Abstract
Purpose
Description of retinal phenotype by structural and functional testing, ornithine plasma levels and mutational data of OAT gene in patients with Gyrate Atrophy (GA).
Methods
Ophthalmologic examination, fundus photography (CFP), autofluorescence (FAF), spectral-domain optical coherence tomography (SD-OCT), Goldmann perimetry (GP), full-field electroretinogram (ffERG) and chromatic perimetry (CP) testing were performed. Ornithine plasma levels were measured. Sanger sequencing mutational analysis of the coding exons and exon–intron junctions of the OAT gene were analyzed.
Results
Twelve eyes of seven Mexican patients with GA were included. CFF showed peripheric patches of chorioretinal atrophy; FAF revealed peripheric oval areas of hypoautofluorescence; SD-OCT exhibited outer retinal tubulations in 58%, cystoid macular edema in 50%, epiretinal membrane in 42%, foveoschisis and staphyloma in 17%, and hyperreflective deposits in 100% of the eyes; GP showed constricted visual fields in 100% of the eyes; ffERG revealed preserved photopic response in 17% and preserved scotopic response in 17% of the eyes; CP exposed a deficit in generalized response of rods and cones in 100% of the eyes. Mean ornithine plasma levels were 509.5 µmol/L. One patient with genetic confirmation of GA had normal ornithine plasma levels (48 µmol/L). Molecular findings in OAT gene detected two novel pathogenic variants: c.796 C > T (p.Gln266*) and c.721_722dupCC (p.Asp242ArgfsTer6).
Conclusion
This study provides new information regarding functional and structural diagnosis in patients with GA, expands the understanding of retinal phenotype in patients with GA, reports two novel mutations and presents the first case of GA confirmed by genetic testing with normal ornithine levels.
Keywords: Gyrate atrophy, OAT gene, Ornithine, Dystrophy, Molecular analysis, Hereditary eye diseases
Introduction
In 1896, E. Fuchs described for the first time a progressive degeneration of the retina and choroid in young patients which was named “atrophia gyrata chorioideae et retinae”[1]; since then, this rare autosomal recessive chorioretinal dystrophy has been widely studied, exhibiting variable clinical characteristics [2]. To date, more than 200 individuals with Gyrate atrophy (GA) have been reported in the literature, of which one third corresponds to the Finnish population. Chorioretinal atrophy in GA is generated by increased levels of ornithine due to a deficiency of ornithine-δ-aminotransferase (OAT) which normally removes ammonia from the cells by the conversion of L-ornithine to proline and glutamic acid [3–5]. GA is caused by biallelic mutations in the OAT gene, with more than 60 different mutations reported to date in individuals from diverse ethnic origins and with no apparent genotype–phenotype correlation [6]. GA is clinically characterized by night vision impairment and loss of peripheral visual field starting in the first and second decades of life. In addition, myopia, cataracts and cystoid macular edema (CME) are common early features [7]. Initial fundoscopic changes are well described as round sharply defined areas of retinal and choroidal atrophy in the periphery and equator which in late stages tend to coalesce and spread centripetally [5, 7–10]. These lesions lead to a marked separation between healthy/normal and atrophic retina with loss of external layers, retinal pigment epithelium, choriocapillaris and most of the medium and large choroidal vessels [11]. Studies in GA mouse models and humans have demonstrated that early correction of ornithine levels, being abnormally elevated up to 20-fold in typical cases, can slow long-term progression of the retinal degeneration [12, 13]. Dietary plans including restricting arginine and low protein intake adding or not supplementations such as vitamin B6 or l-lysine, have been reported useful to lower and stabilize ornithine levels showing good results in vision improvement and slowing the progression. [2, 14–18]. While some case reports and a few case series of GA patients from diverse ethnic groups have been clinically and molecularly characterized [16, 17, 19–21] no information from Latin American patients do exist.
The aim of this study is to describe the retinal phenotype by functional and structural retinal testing in a Mexican cohort of patients diagnosed with GA and to report the pathogenic OAT variants identified in them. This is the first case series of GA reported in the Mexican population and our results expand the understanding of retinal phenotype and the mutational spectrum associated with GA.
Methods and materials
The study was approved by the Institutional Ethics Committee of the Institute of Ophthalmology “Conde de Valenciana” in Mexico City and was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants. The inclusion criteria included patients with clinical characteristics of GA and clear media that allowed adequate clinical and multimodal imaging evaluation of the eye. Patients with suspected GA but no mutation in OAT gene were excluded.
Clinical ophthalmological evaluation
The patients were evaluated at the Clinic for Inherited Retinal Degenerations of the Institute of Ophthalmology Conde de Valenciana by a retina specialist by means of a full ophthalmological evaluation that included slit lamp examination, intraocular pressure measurement by applanation tonometry, and dilated funduscopic evaluation. Demographic data as well as a complete clinical questionnaire were reported. All patients underwent best-corrected visual acuity measurement (BCVA) using Snellen Chart and then converted to Logarithm of Minimum Angle of Resolution (logMAR) values.
Structural retinal evaluation
Wide field color fundus photography (CFP) (Optos California, USA) and a 55 grade color fundus photography (Spectralis; Heidelberg Engineering GmbH; Heidelberg, Germany) or a 45 grade color fundus photography (DRI OCT Triton, Swept source OCT, TOPCON Corporation; Tokyo, Japan) were taken to each patient. To estimate retinal pigment epithelium (RPE) status, retinal autofluorescence (FAF) was obtained using the 488 nm (Spectralis; Heidelberg Engineering GmbH; Heidelberg, Germany) and the 532 nm (Optos California, USA) imaging softwares. Retinal horizontal and vertical cross-sections were obtained with the spectral domain optical coherence tomography (SD-OCT) (Spectralis; Heidelberg Engineering GmbH; Heidelberg, Germany). A 9-mm line scan along the horizontal and vertical meridians crossing the fovea was performed in all patients. The total area of the outer nuclear layer (ONL) in both meridians crossing the fovea, the subfoveal ONL thickness in the horizontal meridian, and the eccentricity of the ellipsoid zone (EZ) line in both meridians were determined by manual segmentation using the Spectralis built-in measurement software.
Functional retinal evaluation
Goldmann kinetic perimetry (GP) was performed in all patients without nystagmus (MonCvONE, Metrovision; Pérenchies, France). Full field ERG (ffERG) (MonPackONE, Metrovision; Pérenchies, France) was performed in all patients using the International Society for Clinical Electrophysiology of Vision (ISCEV) standards. Topographical retinal function of cones and rods were determined by photopic and scotopic visual field testing (Chromatic perimetry; CP) (MonCvONE, Metrovision; Pérenchies, France) under light and dark-adaptation, respectively, in the eye with better BCVA in each patient.
Biochemistry measures
Blood samples were obtained to quantify ornithine plasma levels in all patients and were analyzed by an independent external laboratory.
Mutation screening of OAT gene
Genetic analysis was performed at the Laboratory of Molecular Genetics of the Institute of Ophthalmology Conde de Valenciana. For all patients, genomic DNA (gDNA) was extracted from peripheral blood leukocytes using standard methods after obtaining informed consent. The 9 coding exons and exon–intron junctions of the OAT gene were amplified by PCR using pairs of primers and temperature conditions available on request. Amplicons were sanger sequenced by the dideoxy chain method using a Compact System Sequencer (Promega, Madison, USA). Obtained OAT sequences were manually compared with the canonical gene transcript NM_000274.4. In compound heterozygous cases, available first relatives were also genetically screened for confirmation of trans configuration of the pathogenic variants.
Results
Clinical, structural and functional results
A total of seven patients 12 eyes were included in the study, including 4 females and 3 males. The mean age at the time of the first examination was 34.2 years old (age range, 10–66). The mean age of first symptoms reported by the patients were at 9.2 years old (age range, 5–20), being nyctalopia (N = 3) the main visual symptom in 42.8% of the patients as well as blurry vision in 42.8% (N = 3). Associated systemic features included muscle weakness in 29% (N = 2) of patients, cognitive deficit in 14% (N = 1) of patients, and history of otitis media associated and some degree of hearing loss in 29% (N = 2) of patients. 29% (N = 2) of the patients in the study were related (siblings) (P2 and P3). None of the patients were born to consanguineous parents.
Within the ophthalmological clinical findings, the mean BCVA was 0.5 LogMar (range between 0.0–1) (Snellen 20/63; range between 20/20 and 20/200). 29% (N = 2) of the patients had only one functional eye, one eye had an untreated chronic rhegmatogenous retinal detachment (RRD) with no light perception (P2) and the other eye was eviscerated due to an open ocular trauma (P7). 67% (N = 8) of the eyes had myopia and 50% of the eyes (N = 6) underwent early intervention of cataract surgery (mean age at 20 years) (Table 1).
Table 1.
Clinical characteristics and ornithine plasma levels
| Patient No | Gender | Age at examination (yrs) | Age at presenting symptoms | Main symptom | Visual acuity (LogMar) | Cataract surgery | Ornithine in plasma (µmol/L) | ||
|---|---|---|---|---|---|---|---|---|---|
| Right eye | Left eye | Right eye | Left eye | ||||||
| P1 | F | 10 | 6 | Visual field constriction | 0.2 | 0.1 | No | No | 916 |
| P2* | M | 16 | 6 | Blurry vision | – | 0.2 | - | No | 552 |
| P3* | M | 20 | 15 | Blurry vision | 0 | 0 | No | No | 583 |
| P4 | F | 27 | 6 | Nyctalopia | 0.4 | 0.5 | Yes | Yes | 636 |
| P5 | M | 47 | 20 | Nyctalopia | 0.9 | 0.7 | Yes | No | 48 |
| P6 | F | 54 | 7 | Blurry vision | 1 | 1 | Yes | Yes | 353 |
| P7 | F | 66 | 5 | Nyctalopia | 0.6 | – | Yes | – | 479 |
*Patients 2 and 3 are sibs
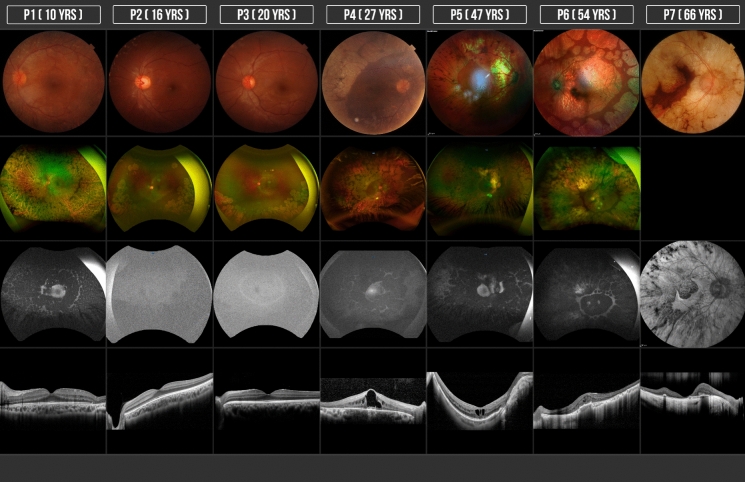
Fundoscopy revealed typical changes of GA in 100% (N = 12) of the eyes, showing sharply demarcated, circular areas of chorioretinal atrophy predominantly distributed in the peripheral retina. 75% (N = 9) of the eyes had macular involvement at the time of the first evaluation of which 25% (N = 3) the fovea was affected. P6 presented a round-shaped atrophic area in the macula with some preserved central foveal tissue with an evident ring-shape area of preserved retina between the coalescent peripheral patches and the posterior pole atrophy (Fig. 1).
Fig. 1.
Structural retinal evaluation: CFC, FAF and SD-OCT of patients with GA. Each column corresponds to the case number (patients 1 through 7) and patients are numbered according to age (youngest to oldest)
All the patients presented round or oval areas of hypoautofluorescence mainly in the periphery that tend to coalesce, spreading to the macula and partially sparing the peripapillary region, leaving some areas of hyperautofluorescence and also at the border of preserved retina in the macular area. P2 and P3 had an hyperautofluorescent ring outside the arcades separating the posterior retina with normal autofluorescence from hypoautofluorescence in the mid-periphery which did not correspond to the hypoautofluorescence of the patchy atrophic lesions in the far periphery (Fig. 1).
The structural features of the retina by SD-OCT evaluation demonstrated structural changes that were concordant between eyes in all patients. In the areas of chorioretinal atrophy we found evident thinning of the retina predominantly the external layers with complete loss of photoreceptor inner segment outer segment junction IS/OS outer retinal as well as the interdigitation zone and external limiting membrane as well as evident choroidal thinning in the most advanced cases, outer retinal tubulations (ORT) were found in 58% (N = 7) of the eyes, cystoid macular edema (CME) was found in 50% (N = 6) of the eyes, 42% (N = 5) of the eyes had an epiretinal membrane (ERM), 17% (N = 2) of the eyes presented foveoschisis and a staphyloma. None of the eyes (N = 0) had focal choroidal excavation. Diffuse hyperreflective deposits were found in 100% (N = 12) of the eyes, most of them were found within the inner retina, mainly in the ganglion cell layer and the inner nuclear layer. These lesions were more evident in the two patients with CME, appearing as a band-shape zone in the ganglion cell layer closer to the inner plexiform layer (P4 and P5) (Fig. 1).
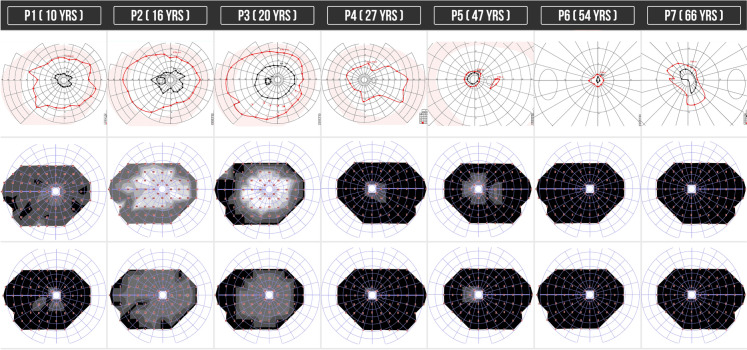
The GP test showed constricted visual fields in the isopters I4e and V4e in 100% (N = 12) of the eyes, P3 had an almost preserved V4e visual field in both eyes. P5, P6 and P7 who presented with a more advanced disease had a markedly constricted visual field with a small central visual field in the isopter V4e corresponding to the preserved retina in the fovea (Fig. 2).
Fig. 2.
Functional retinal evaluation: GP and CP of patients with GA. (ffERG not shown). First CP image reports the scotopic response and second CP image reports the photopic response. Each column corresponds to the case number (patients 1 through 7) and patients are numbered according to age (youngest to oldest)
The ffERG revealed a preserved photopic response in 17% (N = 2) of the eyes and a preserved scotopic response in 17% (N = 2) of the eyes. These nearly preserved responses were observed in two of the younger patients (P2 and P3).
Regarding the functional evaluation by the scotopic and photopic visual field test we could see that all of the patients presented some degree of deficit in the generalized response of rods and cones (more evident in the scotopic tests), finding a more reduced response in patients with more advanced disease, in P2 and P3 with a structural well-preserved macula we found an initial but significant mean deficit in the photopic evaluation (cone response) of 25 dB and 23 dB, respectively, which correlates with the sub-normal findings in the electroretinogram test (Fig. 2).
Ornithine plasma levels results
Mean ornithine plasma levels were 509.5 µmol/L, (ranges between 48 and 916 µmol/L) this reflects an increase of up to more than 4 times its normal value (< 107 µmol/L). *Normal ranges vary between laboratories (22–115 µmol/L are considered normal). An interesting finding is that one patient has ornithine plasma levels within normal ranges (P5) (Table 1).
Molecular findings
Biallelic OAT mutations were identified in all seven patients, including a pair of affected sibs (P2 and P3). A total of three compound heterozygous and three homozygous phenotypes were identified in the 7 probands. Two novel OAT pathogenic variants were identified: c.796 C > T (p.Gln266*) and c.721_722dupCC (p.Asp242ArgfsTer6). The c.721_722dupCC variant was observed in 6 out of 12 pathogenic alleles in our cohort. The most commonly mutated OAT exon in our series was exon 6, harboring 9 out of all 12 disease-causing variants. All three cases of compound heterozygosity demonstrated to carry biallelic OAT mutations (trans configuration) by genetic screening of first-degree relatives (data not shown). Two previously identified mutations in OAT, c.991C > T(p.Arg331*) and c.677C > T (p.Ala226Val) were also recognized (Table 2).
Table 2.
OAT pathogenic variants identified in Mexican GA patients. Two novel mutations were identified
| Patient no | AGE | EXON | OAT variant | Zygosity | ACMG classification | References |
|---|---|---|---|---|---|---|
| P1 | 10 | 6 | c.721_722dupCC; p.Asp242fsTer | Homozygous | Pathogenic | Novel |
| P2* | 16 | 7 | c.796 C > T; p.Gln266Ter | Compound heterozygote | Pathogenic | Novel |
| P3* | 20 | 8 | c.991C > T; p.Arg331Ter | Pathogenic | Kaczmarczyk et al. [35] | |
| P4 | 27 | 6 | c.721_722dupCC; p.Asp242ArgfsTer6 | Compound heterozygote | Pathogenic | Novel |
| 7 | c.796C > T; p.Gln266Ter | Pathogenic | Novel | |||
| P5 | 47 | 6 | c.677C > T; p.Ala226Val | Homozygous | Pathogenic | Michaud et al. [36] |
| P6 | 54 | 6 | c.721_722dupCC; p.Asp242ArgfsTer6 | Compound heterozygote | Pathogenic | Novel |
| 8 | c.991C > T; p.Arg331Ter | Pathogenic | Kaczmarczyk et al. [35] | |||
| P7 | 66 | 6 | c.721_722dupCC; p.Asp242ArgfsTer6 | Homozygous | Pathogenic | Novel |
*Patients 2 and 3 are sibs
Discussion
In the present study we described the anatomical and functional characteristics of seven patients 12 eyes with GA and performed a genetic analysis finding two novel pathogenic variants in the OAT analysis. Most of the features in the patients studied are consistent to previous reports such as age of first symptoms (second decade of life), being night blindness the most common reported symptom, also most of the phakic eyes had mild to high myopia and some degree of cataract with the need of intervention in 50% in the third decade of life [19, 22].The study by Peltola K et al. made in 35 finish patients reported the mean age of diagnosis at 18 years old, in our study the mean age was 34.2 years old which can translate to a late diagnosis. Central and peripheral nervous system abnormalities (muscular weakness, cognitive and hearing impairment) have been demonstrated in patients with GA by electrophysiological studies and these findings were also presented in some patients in our study [23, 24].
In the ophthalmological evaluation the mean BCVA of 0.5 logMar (Snellen 20/63) was worse than a similar case series of 7 patients with GA where a mean BCVA of 0.26 logMar was reported, this can be explained by the small number of patients studied in both series and the heterogeneity of disease severity. P2 16 years old was blind in one eye secondary to an untreated RRD, interestingly some cases have been reported related to this finding in pediatric patients with GA, with a novel OAT mutation confirmation in one case [25, 26], as pediatric RRD is uncommon even in myopic patients and GA has a very low prevalence worldwide it is important to evaluate if this complication might be another feature included in the clinical manifestations of GA.
In the fundoscopy all eyes presented patches of chorioretinal atrophy in different amounts which tended to be more coalescent in the older patients except for P1 who was 10 years old and presented an advanced stage of the disease. Another clinical finding which needs a special mention was presented in P6 (54 years old), with a well-defined area of atrophy in the macula separated from the peripheral atrophic patches by an area of preserved retina at the equator in both eyes, this is different to what has been classically described where the atrophy starts in the mid-periphery and spreads to the macula and the ora serrata in the more advanced stages [7], as we don’t have previous images of this patient we can only suggest but not proof a different pattern of atrophic changes in patients with GA that also start at one point of the disease in the posterior pole and not only in the periphery (Fig. 1). We found this pattern with similar characteristics by the fundus image of two patients presented by Sergouniotis et al. [19].
FAF findings were consistent to the atrophic area in all cases, with some degree of hyperautofluorescence in the border of the preserved retina as mentioned before [19]. With the use of ultra-wide field autofluorescence, P2 and P3 (siblings) showed an interesting pattern with an evident hyperautofluorescent ring outside the arcades that separated the retina with normal autofluorescence posteriorly from the mid-periphery retina anteriorly with subtle hypoautofluorescence that did not show the same degree of autofluorescence found in the chorioretinal patches in far-periphery retina (Fig. 1). By this finding in these two patients 3 eyes, we can suggest that an initial RPE damage that is not clinically visible can precede the chorioretinal patches of atrophy which might be evidenced by the FAF. Measuring this hyper-AF ring in patients with initial damage could work as an indicator of progression and to do a more accurate follow-up.
In our study, CME was found in 50% of the eyes studied which differs from the 100% of eyes described in Sergouniotis et al. case series [19]. This specific finding can be explained by a disruption of the outer blood-retinal barrier in the parafoveal area leading to a diffusion of fluid toward intraretinal spaces, maybe a lower incidence of this finding in our study could be attributed to the fact that the patients who did not present intraretinal cyst had a more preserved parafoveal area. CME in this type of retinal dystrophy has been treated with topical and oral carbonic anhydrase inhibitors, topical non-steroidal anti-inflammatory drugs, intravitreal or subtenon steroid injections, restriction of arginine in diet and vitamin B6 supplementation with widely range of response [27, 28]. Half of the patients presented ORT, the youngest patient (P1, 10 years old) with very advanced damage also presented this finding which demonstrates that the ORT can be considered as an indicator of the stage and marker of severity of the disease as mentioned before [29]. The intraretinal hyperreflective deposits found in the ganglion cell layer had been reported previously [19], usually found near the chorioretinal atrophy, in our study found these deposits along all the preserved macula, a gliotic process due to cell death has been hypothesized in a mice model study. In our study we found more accumulation of these deposits in the patients with chronic macular edema, where they seem to coalesce and form a band-shape zone.
Interestingly, the visual field constriction found in the GP mainly in isopter I4e and the generalized decrease in sensitivity response by the topographic functional evaluation of the cones and rods response separately with scotopic and photopic perimetry in all of eyes was more severe than the clinical changes visible on fundoscopy, making these two functional objective studies parts of the follow-up evaluation of patients with GA (Fig. 2).
Although it was not possible to make a correlation between the severity of retinal degeneration and ornithine levels as the measurements were done in a cross-sectional manner, we found a lower mean of elevated plasma ornithine levels of 586 µmol/L (excluding the patient with normal values) than reported in the finnish and japanese case series, 960 and 975 µmol/L respectively [19, 22], nevertheless the variability of the disease’s severity was quite similar. The possible explanation for this finding could be related to the differences in diet between populations, but this must be properly studied. Furthermore, P5 (47 years old) with a homozygous OAT pathogenic variant had normal ornithine plasma levels at the time of the diagnosis, without any diet modification or treatment (Table 1). Previous studies have reported patients with normal ornithine levels and clinical findings corresponding to GA but with no genetic confirmation [30–34] naming these patients “GA-like phenotype”.
To date, this is the first reported case of GA confirmed by genetic testing with normal ornithine levels. It is important to note that we didn’t have the opportunity to repeat the ornithine level measurements in this patient. In such cases, it is recommended to perform the measurements at least three times to confirm the results. This supports the theory proposed by the authors mentioned before that there might be more than one pathophysiological mechanism within the metabolic pathways of ornithine and arginine, leading to an accumulation or a decrease of substrates that eventually produce chorioretinal degeneration. This should be evaluated in more studies in the future.
Pathogenic OAT variants were identified in all GA patients from this cohort. Interestingly, 50% of disease-causing mutations (6/12) corresponded to the novel c.721_722dupCC (p.Asp242ArgfsTer6) variant, which was observed homozygously in two cases and in compound heterozygosity in two additional patients. While additional haplotype studies will be needed, our preliminary data supports that the c.721_722dupCC variant could be a founder OAT mutation in Mexico. A second novel OAT variant, corresponding to c.796C > T (p.Gln266*), was identified in a single allele in this group of patients (Table 2).
Patients included in this study were referred to a specialist in metabolic nutrition to implement an arginine-restricted diet, ensuring careful consideration of nutraceutical components to prevent malnutrition-related disorders. This dietary intervention was meticulously designed to balance the reduction of arginine intake while providing all essential nutrients, thereby safeguarding against the potential risks of dietary insufficiencies. The collaboration with nutritional experts highlights the importance of a multidisciplinary approach in managing complex metabolic conditions.
In conclusion, the present study expands our knowledge of the clinical and genetic features of GA in the Mexican population. This study provides new information regarding functional and structural diagnosis to use in the future and evaluate if these tools can help us make a more accurate diagnosis on staging of the disease and a better follow-up to the patients under treatment. The most frequent mutations as well as the novel molecular findings in the Mexican population have important implications for the future genetic diagnosis of GA, allowing us to identify carriers of the disease and also study the genotype–phenotype correlation in larger studies in the future.
Clinical studies on retinal dystrophies are imperative for a deeper understanding of the disease’s clinical course. Identifying the most precise clinical variables that measure disease progression is crucial, as these indicators are integral to the meticulous planning of gene therapy clinical trials aimed at slowing or halting disease advancement. Such trials depend heavily on accurate, reproducible clinical endpoints that not only reflect the true nature of the disease's progression but also offer measurable targets for therapeutic intervention.
Acknowledgements
The authors wish to acknowledge the assistance of Gerardo Alan Martínez Aguilar and Tatiana Urrea-Victoria M.D from the Retina and Vitreous Department, Institute of Ophthalmology “Conde de Valenciana”, Mexico City and Luis Ángel Montes Almanza from the Genetics Department, Institute of Ophthalmology “Conde de Valenciana”, Mexico City.
Author contributions
All authors contributed to the study conception and design, writing, material preparation, data collection, and analysis. All authors read and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the images in this manuscript.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ana Lía Díazceballos-García, Email: analiadiazceballos@gmail.com.
Rodrigo Matsui, Email: romatsui@institutodeoftalmologia.org.
References
- 1.Saebo J (1948) Atrophia gyrata choroideae et retinae. Br J Ophthalmol 32(11):824–847. 10.1136/bjo.32.11.824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takki K (1974) Gyrate atrophy of the choroid and retina associated with hyperornithinaemia. Br J Ophthalmol 58(1):3–23. 10.1136/bjo.58.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Donnell JJ, Sandman RP, Martin SR (1978) Gyrate atrophy of the retina: inborn error of L-ornithin:2-oxoacid aminotransferase. Science 200:200–201. 10.1126/science.635581 [DOI] [PubMed] [Google Scholar]
- 4.Shen BW, Hennig M, Hohenester E, Jansonius JN, Schirmer T (1998) Crystal structure of human recombinant ornithine aminotransferase. J Mol Biol 277(1):81–102. 10.1006/jmbi.1997.1583 [DOI] [PubMed] [Google Scholar]
- 5.Simell O, Takki K (1973) Raised plasma – ornithine and gyrate atrophy of the choroid and retina. Lancet 1(7811):1031–1033. 10.1016/s0140-6736(73)90667-3 [DOI] [PubMed] [Google Scholar]
- 6.Rojas, S. (2012). Retina y Vítreo: Desórdenes hereditarios y distrofias de retina. (2nd edn). Manual Moderno. México.
- 7.Takki KK, Milton RC (1981) The natural history of gyrate atrophy of the choroid and retina. Ophthalmology 88(4):292–301. 10.1016/s0161-6420(81)35031-3 [DOI] [PubMed] [Google Scholar]
- 8.Ramesh V, Benoit LA, Crawford P, Harvey PT, Shows TB et al (1988) The ornithine aminotransferase (OAT) locus: analysis of RFLPs in gyrate atrophy. Am J Hum Genet 42(2):365–372 [PMC free article] [PubMed] [Google Scholar]
- 9.Krill A, Archer D (1971) Classification of the Choroidal Atrophies. Am J Ophthalmol 72(3):562–585. 10.1016/0002-9394(71)90854-3 [DOI] [PubMed] [Google Scholar]
- 10.Vannas-Sulonen K (1987) Progression of gyrate atrophy of the choroid and retina: a long-term follow-up by fluorescein angiography. Acta Ophthalmol 65(1):101–109. 10.1111/j.1755-3768.1987.tb08499.x [DOI] [PubMed] [Google Scholar]
- 11.Wilson DJ, Weleber RG, Green WR (1991) Ocular clinicopathologic study of gyrate atrophy. Am J Ophthalmol 111:24–33. 10.1016/s0002-9394(14)76892-8 [DOI] [PubMed] [Google Scholar]
- 12.Kaiser-Kupfer MI, Caruso RC, Valle D, Reed GF (2004) Use of arginine- restricted diet to slow progression of visual loss in patients with gyrate atrophy. Arch Ophthalmol 122(7):982–984. 10.1001/archopht.122.7.982 [DOI] [PubMed] [Google Scholar]
- 13.Santinelli R, Costagliola C, Tolone C, D’Aloia A, D’Avanzo A et al (2004) Low-protein diet and progression of retinal degeneration in gyrate atrophy of the choroid and retina: a twenty-six-year follow-up. J Inherit Metab Dis 27(2):187–196. 10.1023/B:BOLI.0000028779.29966.05 [DOI] [PubMed] [Google Scholar]
- 14.Cui X, Jauregui R, Park K, Tsang S (2018) Multimodal characterization of a novel mutation causing vitamin B6-responsive gyrate atrophy. Ophthalmic Genet 39(4):512–516. 10.1080/13816810.2018.1474370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heller D, Weiner C, Nasie I, Anikster Y, Landau Y et al (2017) Reversal of cystoid macular edema in gyrate atrophy patients. Ophthalmic Genet 38(6):549–554. 10.1080/13816810.2017.1301966 [DOI] [PubMed] [Google Scholar]
- 16.Kim SJ, Lim DH, Kim JH, Kang SW (2013) Gyrate atrophy of the choroid and retina diagnosed by ornithine-δ-aminotransferase gene analysis: a case report. Korean J Ophthalmol 27(5):388–391. 10.3341/kjo.2013.27.5.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zekušić M, Škaričić A, Fumić K, Rogić D, Žigman T et al (2018) Metabolic follow-up of a Croatian patient with gyrate atrophy and a new mutation in the OAT gene: a case report. Biochem Med (Zagreb) 28(3):030801. 10.11613/BM.2018.030801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elpeleg N, Korman SH (2001) Sustained oral lysine supplementation in ornithine delta-aminotransferase deficiency. J Inherit Metab Dis 24(3):423–424. 10.1023/a:1010545811361 [DOI] [PubMed] [Google Scholar]
- 19.Sergouniotis PI, Davidson AE, Lenassi E, Devery SR, Moore AT et al (2012) Retinal structure, function, and molecular pathologic features in gyrate atrophy. Ophthalmology 119(3):596–605. 10.1016/j.ophtha.2011.09.017 [DOI] [PubMed] [Google Scholar]
- 20.Huang J, Fu J, Fu S, Yang L, Nie K et al (2019) Diagnostic value of a combination of next-generation sequencing, chorioretinal imaging and metabolic analysis: lessons from a consanguineous Chinese family with gyrate atrophy of the choroid and retina stemming from a novel OAT variant. Br J Ophthalmol 103(3):428–435. 10.1136/bjophthalmol-2018-312347 [DOI] [PubMed] [Google Scholar]
- 21.Katagiri S, Gekka T, Hayashi T, Ida H, Ohashi T et al (2014) OAT mutations and clinical features in two Japanese brothers with gyrate atrophy of the choroid and retina. Doc Ophthalmol 128(2):137–148. 10.1007/s10633-014-9426-1 [DOI] [PubMed] [Google Scholar]
- 22.Peltola KE, Näntö-Salonen K, Heinonen OJ, Jääskeläinen S, Heinänen K et al (2001) Ophthalmologic heterogeneity in subjects with gyrate atrophy of choroid and retina harboring the L402P mutation of ornithine aminotransferase. Ophthalmology 108(4):721–729. 10.1016/s0161-6420(00)00587-x [DOI] [PubMed] [Google Scholar]
- 23.Valtonen M, Näntö-Salonen K, Jääskeläinen S, Heinänen K, Alanen A et al (1999) Central nervous system involvement in gyrate atrophy of the choroid and retina with hyperornithinaemia. J Inherit Metab Dis 22(8):855–866. 10.1023/a:1005602405349 [DOI] [PubMed] [Google Scholar]
- 24.Peltola KE, Jääskeläinen S, Heinonen OJ, Falck B, Näntö-Salonen K et al (2002) Peripheral nervous system in gyrate atrophy of the choroid and retina with hyperornithinemia. Neurology 59(5):735–740. 10.1212/wnl.59.5.735 [DOI] [PubMed] [Google Scholar]
- 25.Magliyah M, Alsalamah AK, AlOtaibi M, Nowilaty SR (2021) A novel c.980C>G variant in OAT results in identifiable gyrate atrophy phenotype associated with retinal detachment in a young female. Ophthalmic Genet 42(2):204–208. 10.1080/13816810.2020.1843185 [DOI] [PubMed] [Google Scholar]
- 26.Barnett CP, Lam WC, Schulze A (2009) Retinal detachment causing unilateral blindness in a 12-year-old girl with gyrate atrophy. J Inherit Metab Dis 32(5):670. 10.1007/s10545-009-1220-y [DOI] [PubMed] [Google Scholar]
- 27.Çavdarlı C, Şahlı E, Çavdarlı B, Alp MN (2020) Regression of macular edema with topical brinzolamide and nepafenac alone and identification of a novel gyrate atrophy mutation. Arq Bras Oftalmol 83(2):149–152. 10.5935/0004-2749.20200028 [DOI] [PubMed] [Google Scholar]
- 28.Casalino G, Pierro L, Manitto MP, Michaelides M, Bandello F (2018) Resolution of cystoid macular edema following arginine-restricted diet and vitamin B6 supplementation in a case of gyrate atrophy. J AAPOS 22(4):321–323. 10.1016/j.jaapos.2017.12.016 [DOI] [PubMed] [Google Scholar]
- 29.Goldberg NR, Greenberg JP, Laud K, Tsang S, Freund KB (2013) Outer retinal tubulation in degenerative retinal disorders. Retina 33(9):1871–1876. 10.1097/IAE.0b013e318296b12f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saito T, Hayasaka S, Yabata K, Omura K, Mizuno K et al (1981) Atypical gyrate atrophy of the choroid and retina and iminoglycinuria. Tohoku J Exp Med 135(3):331–332. 10.1620/tjem.135.331 [DOI] [PubMed] [Google Scholar]
- 31.Bargum R (1986) Differential diagnosis of normoornithinaemic gyrate atrophy of the choroid and retina. Acta Ophthalmol (Copenh) 64(4):369–373. 10.1111/j.1755-3768.1986.tb06937.x [DOI] [PubMed] [Google Scholar]
- 32.Kellner U, Weleber RG, Kennaway NG, Fishman GA, Foerster MH (1997) Gyrate atrophy-like phenotype with normal plasma ornithine. Retina 17(5):403–413. 10.1097/00006982-199709000-00008 [DOI] [PubMed] [Google Scholar]
- 33.Labiano AT, Arroyo MH (2020) Gyrate atrophy-like phenotype with normal plasma ornithine and low plasma taurine. GMS Ophthalmol Cases. 27(10):04. 10.3205/oc000131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pauleikhoff L, Weisschuh N, Lentzsch A, Spital G, Krohne TU, Agostini H, Lange CAK (2024) Clinical characteristics of gyrate atrophy compared with a gyrate atrophy-like retinal phenotype. Eur J Ophthalmol 34(1):79–88. 10.1177/11206721231178147 [DOI] [PubMed] [Google Scholar]
- 35.Kaczmarczyk A, Baker M, Diddle J, Yuzyuk T, Valle D et al (2022) A neonate with ornithine aminotransferase deficiency; insights on the hyperammonemia-associated biochemical phenotype of gyrate atrophy. Mol Genet Metab Rep. Mar 16;31:100857. 10.1016/j.ymgmr.2022.100857 [DOI] [PMC free article] [PubMed]
- 36.Michaud J, Thompson GN, Brody LC, Steel G, Obie C et al (1995) Pyridoxine-responsive gyrate atrophy of the choroid and retina: clinical and biochemical correlates of the mutation A226V. Am J Hum Genet. Mar;56(3):616–622 [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.