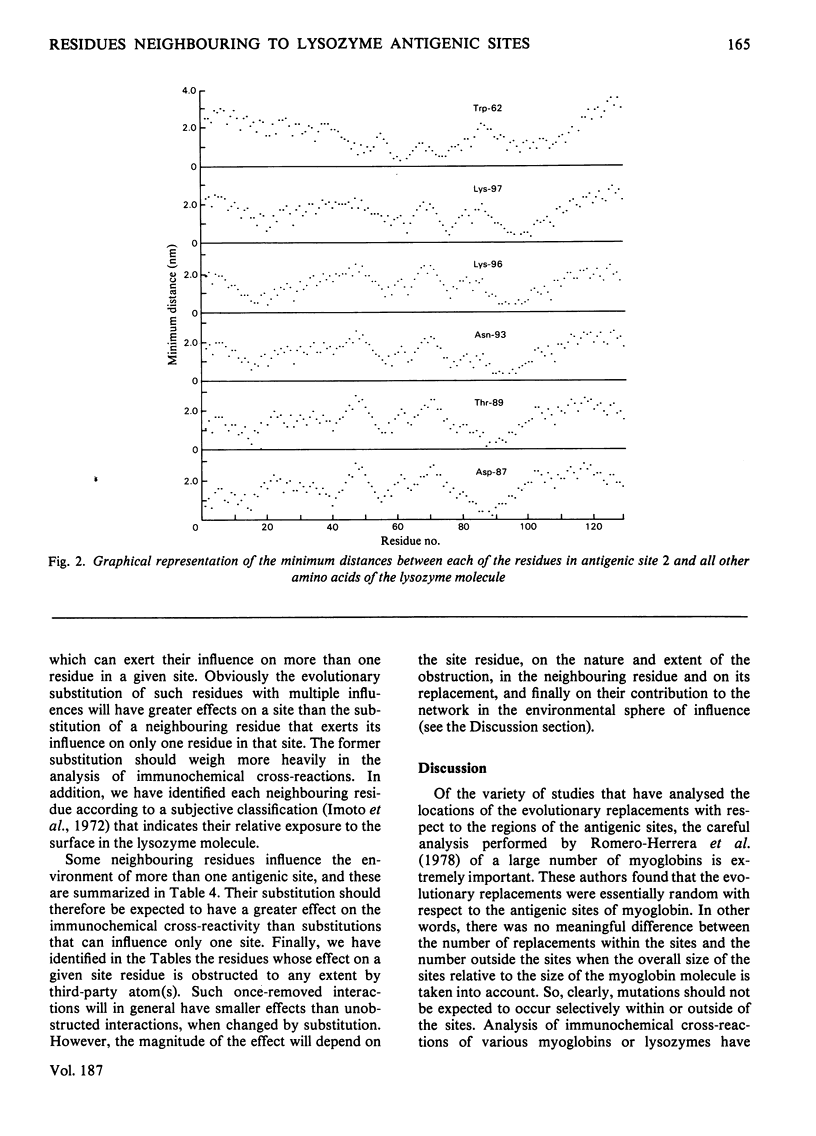
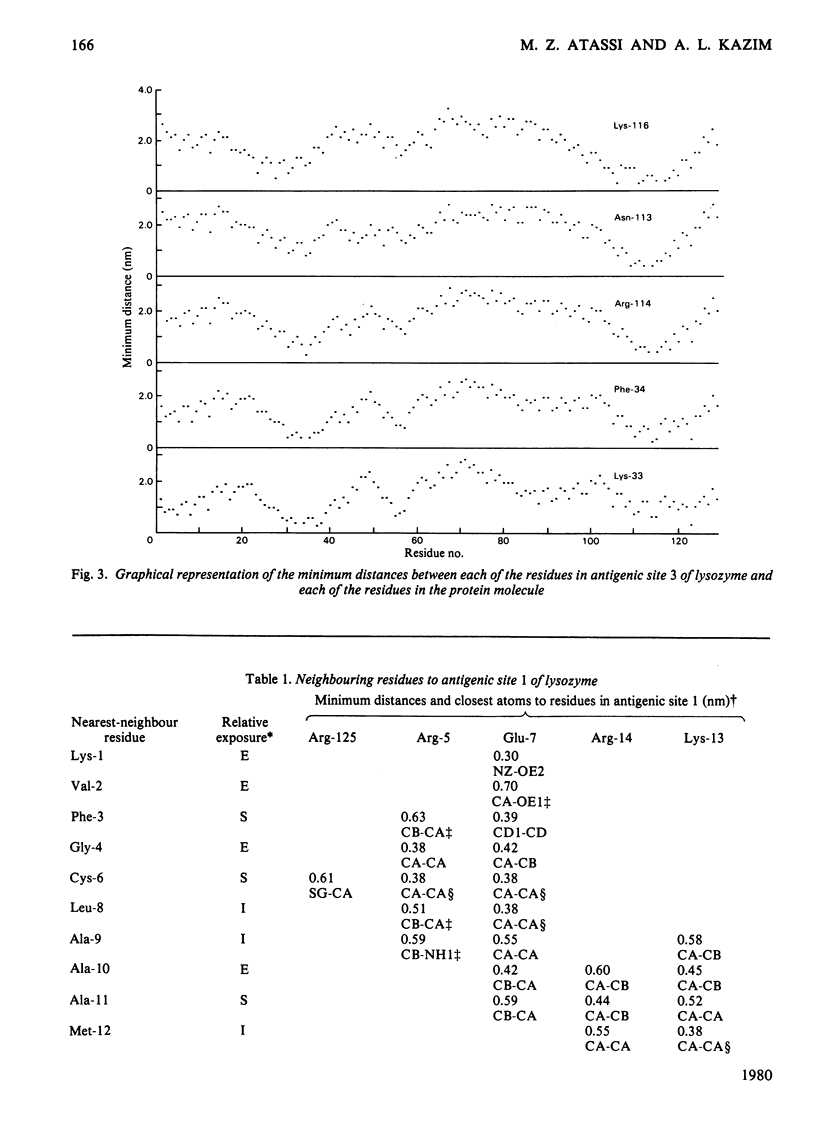
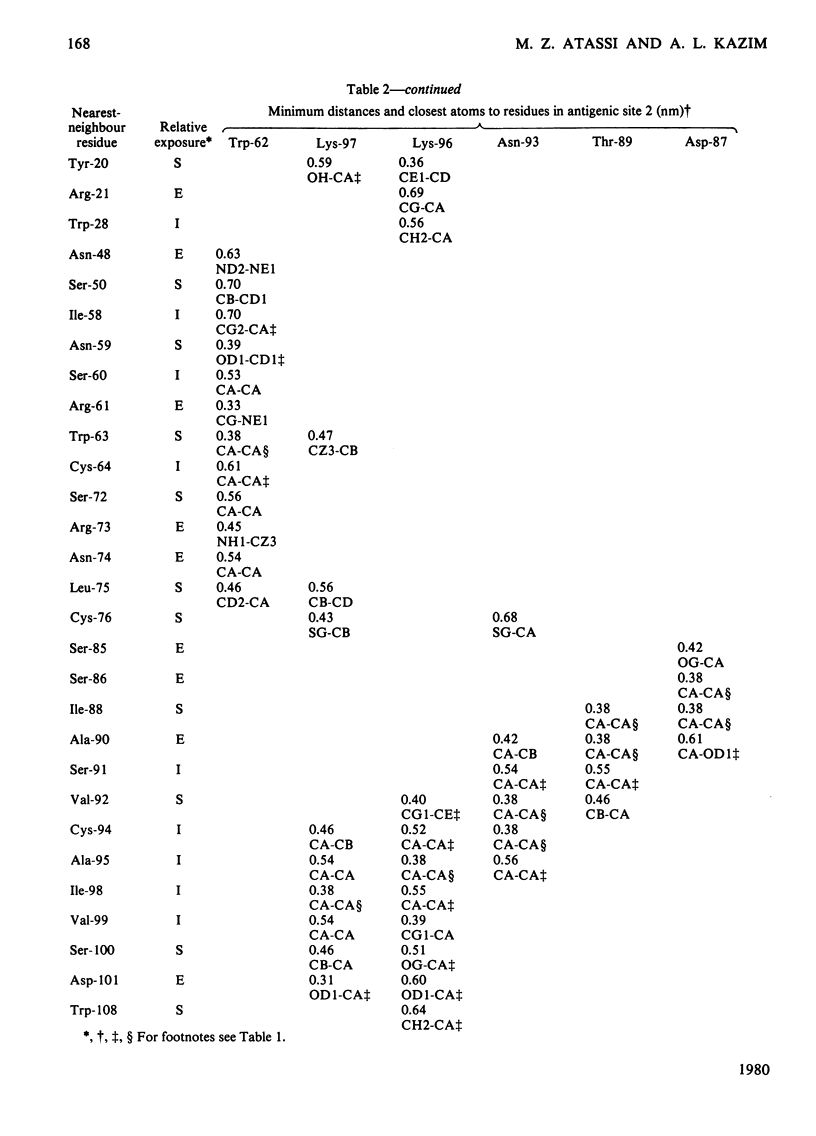
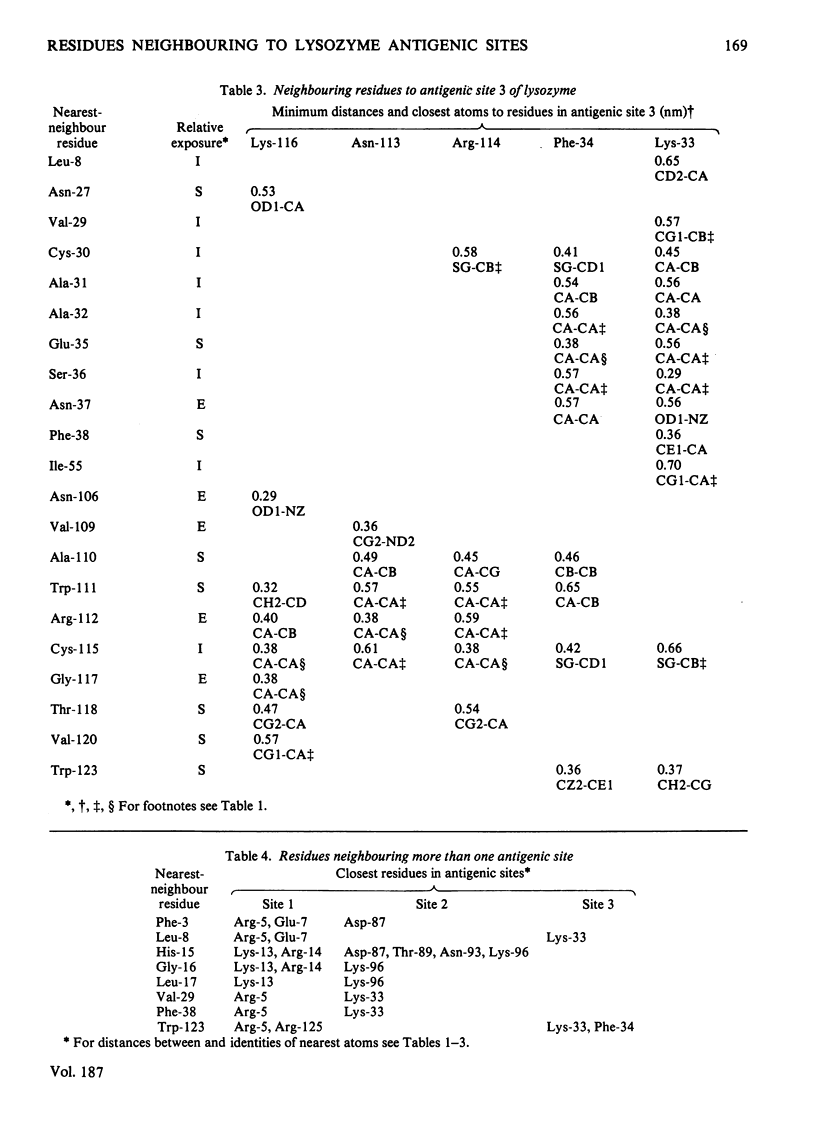
By using the antigenic structure of lysozyme determined in this laboratory and the X-ray co-ordinates we have calculated the closest-atom distances between each of the residues in the three antigenic sites and all the other amino acids of the lysozyme molecule. These calculations enabled us to identify the nearest neighbours to each of the site residues. Thus the immediate environment of each site residue is described. For the three antigenic sites there is a total of 71 neighbouring residues. The effects of evolutionary amino acid substitutions in site-neighbouring residues on the binding capacity of protein binding sites in general and on protein antigenic sites in particular are discussed. These, together with the direct replacements in site residues, will acount for the major effects. However, the limitations of this treatment are stressed. The smaller effects on antigenic sites of replacements at once-removed and even at more distant locations, which, when they become cumulative, could be considerable, are brought to attention, together with any influences of conformational readjustments that can take place as a result of evolutionary amino acid replacements.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atassi M. Z. Antigenic structure of myoglobin: the complete immunochemical anatomy of a protein and conclusions relating to antigenic structures of proteins. Immunochemistry. 1975 May;12(5):423–438. doi: 10.1016/0019-2791(75)90010-5. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Habeeb A. F., Rydstedt L. Lack of immunochemical cross-reaction between lysozyme and alpha-lactalbumin and comparison of their conformations. Biochim Biophys Acta. 1970 Jan 20;200(1):184–187. doi: 10.1016/0005-2795(70)90061-9. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z. Immunochemistry of sperm whale myoglobin. 3. Modification of the three tyrosine residues and their role in the conformation and differentiation of their roles in the antigenic reactivity. Biochemistry. 1968 Sep;7(9):3078–3085. doi: 10.1021/bi00849a008. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z. Immunochemistry of sperm whale myoglobin. VI. Preparation and conformational analysis of eight mammalian myoglobins. Biochim Biophys Acta. 1970 Dec 22;221(3):612–622. doi: 10.1016/0005-2795(70)90233-3. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Lee C. L. The precise and entire antigenic structure of native lysozyme. Biochem J. 1978 May 1;171(2):429–434. doi: 10.1042/bj1710429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi M. Z. Precise determination of the entire antigenic structure of lysozyme: molecular features of protein antigenic structures and potential of "surface-simulation" synthesis--a powerful new concept for protein binding sites. Immunochemistry. 1978 Dec;15(12):909–936. doi: 10.1016/0161-5890(78)90126-8. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Tarlowski D. P., Paull J. H. Immunochemistry of sperm whale myoglobin. VII. Correlation of immunochemical cross-reaction of eight myoglobins with structural similarity and its dependence on conformation. Biochim Biophys Acta. 1970 Dec 22;221(3):623–635. doi: 10.1016/0005-2795(70)90234-5. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Thomas A. V. Immunochemistry of sperm whale myoglobin. IV. The role of the arginine residues in the conformation and differentiation of their roles in the antigenic reactivity. Biochemistry. 1969 Aug;8(8):3385–3394. doi: 10.1021/bi00836a037. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Mair G. A., North A. C., Phillips D. C., Sarma V. R. On the conformation of the hen egg-white lysozyme molecule. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):365–377. doi: 10.1098/rspb.1967.0034. [DOI] [PubMed] [Google Scholar]
- Habeeb A. F., Atassi M. Z. Enzymic and immunochemical properties of lysozyme. IV. Demonstration of conformational differences between alpha-lactalbumin and lysozyme. Biochim Biophys Acta. 1971 Apr 27;236(1):131–141. doi: 10.1016/0005-2795(71)90158-9. [DOI] [PubMed] [Google Scholar]
- Hurrell J. G., Smith J. A., Todd P. E., Leach S. J. Cross-reactivity between mammalian myoglobins: linear vs spatial antigenic determinants. Immunochemistry. 1977 Apr;14(4):283–288. doi: 10.1016/0019-2791(77)90251-8. [DOI] [PubMed] [Google Scholar]
- Ibrahimi I. M., Prager E. M., White T. J., Wilson A. C. Amino acid sequence of California quail lysozyme. Effect of evolutionary substitutions on the antigenic structure of lysozyme. Biochemistry. 1979 Jun 26;18(13):2736–2744. doi: 10.1021/bi00580a008. [DOI] [PubMed] [Google Scholar]
- Lee C. L., Atassi M. Z. Enzymic and immunochemical properties of lysozyme. Accurate definition of the antigenic site around the disulphide bridge 30-115 (site 3) by 'surface-simulation' synthesis. Biochem J. 1977 Dec 1;167(3):571–581. doi: 10.1042/bj1670571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Herrera A. E., Lehmann H., Joysey K. A., Friday A. E. On the evolution of myoglobin. Philos Trans R Soc Lond B Biol Sci. 1978 May 9;283(995):61–163. doi: 10.1098/rstb.1978.0018. [DOI] [PubMed] [Google Scholar]
- Twining S. S., Atassi M. Z. Use of immunoadsorbents for the study of antibody binding to sperm whale myoglobin and its synthetic antigenic sites. J Immunol Methods. 1979;30(2):139–151. doi: 10.1016/0022-1759(79)90088-7. [DOI] [PubMed] [Google Scholar]


