Abstract
Polycythemia vera (PV) is a chronic myeloproliferative disorder characterized by increased red blood cell mass, leading to a heightened risk for thrombosis and hemorrhage. While thrombotic complications such as stroke, deep vein thrombosis, and pulmonary embolism are commonly associated with PV, coronary artery syndromes, as the initial presentation, are rare. Here, we present the case of a 73-year-old male who presented with severe chest pain and was diagnosed with non-ST–elevation myocardial infarction (NSTEMI). During his hospitalization, the patient experienced spontaneous psoas muscle hemorrhage, which prompted further investigation. Laboratory workup revealed elevated hemoglobin levels and a positive JAK2 V617F mutation, confirming a diagnosis of polycythemia vera. This case highlights the importance of considering myeloproliferative disorders in patients with atypical thrombotic and hemorrhagic events. It emphasizes the need for early diagnosis and appropriate treatment to optimize patient outcomes.
Keywords: Polycythemia vera, Acute coronary syndrome, Myeloproliferative disorders, Thromboembolic events
Introduction
Polycythemia vera (PV) is a chronic myeloproliferative disease characterized by an abnormal red blood cell mass increase, leading to thicker blood and a higher likelihood of blood clots [1]. Reported complications of PV range from 15% to 35% [2]. It increases the risk of bleeding and thrombosis, contributing to morbidity and mortality in 40% to 60% of affected patients [3]. The suggested causes of thrombosis involve irregularities in red blood cells, white blood cells, platelets, endothelial cells, coagulation factors, and various risk factors associated with patients [4]. Approximately 20% of patients with PV are identified as having thrombotic events as their initial symptom [5,6]. Bleeding episodes may be linked to the use of aspirin or anticoagulants, coexisting Von Willebrand disease, or associated thrombotic events.
While thrombotic complications such as stroke, deep vein thrombosis, and pulmonary embolism are more commonly associated with PV, coronary artery syndrome as an initial manifestation is extremely rare. Here, we present a unique case of a patient who initially presented with non-ST–elevation myocardial infarction and later developed bleeding, which led to the diagnosis of PV. Through this case, we want to highlight the importance of considering underlying myeloproliferative disorders like PV in patients presenting with atypical combinations of thrombotic and hemorrhagic events, as early recognition and appropriate management are critical for improving outcomes.
Case presentation
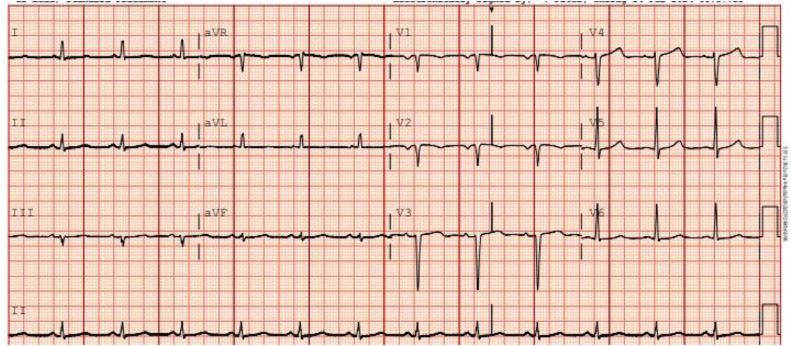
A 73-year-old male with a past medical history of heart failure with recovered EF (60%), HTN, HLD, and Diabetes Mellitus presented to the hospital with complaints of severe chest pain that started 2 days ago. The pain was worse with exertion, pressure like in nature, 9/10 intensity. The physical exam was, however, unremarkable. On admission, vitals were remarkable, with a heart rate of 60 beats per minute, blood pressure of 119/58, and respiratory of 18 breaths. Immediately after arrival. An electrocardiogram was done, which showed normal sinus rhythm (Fig. 1).
Fig. 1.
Initial electrocardiogram showing normal sinus rhythm with left atrial enlargement.
Initial lab values showed hemoglobin of 16 g/dl (13.1-5.5 g/dL), platelet count of 380K/cm (140-400 K/cm), white cell count 8.1 K/cm (4.8-10.8K/cm), creatinine 2 mg/dl (0.7-1.3 mg/dl), sensitive troponin >5000 ng/L (<6 ng/L), B- type natriuretic peptide 2810 pg/mL (100 pg/mL). The patient was admitted to the cardiology unit for management of non-ST elevated myocardial infarction (NSTEMI). He was started on a heparin drip and antiplatelet medications for further management of NSTEMI.
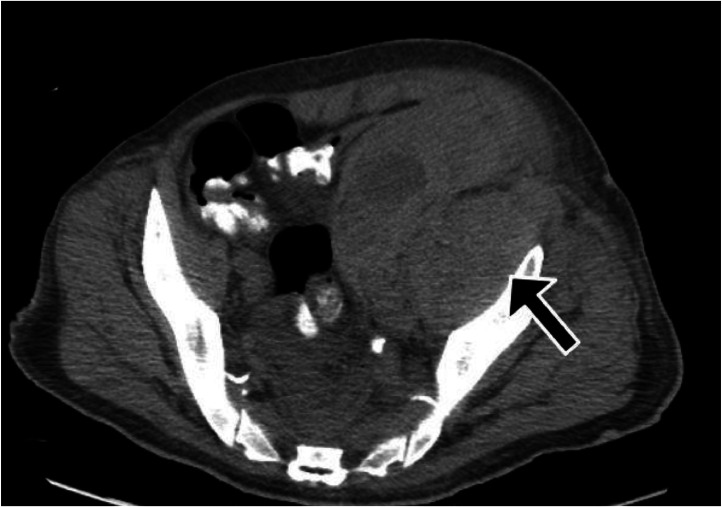
However, by the next day, the patient started complaining of severe lower abdominal and left thigh pain. Due to the severity of abdominal pain, a CT scan of the abdomen and pelvis was performed, which showed marked swelling of the left psoas and left iliac muscle, most pronounced at the level of the pelvis with mass-effect on the adjacent bowel with heterogeneous density, indicative of hemorrhage of varying age (Fig. 2).
Fig. 2.
CT scan of abdomen and pelvis showing marked swelling of the left psoas and left iliacus with mass-effect on the adjacent bowel with heterogeneous density indicative of hemorrhage.
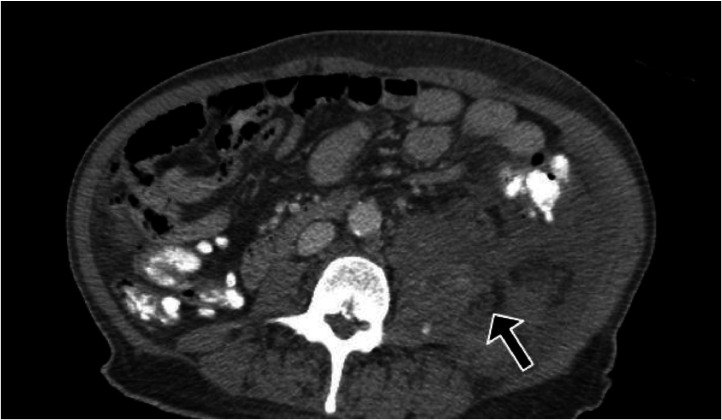
Due to the hematoma, heparin drip and all other antiplatelet medications were stopped and surgery was immediately consulted. They recommended performing a CT angiogram (CTA) to rule out acute bleeding. CTA showed hypodense areas within these 2 muscles, which likely reflect liquefaction of hemorrhage and Active extravasation of IV contrast into the swollen hemorrhagic left psoas muscle indicative of active bleeding. A few small foci of extravasated contrast exist in the left psoas muscle (Fig. 3).
Fig. 3.
CT angiogram of the abdomen and pelvis shows Active extravasation of IV contrast into the swollen hemorrhagic left psoas muscle indicative of active bleeding.
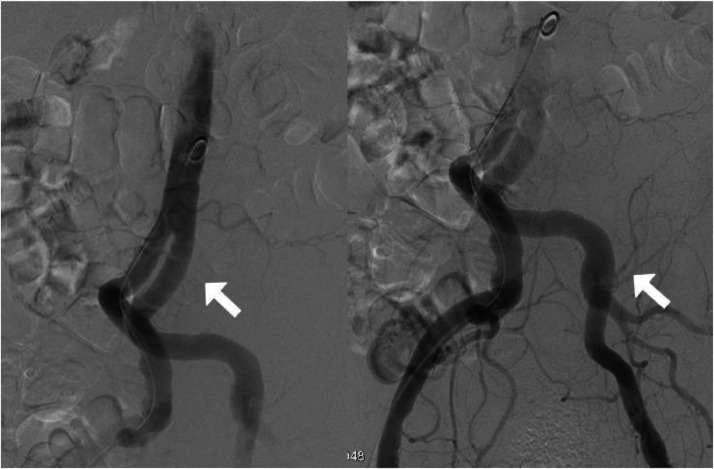
Interventional radiology was consulted, and the patient was taken for immediate embolization to control the bleeding. However, interventional radiologists could not notice any arterial extravasation to confirm the arterial bleeding. Arteriography of the abdominal aorta and iliac arteries showed no evidence of contrast extravasation to denote an active bleed (Figs. 4 A and B). Closure of the right common femoral arteriotomy via an Angio-Seal device and manual pressure. Pain was controlled with morphine, and intravenous fluids were continued.
Fig. 4.
(A) (B): Arteriography of the abdominal aorta and iliac arteries without evidence of contrast extravasation to denote an active bleed.
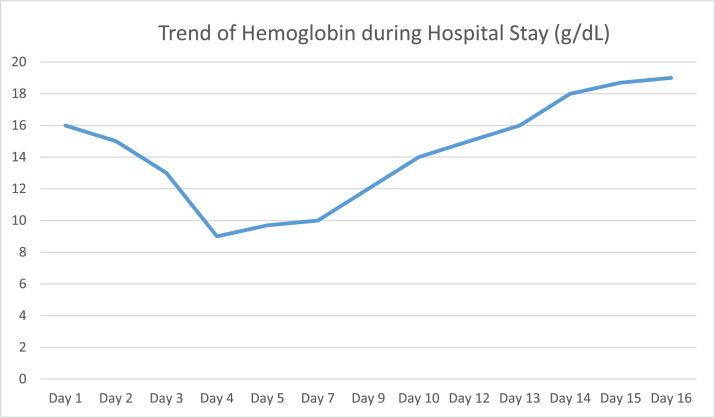
After the patient was stabilized and bleeding stopped, the hemoglobin started increasing and rising to 18 mg/dl. The trend of hemoglobin during hospitalization is shown in Fig. 5.
Fig. 5.
Graph showing the trend of hemoglobin during the hospital stay.
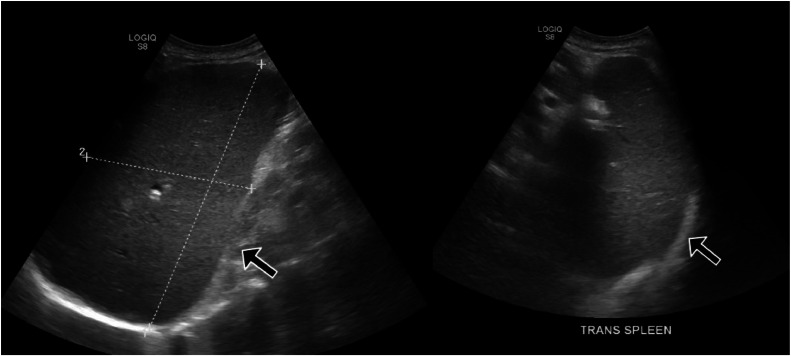
On hematology recommendations, an hematology workup was immediately sent (Table 1). VW antigen was normal. Flow cytometry showed 0.5% myeloblast. Ultrasound of the spleen showed an enlarged spleen measuring 21 × 12.5 × 8.6 cm, corresponding to a volume of 1196 cc (Fig. 6). The patient was diagnosed with PV as he met WHO criteria (Table 1). The patient also started complaining of vision changes during the hospital stay. Hematology recommended immediate apheresis due to possible PV. Symptoms improved significantly after the procedure. The patient was also started on hydroxyurea. With the resolution of symptoms, hemoglobin started to improve. The patient was discharged on hydroxyurea and advised to follow up as an outpatient in the hematology clinic.
Table 1.
The table shows results for hematological workup.
| Lab Test | Result | Reference range |
|---|---|---|
| BCR ABL1 gene rearrangement | Not detected | Not detected |
| Ristocetin cofactor | 49% | 42%-200% |
| JAK2 mutation | JAK2 V617 Detected | Not Detected |
| Factor VIII acitvity | 76 | 50-180 |
| Erythropoietin level | 2 mU/mL | 4-26 mU/mL |
Fig. 6.
Ultrasound of the abdomen showing enlarged spleen measuring 21 × 12.5 × 8.6 cm which corresponds to volume of 1196 cc.
Discussion
Myeloproliferative neoplasms (MPNs) are a diverse set of blood disorders characterized by the abnormal overproduction of myeloid lineage cells. The 4 main types of MPNs include chronic myeloid leukemia (CML), PV, essential thrombocythemia (ET), and primary myelofibrosis (PMF) [7].
The 2007 WHO criteria for diagnosing PV include 2 primary and 3 minor criteria (Table 2) [8].
Table 2.
Shows WHO diagnostic criteria for polycythemia vera (PV).
| Major criteria |
|---|
| 1- Hemoglobin >18.5 g/dL in men, 16.5 g/dL in women, or other evidence of increased red cell volume (RCV) |
| 2- Presence of JAK2V617F or other functionally similar mutation |
| Minor criteria |
| 1- Bone marrow biopsy specimen showing hypercellularity for age |
| 2- Serum erythropoietin (EPO) level below the reference range for normal |
| 3- Endogenous erythroid colony formation in vitro |
We need both major and 1 minor criterion to diagnose PV or just the first significant criterion alongside 2 minor criteria. Our patient fulfilled both the primary criteria (Hemoglobin >18.5, presence of JAK2 mutation) and 1 minor criterion (EPO level was below normal range).
The differential diagnosis in this case included secondary polycythemia, psoas muscle hematoma secondary to trauma, heparin induced thrombocytopenia or disseminated intravascular coagulation. However, the erythropoietin in our patient was low, making secondary polycythemia less likely. The patient also denied history of trauma. Heparin induced thrombocytopenia was also less likely as the platelet level remained normal. Moreover, the platelet and fibrin levels were also normal in the patient, ruling out DIC.
Thrombotic events are closely linked to PV development, which is pivotal in determining patient risk assessments and treatment strategies. A retrospective analysis of 9429 patients with myeloproliferative neoplasms (MPNs) from the Swedish Cancer Registry, including 3001 with PV, examined data from 1987 to 2009 with follow-up until 2010. It found that, 3 months after diagnosis, patients with PV had about a 3-fold higher risk of arterial thrombosis and a 13-fold higher risk of venous thrombosis than age- and sex-matched controls [9].
Individuals without a history of thrombotic events and who are younger than 60 are classified as low risk, while those with prior thrombotic events or aged 60 and older are considered high risk [10,11]. The exact pathophysiology of thromboembolic events in polycythemia remains unclear, but several contributing factors are recognized. These include elevated hematocrit levels and blood hyperviscosity, enhanced platelet aggregation and thrombogenesis, leukocytosis, membrane rigidity, and intimal proliferation [12].
Existing studies indicate that the incidence of cardiovascular complications associated with MPNs varies between 4% and 21%. PV generally impacts the significant arteries within the cardiovascular and cerebrovascular systems [13]. In 1 study, over a 10-year follow-up of patients with PV, coronary events were commonly encountered, with an incidence rate of 11.4% [14], and in a study conducted by Vianello et al. assessed coronary flow reserve in patients with PV and Essential thrombocytosis (ET) who had no prior history of cardiac disease. Their findings revealed that asymptomatic individuals with PV and ET exhibited coronary microvascular dysfunction. Moreover, they identified a correlation between JAK2 gene mutations and impaired coronary flow reserve, which may elevate cardiovascular risk.
In patients with PV, bleeding tendencies are usually mucocutaneous, presenting as easy bruising, nosebleeds, and gum bleeding. The use of aspirin and other antithrombotic medications has been frequently associated with an increased risk of both minor and major bleeding events [15,16]. An observational prospective study showed a combination of aspirin and anticoagulants in patients with PV significantly increases the risk of hemorrhage compared to aspirin alone [17]. Our patient initially presented with acute coronary syndrome as the initial presentation of PV. The patient experienced a hemorrhagic episode in the psoas muscle, likely triggered by the use of anticoagulation and antiplatelet therapies during the hospital stay.
Acute coronary syndrome (ACS) presenting as the initial manifestation of PV is rare. Our review of the literature revealed a few case reports with similar presentations (Table 3).
Table 3.
Overview of case reports on cardiac complications as initial presentations of polycythemia vera (PV).
| Author | Gender | Age | Initial presentation | Initial hemoglobin / Hct Levels | Cardiac complication | Myeloproliferative neoplasm | JAK2 mutation | Treatment |
|---|---|---|---|---|---|---|---|---|
| Raza et al. [18] | Male | 22 Y | Chest pain and vomiting | 18.5 g/dL | ST segment elevation (STEMI) | PV | Positive | Streptokinase, Hydroxyurea, Aspirin |
| Davis et al. [19] | Male | 30Y | Cardiac Arrest | 18.4g/dL | Cardiac Arrest, cardiogenic shock | PV | Positive | Anticoagulation, phlebotomy, Hydroxyurea |
| Hosoya et al. [20] | Male | 50Y | Epigastric discomfort and right hand clumsiness | 13.5 g/dL | Non-ST–segment elevation myocardial infarction (NSTEMI) | PV | NA | Antiplatelet therapy, Hydroxyurea |
| Inami et al. [21] | Male | 64Y | Chest pain | NA | STEMI | PV | Positive | Balloon angioplasty, Phlebotomy, hydroxyurea |
| Shao et al. [22] | Male | 60Y | Chest pain | Normal | STEMI | PV | Positive | Antiplatelets, Hydroxyurea and Interferon |
| Nishiguchi et al. [23] | Female | 78F | Chest Pain | Hct 37-42% | STEMI | PV | NA | NA |
| Nahler et al. [24] | Male | 22Y | Recurrent chest pain | Hb 19.8g/dL | STEMI | PV | Positive | Hydroxyurea |
Emergency management for patients with acute coronary syndrome (ACS) should be tailored to the specific type of myocardial infarction (MI) they are experiencing. The early diagnosis and management of myocardial infarction in PV can be complex and require a multidisciplinary approach for optimal care. Our patient was started on antiplatelet therapy and a heparin drip, which was later stopped due to bleeding. Cardiac catheterization was, however, postponed due to complications such as bleeding. The patient's symptomatic status determines the management of PV. Treatment options primarily involve cytoreductive and anticoagulant medications [25]. For patients younger than 60 without a prior history of thrombosis, venous exsanguination, and low-dose aspirin are suggested. In comparison, for those over 60 or with a history of thrombosis, treatment should include hydroxyurea to help reduce cell mass. Following a thrombotic episode in patients with PV, treatment typically includes both aspirin and hydroxyurea.
However, our case has certain limitations. As a single case, the findings may not be generalizable to all PV patients presenting with ACS and related complications. The rare presentation of ACS as the first symptom of PV might have led to diagnostic delays, as the initial focus was on managing ACS, with PV diagnosis occurring later in the course of treatment. Despite these limitations, this case highlights the complexities of managing patients with PV who present with ACS, particularly in balancing thrombotic and bleeding risks.
Conclusion
This case highlights the rare presentation of acute coronary syndrome (ACS) as the initial manifestation of PV, with a unique complication of bleeding during hospitalization. Early diagnosis and careful management, particularly balancing anticoagulation therapy, are essential for preventing further complications in such patients. Clinicians should closely monitor for signs of hemorrhage when initiating anticoagulants or antiplatelets and consider alternative strategies, such as reducing the intensity of anticoagulation or using cytoreductive therapy to lower the risk of thrombosis while minimizing bleeding risks. More studies are needed to establish optimal treatment protocols in patients with PV and ACS.
Patient consent
Verbal and Signed consent was taken from the patient.
Footnotes
Competing Interests: The authors declare no conflict of interest.
Generative AI: Generative AI was used to improve the language and remove grammatical mistakes.
References
- 1.Stuart BJ, Viera AJ. Polycythemia vera. Am Fam Physician. 2004;69(9):2139–2144. [PubMed] [Google Scholar]
- 2.Yesilova AM, Yavuzer S, Yavuzer H, Cengiz M, ID Toprak, Hanedar E., et al. Analysis of thrombosis and bleeding complications in patients with polycythemia vera: a Turkish retrospective study. Int J Hematol. 2016;105:70–78. doi: 10.1007/s12185-016-2105-0. [DOI] [PubMed] [Google Scholar]
- 3.Adel G, Aoulia D, Amina Y, Aymen BA, Abdel-Hamid NM. Polycythemia vera and acute coronary syndromes: pathogenesis, risk factors and treatment. J Hematol Thromb Dis. 2013;1:107–112. [Google Scholar]
- 4.Barbui T, Finazzi G, Falanga A. Myeloproliferative neoplasms and thrombosis. Blood. 2013;122:2176–2184. doi: 10.1182/blood-2013-03-460154. [DOI] [PubMed] [Google Scholar]
- 5.Gruppo Italiano Studio Policitemia Polycythemia vera: the natural history of 1213 patients followed for 20 years. Gruppo Italiano Studio Policitemia. Ann Intern Med. 1995;123:656–664. doi: 10.7326/0003-4819-123-9-199511010-00003. [DOI] [PubMed] [Google Scholar]
- 6.Tefferi A, Rumi E, Finazzi G, Gisslinger H, Vannucchi AM, Rodeghiero F, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27:1874–1881. doi: 10.1038/leu.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spivak JL, Barosi G, Tognoni G, Barbui T, Finazzi G, Marchioli R, et al. Chronic myeloproliferative disorders. Hematology Am Soc Hematol Educ Program. 2003;2003(1):200–224. doi: 10.1182/asheducation-2003.1.200. [DOI] [PubMed] [Google Scholar]
- 8.Silver RT, Chow W, Orazi A, Arles SP, Goldsmith SJ. Evaluation of WHO criteria for diagnosis of polycythemia vera: a prospective analysis. Blood. 2013;122(11):1881–1886. doi: 10.1182/blood-2013-06-508416. [DOI] [PubMed] [Google Scholar]
- 9.Hultcrantz M, Bjorkholm M, Dickman PW, Landgren O, Derolf AR, Kristinsson SY, et al. Risk for arterial and venous thrombosis in patients with myeloproliferative neoplasms: a population-based cohort study. Ann Intern Med. 2018;168:317–325. doi: 10.7326/M17-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tefferi A. Polycythemia vera and essential thrombocythemia: 2013 update on diagnosis, risk-stratification, and management. Am J Hematol. 2013;88:507–516. doi: 10.1002/ajh.23417. [DOI] [PubMed] [Google Scholar]
- 11.Passamonti F. How I treat polycythemia vera. Blood. 2012;120:275–284. doi: 10.1182/blood-2012-02-366054. [DOI] [PubMed] [Google Scholar]
- 12.Hermanns B., Handt S., Kindler J., Füzesi L. Coronary vasculopathy in polycythemia vera. Pathology & Oncology Research. 1998;4:37–39. doi: 10.1007/BF02904693. [DOI] [PubMed] [Google Scholar]
- 13.Cengiz B, Aytekin V, Bildirici U, Sahin ST, Yurdakul S, Aytekin S, et al. A rare cause of acute coronary syndromes in young adults - myeloproliferative neoplasms: a case series. Rev Port Cardiol (Engl Ed) 2019;38:613–617. doi: 10.1016/j.repc.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Rossi C, Randi ML, Zerbinati P, Rinaldi V, Girolami A. Acute coronary disease in essential thrombocythemia and polycythemia vera. J Intern Med. 1998;244(1):49–53. doi: 10.1046/j.1365-2796.1998.00314.x. [DOI] [PubMed] [Google Scholar]
- 15.Adverse effects of antiaggregating platelet therapy in the treatment of Polycythemia Vera. Semin Hematol. 1986;23:172–176. [PubMed] [Google Scholar]
- 16.Incidence and clinical risk factors for bleeding and thrombotic complications in myeloproliferative disorders. A retrospective analysis of 260 patients. Ann Hemtol. 1991;63:101–106. doi: 10.1007/BF01707281. [DOI] [PubMed] [Google Scholar]
- 17.Zwicker JI, Paranagama D, Lessen DS, Colucci PM, Grunwald MR. Hemorrhage in patients with polycythemia vera receiving aspirin with an anticoagulant: a prospective, observational study. Haematologica. 2022;107(5):1106–1110. doi: 10.3324/haematol.2021.279032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raza S, Khan J, Shah AM, Khan M. Myocardial infarction in young individual: a case report of polycythemia vera-induced acute inferior wall myocardial infarction. SAGE Open Med Case Rep. 2024;12 doi: 10.1177/2050313X241253741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis MI, Courtney BK, Cohen G, Poon S, Madan M. Polycythemia Vera presenting as cardiac arrest: novel management strategies. Case Rep Cardiol. 2019;2019 doi: 10.1155/2019/9656387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosoya H, Levine JJ, Henry DH, Goldberg S. Double the trouble: acute coronary syndrome and ischemic stroke in polycythemia vera. Am J Med. 2017;130(6):e237–e240. doi: 10.1016/j.amjmed.2017.02.016. 2017. [DOI] [PubMed] [Google Scholar]
- 21.Inami T, Okabe M, Matsushita M, Kobayashi N, Inokuchi K, Hata N, et al. JAK2 mutation and acute coronary syndrome complicated with stent thrombosis. Heart Vessels. 2016;31(10):1714–1716. doi: 10.1007/s00380-016-0798-x. [DOI] [PubMed] [Google Scholar]
- 22.Shao X, Liu Z, Qin C, Xiao F. Acute myocardial infarction followed by cerebral hemorrhagic infarction in Polycythemia Vera: case report and literature review. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.660999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishiguchi T, Tanaka A, Yamano T, Kubo T, Akasaka T. Intimal exfoliation following abnormal circular proliferation as a cause for acute coronary syndrome in a patient with polycythemia vera. Int J Cardiol. 2015;199:239–240. doi: 10.1016/j.ijcard.2015.07.059. [DOI] [PubMed] [Google Scholar]
- 24.Nahler A, Fuchs D, Reiter C, Kiblböck D, Steinwender C, Lambert T. Myocardial infarction with proximal occlusion of the left anterior descending coronary artery in a 22-year-old patient with polycythaemia vera. Clin Med (Lond) 2017;17(1):46–47. doi: 10.7861/clinmedicine.17-1-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vianello F, Cella G, Osto E, Ballin A, Famoso G, Tellatin S, et al. Coronary microvascular dysfunction due to essential thrombocythemia and policythemia vera: the missing piece in the puzzle of their increased cardiovascular risk? Am J Hematol. 2015;90:109–113. doi: 10.1002/ajh.23881. [DOI] [PubMed] [Google Scholar]