Abstract
Rectal migration of an intrauterine device (IUD) is a rare but potentially serious complication requiring prompt diagnosis and management. We present a rare case of rectal migration of an intrauterine device (IUD) in a 26-year-old female, highlighting the clinical presentation, diagnostic evaluation, laparoscopic removal, and postoperative outcomes. This case emphasizes the critical importance of vigilant monitoring, early intervention, and close follow-up in managing IUD migration to ensure optimal patient outcomes. Timely recognition and intervention resulted in successful symptom resolution and a favorable long-term prognosis.
Keywords: Intrauterine device, Case report, Perforation, Migration, Rectum
Introduction
The intrauterine device (IUD) is a highly effective contraceptive method [1], boasting an efficacy exceeding 99% [2]. However, it can occasionally cause complications, such as uterine perforation and subsequent migration into adjacent organs.
Rectal migration and its complications still an exceedingly rare event. We present a case of rectal migration of an IUD in a 26-year-old woman, highlighting the clinical presentation, diagnostic approach, surgical intervention, and long-term outcomes.
Case presentation
A 26-year-old female, treated for hypothyroidism and hyperprolactinemia, presented to the emergency department with menorrhagia and rectal bleeding ongoing for 7 months following the placement of an intrauterine device (IUD). The rectal bleeding only occurred during her menstrual cycle. She also reported abdominal pain and dysmenorrhea.
Her medical history included multiple vaginal deliveries, and she had been using an IUD for contraception for the past 7 months.
Upon admission, her vital signs were normal, and hypogastric tenderness without a palpable mass was noted.
An anorectal digital examination showed regular mucosa with the presence of blood. A gynecological examination did not find the IUD string in the vaginal canal.
Routine laboratory investigations, including urinalysis, returned normal results.
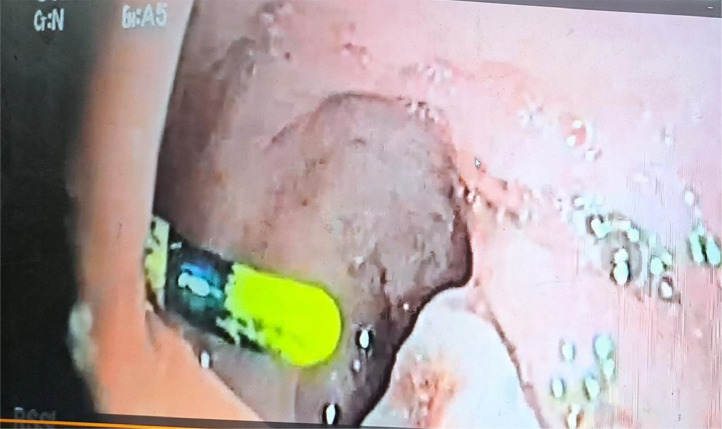
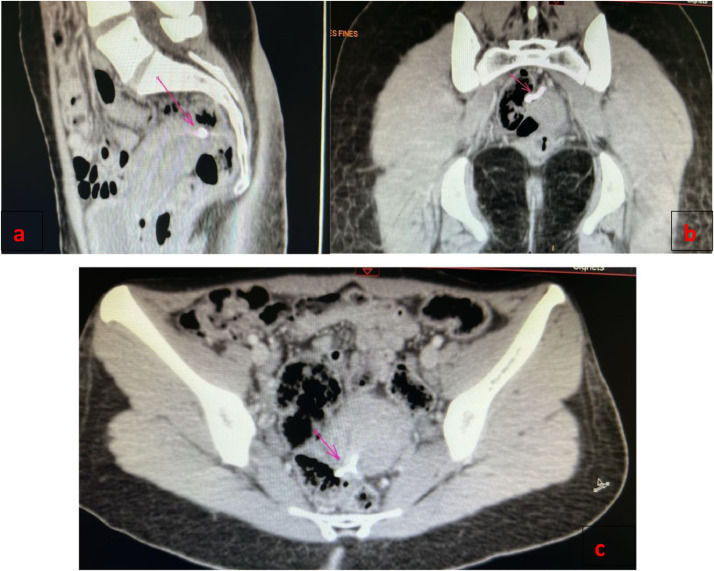
A rectosigmoidoscopy revealed a foreign body located 15 cm from the anal margin (Fig. 2). An abdominopelvic CT scan showed that the IUD was located in the lower uterine segment, had perforated the postero-lateral uterine wall, and the upper rectal wall at a distance of 14 cm from the anal margin (Fig. 1). The diagnosis of secondary uterine perforation with intrarectal migration of the IUD was confirmed.
Fig. 2.
Intrauterine device embedded in the wall of the rectum.
Fig. 1.
Abdominal computerised tomography scan showed the intrauterine device (A) and the IUD outside the cavity, closely related to the rectum (B, C) IUD marked with an arrow.
After multidisciplinary consultation, the decision was made to remove the IUD hysteroscopically. Intraoperative findings revealed that the vertical rod of the IUD had penetrated the myometrium in a lateral-posterior position. The IUD was successfully extracted using endoscopic forceps, and the patient's postprocedural recovery was uneventful.
At the 1-week follow-up, there was no evidence of recurrent bleeding, and a subsequent endoscopy showed erythema 2 cm from the anal margin, indicating the absence of further complications.
Discussion
According to the World Health Organization (WHO) and the American College of Obstetricians and Gynecologists (ACOG), intrauterine devices (IUDs) have emerged as highly effective and widely utilized contraceptive methods since 1965 due to their low cost, easy availability, and minimal side effects [1,3].
The insertion of an IUD, while not technically difficult, involves multiple complications, particularly when proper usage protocols are not followed.
These complications include syncope, seizures, pelvic inflammatory disease, ectopic pregnancy, and septic abortion [4,5].
The most serious complication linked to IUD insertion is uterine perforation, which can lead to migration into adjacent structures such as the urinary bladder, bowel, omentum, and retroperitoneum [6,3]. The risk of perforation is less than 1 in 1,000 insertions for currently available IUDs [4], but it is heightened during the period 4 to 8 wk postpartum.
The mechanism of perforation is attributed either to the insertion procedure or to a chronic inflammatory reaction that causes gradual erosion through the uterine wall [3]. Several factors influence the incidence of perforation, including uterine size, position, timing of insertion, congenital uterine anomalies, myometrial defects (whether pre-existing or iatrogenic from IUD placement), lactation, placement of the IUD within 6 months postpartum, nulliparity, and previous miscarriages [[7], [8], [9], [10]].
In a minority of cases, an IUD may perforate through all layers of the uterus (endometrium, myometrium, serosa), resulting in complete severance of its uterine connection and allowing it to lie freely within the peritoneal cavity. Alternatively, the IUD may become enveloped by the omentum or, less commonly, be found in the broad ligament or other extrauterine locations. This condition is known as complete perforation. In contrast, partial perforation refers to cases where only a portion of the uterus is involved [3].
Complications associated with rectal migration of an IUD can vary in severity and may require prompt recognition and intervention to prevent adverse outcomes. Potential complications include infection, the development of abscesses, bleeding, or perforations involving other intraperitoneal organs. A literature review reveals that the most common visceral involvement occurs in the omentum, followed by the bladder [3]. A few cases have been reported in the rectum.
Several classifications of uterine perforation have been proposed:
-
1.Esposito Classification:
-
○Type A: Complete perforation
-
○Type B: Partial perforation
-
○
-
2.Mahran Classification:
-
○First Degree: Minor extent of partial perforation
-
○Second Degree: Greater extent of partial perforation
-
○Third Degree: Complete perforation
-
○
-
3.Ansari Classification:
-
○Category A: Minor partial perforation
-
○Category B: Significant partial perforation
-
○Category C: Complete perforation
-
○Category D: Complete perforation with the string absent at the external os [3]
-
○
This extended classification by Ansari incorporates both the degree of perforation and the presence or absence of the IUD string at the external os, providing a more comprehensive assessment of uterine perforation.
The clinical presentation of rectal migration of an IUD can vary depending on the extent of migration, associated complications, and individual patient factors. Common signs and symptoms include (Table 1).
Table 1.
This table organizes the symptoms clearly, with columns for the symptom, its description,and the context in which it might occur.
| Symptoms | Description | Context |
| Asymptomatic expulsions | Occurs in approximately 38.3% of cases [9] | Represents a significant proportion of cases where no symptoms are reported [9] |
| Rectal bleeding | Associated with vaginal spotting, tenesmus | Could suggest migration of the IUD to adjacent structures. |
| Pelvic pain or cramping | Localized or diffuse abdominal pain, constant or intermittent | Frequent symptom [11] |
| Gynecological symptoms | Vaginal discharge, dyspareunia | Associated with abnormal positioning or complications of IUD [11] |
| Recurrent infections | Increased risk of pelvic infections or abscesses | Potential for chronic infections due to foreign body presence. |
| Gastrointestinal symptoms | Constipation, diarrhea | The triad of chronic abdominal pain, fever, and intermittent diarrhea associated with a missing IUD has been considered as symptoms of intestinal injury |
| Urinary symptoms | Dysuria, pollakiuria, urinary urgency | Could indicate migration of the IUD into the urinary system. |
Patients with an IUD should be informed about the possibility of its migration. Regular self-examination for "missing threads" is essential for the early detection of IUD migration. If the string of the device is not visible, it is crucial to use imaging techniques to locate the IUD within the uterus and confirm its correct positioning.
Imaging plays a crucial role in confirming the diagnosis, assessing the extent of migration, identifying associated complications, and guiding surgical planning for device retrieval. Various imaging modalities include (Table 2).
Comparative analysis of imaging Modalities,Endoscopic evaluation and laparoscopy
| Imaging modality | Advantages | Disadvantages |
| Pelvic ultrasound | Noninvasive, widely available, real-time imaging | Limited soft tissue detail, operator-dependent |
| X-ray | Good for visualizing the IUD's location relative to bony structures | Limited soft tissue detail, radiation exposure |
| CT Scan | Detailed cross-sectional images, excellent for evaluating extent of migration and complications | High radiation exposure, less soft tissue contrast than MRI |
| MRI | Offers high-resolution images of soft tissues and is superior in visualizing the exact location of the IUD and its relation to pelvic structures without radiation exposure | Expensive, less available, longer scan times |
| Barium enema | Outlines the gastrointestinal tract, good for assessing bowel injury or obstruction | Invasive, not specific for IUD visualization |
| Endoscopic evaluation | Direct visualization allows for potential retrieval | Invasive, requires sedation/anesthesia |
| Diagnostic laparoscopy | Direct visualization allows for simultaneous diagnosis and treatment | Invasive, surgical risks, requires anesthesia |
The position of the IUD is determined by measuring the distance between the uterine fundus and the edge of the IUD closest to the fundus. It is considered correctly positioned when this distance measures less than 2 cm or, more specifically, if it is no greater than 4/3 of the mean thickness of the uterine wall [12]. These imaging techniques are essential for accurate diagnosis, effective management of IUD migration, and appropriate planning for surgical retrieval if necessary.
The management of rectal migration of an intrauterine device (IUD) requires a multidisciplinary approach involving gynecologists, colorectal surgeons, and other specialists. Treatment strategies are tailored based on the patient's symptoms, the extent of migration, and the presence of complications. The management options include (Table 3).
Management Strategies for Rectal Migration of an Intrauterine Device.
| Management strategies | Indication |
| Endoscopic removal (Colonoscopy) | For a minority of uncomplicated perforations. Where the device is situated within the lumen or embedded in the internal aspect of the wall. Coloscopic retrieval may present difficulties if the device is partially integrated into adjacent structures and surrounded by granulation tissue. |
| Surgical removal (laparoscopy or laparotomy) | Necessary when the IUD is inaccessible endoscopically or if complications such as bowel perforation, abscess formation, or bowel obstruction are present. |
| Antibiotic therapy | Required when there is evidence of infection or abscess. |
| Pain management | Use of analgesic medications to alleviate symptoms. |
| Psychological support | Counseling and support for anxiety or emotional distress. |
Table 4 summarizes the cases of intra-rectal migration of intrauterine devices, comparing our findings with the literature. Our case involved a 26-year-old multiparous female with rectal migration diagnosed after 7 months, revealed by gastrointestinal bleeding. This finding aligns with the literature, indicating that such cases are relatively rare but serious, requiring timely intervention.
Summarizes the cases of intra-rectal migration of intrauterine devices, comparing our findings with the literature. Our case involved a 26-year-old multiparous female with rectal migration diagnosed after seven months, revealed by gastrointestinal bleeding. This finding aligns with the literature, indicating that such cases are relatively rare but serious, requiring timely intervention.
| Case | Age | Gynecological History | IUD | Period of Insertion of IUD | Risk Factors | Symptoms | Treatment |
| Boushehry & et al. | 30 | P3G3 | Copper T | 1 y postpartum | Lactation | Abdominal pain | Combined laparoscopic-colonoscopic surgical intervention |
| Rui Li & et al. | 45 | P1G1 | - | 1 y postpartum | - | Abdominal pain, Dyspareunia | Laparoscopic removal |
| Isikhuemen & et al. | 32 | Multipara | Copper T | 6 mo postpartum | Pregnancy, Intrauterine insertion period (6 mo postpartum) | Inability to feel the IUD thread | IUD was retrieved with minimal pulling |
| B Macalou & et al. | 30 | Multipara | Gyne T 380 | 3 y before | Pregnancy | Abdominal pain, Diarrhea, Detection of IUD thread at the anal canal during defecation | IUD was retrieved with minimal pulling |
| Wuen Lynn Toh & et al. | 30 | G1P1 | Copper T | 6 wk postpartum | Intrauterine insertion period | Abdominal pain, Upper urinary infection, Fever | Partial rectotomy |
| María Antonia Huertas-Velasco & et al. | 30 | G2P2 | Copper T (Nova T380) | 4 mo before | Pregnancy | Vaginal bleeding | Colonoscopy (endoscopic removal with forceps) |
| Anisha Turner & et al. | 33 | G4P4 | - | - | Pregnancy | Rectal bleeding | Laparoscopic removal |
| Rola S Al Mukhtar & et al. | 37 | G4P4 | Copper T | One and a half years postpartum | Pregnancy | Rectal bleeding | Sigmoidoscopy endoscopic removal using a grasper and 2 endoclips were applied at the site of retrieval |
| O.O Bello & et al. | 31 | G2P2 | Copper-T 380A | - | Pregnancy | IUD strings protruding from her anus | IUD was removed under direct vision by slightly pulling on the strings |
| Dzib-Calan EA & et al. | 26 | Nulliparous | - | - | Nulliparity | Menstrual rhythm disorders, Dyspareunia | Colonoscopic removal |
| Lauren Shute & et al. | 55 | - | - | 30 y ago | - | Confusion, Dysuria, Lower back pain | Laparoscopic removal |
| Sophie Schoenen & et al. | 50 | G3P3 | Copper T | - | - | Pain in the hypogastrium and both iliac fossae, Hemoccult test (+) | Laparoscopic removal |
| Raleene Gatmaitan & et al. | 36 | G9P7 | - | Previous miscarriages | Abdominal pain, Nausea | Colonoscopic removal | |
| Prashant Joshi & et al. | 20 | Primiparous | - | 1 month postpartum | Lactation, Intrauterine insertion period | Abdominal pain | Laparoscopic removal |
| Li K.E & et al. | 34 | G1P1 | Copper device (MLZ380) | 2 y before | - | Abdominal pain | Anterior resection of the rectum, on-table lavage, and primary anastomosis via a midline laparotomy |
| Our Case | 26 | Multiparous | - | 1 y postpartum | Lactation | Menorrhagia and rectal bleeding | Hysteroscopic removal |
Preventing IUD migration involves ensuring proper insertion technique by trained healthcare providers, patient education about signs of complications, and routine follow-up visits to monitor the IUD's position. Providers should carefully assess the uterine position and size prior to insertion and confirm correct placement postinsertion through appropriate follow-up visits. Proper training in insertion techniques, the use of ultrasound guidance, and patient education on the signs of migration are crucial for enhancing patient safety and reducing the likelihood of complications. As physicians gain experience in IUD insertion, the risk of perforation diminishes. Selecting an IUD that matches the size of the endometrial cavity and strictly adhering to the manufacturer's insertion instructions is essential [4,13,10].
Conclusion
In conclusion, meticulous and regular monitoring of intrauterine devices (IUDs) is essential to prevent the rare but serious complication of organ perforation. Consistent oversight and timely removal of IUDs when necessary can significantly reduce the risks associated with their migration into surrounding organs. This proactive approach is vital for ensuring patient safety and mitigating potential adverse outcomes.
Patient consent
I confirm that the patient has given their consent.
Footnotes
Competing Interests: The authors declare that they have no conflicts of interest related to this article.
References
- 1.Buhling K.J., Zite N.B., Lotke P., Black K., Group Worldwide IUD Working. Worldwide use of intrauterine contraception: a review. Contraception. 2014;89(3):162–173. doi: 10.1016/j.contraception.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Lanzola E.L., Ketvertis K. In StatPearls. StatPearls Publishing; Treasure Island (FL): 2024. Intrauterine device. [PubMed] [Google Scholar]
- 3.Heartwell S., Schlesselman S. Risk of uterine perforation among users of intrauterine devices. Obstetr Gynecology. 1983;61(1):31–36. [PubMed] [Google Scholar]
- 4.Sitruk-Ware R., Nath A., Mishell D.R. Contraception technology: past, present and future. Contraception. 2013;87(3):319–330. doi: 10.1016/j.contraception.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barwin B.N., Tuttle S., Jolly E.E. The intrauterine contraceptive device. Can Med Assoc J. 1978;118(1):53–58. [PMC free article] [PubMed] [Google Scholar]
- 6.Prabhu J.K., Rani R., Nayak N.K., Natarajan P. Migration of intrauterine contraceptive device into sigmoid colon. J Clin Diagnost Res. 2018;12(8):QD04–QD05. doi: 10.7860/JCDR/2018/36172.11860. [DOI] [PubMed] [Google Scholar]
- 7.Peri N., Graham D., Levine D. Imaging of contraceptive devices. J Ultrasound Med. 2007;26(11):1389–1401. doi: 10.7863/jum.2007.26.11.1389. [DOI] [PubMed] [Google Scholar]
- 8.Arslan A., Kanat-Pektas M., Yesilyurt H., Bilge U. Colon penetration by a copper intrauterine device: a case report with literature review. Arch Gynecol Obstetr. 2009;279(3):395–397. doi: 10.1007/s00404-008-0727-7. [DOI] [PubMed] [Google Scholar]
- 9.Zakin D., Stern W.Z., Rosenblatt R. Complete and partial uterine perforation and embedding following insertion of intrauterine devices. Obstetr Gynecolo Survey. 1981;36(7):335–353. doi: 10.1097/00006254-198107000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Velazquez Velasco J.R., Vilchis Nava P., Nevarez Bernal R.A., Kably Ambe A. Uterine and jejunum perforation due to intrauterine device: a report of a case and literature review. Ginecología y Obstetricia de México. 2006;74(8):435–438. [PubMed] [Google Scholar]
- 11.Merz E. Thieme; New York: 2007. Ultrasound in Obstetrics and Gynecology. [Google Scholar]
- 12.Stuckey A., Dutreil P., Aspuru E., Nolan T.E. Symptomatic cecal perforation by an intrauterine device with appendectomy removal. Obstetr Gynecol. 2005;105(5 Pt 2):1239–1241. doi: 10.1097/01.AOG.0000158868.95772.21. [DOI] [PubMed] [Google Scholar]
- 13.Caliskan E., Oztürk N., Dilbaz B.O., Dilbaz S. Analysis of risk factors associated with uterine perforation by intrauterine devices. Eur J Contracept Reproduct Health Care. 2003;8(3):150–155. doi: 10.1080/713604412. [DOI] [PubMed] [Google Scholar]