Abstract
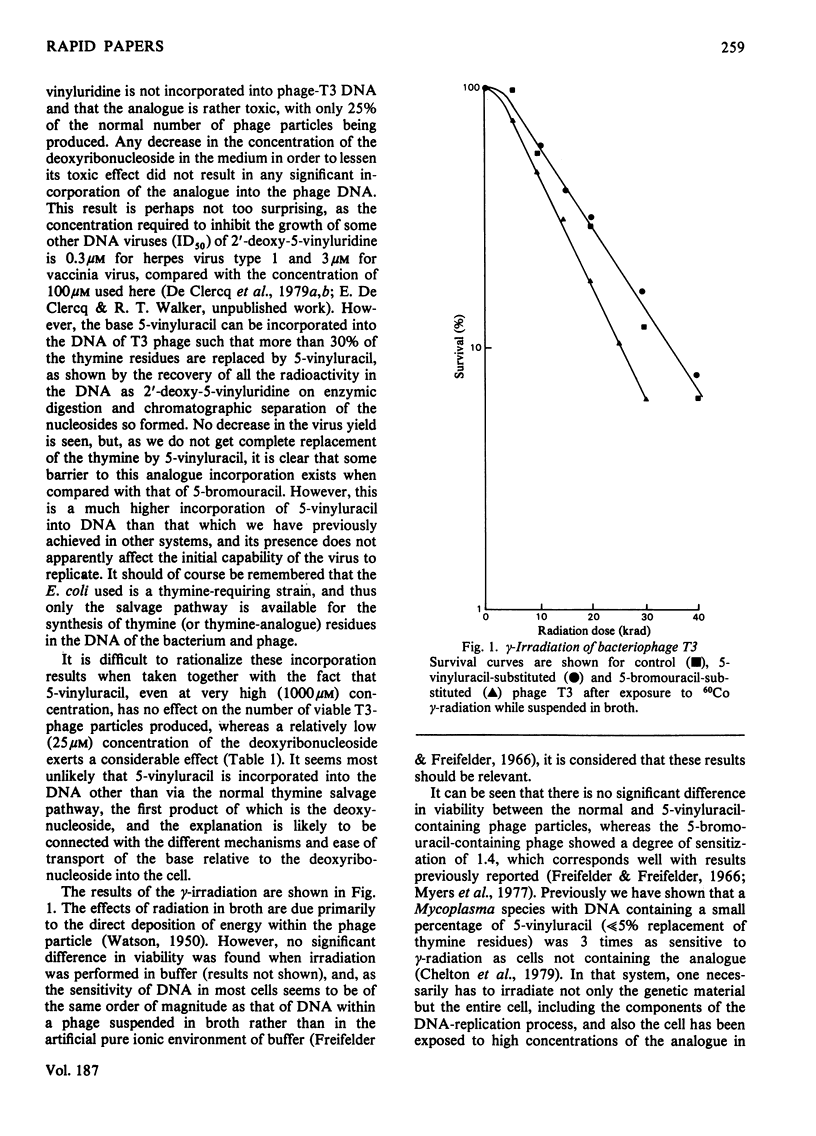
Bacteriophage T3 was produced in a form that contained 32% of its normal DNA thymine residues replaced with 5-vinyluracil residues by infecting a thymine-requiring strain of Escherichia coli with phage T3 in a medium containing 5-vinyluracil. When 2'-deoxy-5-vinyluridine was added to the medium instead, no incorporation was observed into the phage DNA, and the presence of the deoxyribonucleoside severely decreased the number of viable phage particles produced. The analogue-containing phage, although initially viable, rapidly lost viability when stored, but it was no more sensitive than was normal phage T3 to the effect of gamma-radiation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chelton E. T., Evans C. H., Jones A. S., Walker R. T. Incorporation of 5-substituted uracil derivatives into nucleic acids. II. Incorporation of 5-vinyluracil into the DNA of Escherichia coli. Biochim Biophys Acta. 1973 Jun 8;312(1):38–44. doi: 10.1016/0005-2787(73)90050-6. [DOI] [PubMed] [Google Scholar]
- Chelton E. T., Jones A. S., Walker R. T. The sensitivity of Mycoplasma mycoides var. capri cells to gamma-radiation after growth in a medium containing the thymine analogue 5-vinyluracil. Biochem J. 1979 Sep 1;181(3):783–785. doi: 10.1042/bj1810783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Descamps J., De Somer P., Barr P. J., Jones A. S., Walker R. T. (E)-5-(2-Bromovinyl)-2'-deoxyuridine: a potent and selective anti-herpes agent. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2947–2951. doi: 10.1073/pnas.76.6.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D., Freifelder D. R. Mechanism of x-ray sensitization of bacteriophage T7 by 5-bromouracil. Mutat Res. 1966 Jun;3(3):177–184. doi: 10.1016/0027-5107(66)90059-5. [DOI] [PubMed] [Google Scholar]
- JONES A. S., WALKER R. T. Studies on the deoxyribonucleic acid of Serratia marcescens. J Gen Microbiol. 1963 May;31:187–194. doi: 10.1099/00221287-31-2-187. [DOI] [PubMed] [Google Scholar]
- LARK K. G., REPKO T., HOFFMAN E. J. THE EFFECT OF AMINO ACID DEPRIVATION ON SUBSEQUENT DEOXYRIBONUCLEIC ACID REPLICATION. Biochim Biophys Acta. 1963 Sep 17;76:9–24. [PubMed] [Google Scholar]
- Myers D. K., Childs J. D., Jones A. R. Sensitization of bacteriophage T4 to 60 Co-gamma radiation and to low-energy X radiation by bromouracil. Radiat Res. 1977 Jan;69(1):152–165. [PubMed] [Google Scholar]
- Sharma R. A., Bobek M. Synthesis of 5-vinyluridine and 5-vinyl-2'-deoxyuridine as new pyrimidine nucleoside analogs. J Org Chem. 1975 Aug 8;40(16):2377–2379. doi: 10.1021/jo00904a025. [DOI] [PubMed] [Google Scholar]
- WATSON J. D. The properties of x-ray inactivated bacteriophage. I. Inactivation by direct effect. J Bacteriol. 1950 Dec;60(6):697–718. doi: 10.1128/jb.60.6.697-718.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]


