Abstract
Background
The results of Study 1 we have published proved that medium and high doses of Gegen Qinlian Decoction (GQD) were effective in treating type 2 diabetes (T2D) with damp-heat syndrome. However, whether the main drug of GQD in treating T2D was Puerariae Lobatae Radix or Coptidis Rhizoma has always been a hot topic of debate among many doctors. Therefore, we conducted Study 2 and Study 3 to determine the main drug of GQD for treating T2D.
Methods
Both Study 2 and Study 3 were randomized, double-blind, dose-parallel controlled, multicenter trials. In Study 2, Puerariae Lobatae Radix was used as the main drug, and in Study 3, Coptidis Rhizoma was used as the main drug. About 120 patients with newly diagnosed T2D were enrolled in each study and randomized 1:1:1 to three treatment groups. The three treatment groups were named HD, MD, and LD groups according to the high, medium, and low doses of the main drug. The course of treatment was 12 weeks. The primary outcomes were the changes in HbA1c.
Results
In Study 2, the HbA1c decreased by 0.58 (0.87), 0.28 (1.17), and 0.55 (0.85) in the HD, MD, and LD groups, respectively, with no significant difference between treatment groups according to covariance analysis (F=0.66, P=0.5206). In Study 3, the HbA1c decreased by 0.75 (0.82), 0.34 (0.71), and 0.26 (0.79) in the HD, MD, and LD groups respectively. By analysis of covariance, the change values of HbA1c were significantly different among the three groups (F=3.11, P=0.0492).
Conclusion
The changes in HbA1c were positively correlated with the dose of Coptidis Rhizoma, but not significantly with the dose of Puerariae Lobatae Radix. It demonstrated that the main drug of GQD in treating T2D patients is Coptidis Rhizoma.
Keywords: Coptidis rhizoma, Huanglian, Gegen Qinlian Decoction, type 2 diabetes, dose-parallel controlled, clinical trial
Introduction
Type 2 diabetes (T2D) is a common chronic metabolic disease characterized by hyperglycemia.1 Diabetic patients, especially those with poor glycemic control, usually experience various complications, such as kidney disease, cardiovascular disease, diabetic retinopathy, foot injury, etc., which will cause significant harm to their physical and mental health.2,3 It is estimated that the global prevalence of diabetes is 10.5% (536.6 million people) in 2021, and will rise to 12.2% (783.2 million) in 2045.4 Healthcare expenditures resulting from diabetes posed a huge social, financial, and health system burden worldwide.5,6 Traditional Chinese medicine (TCM), as a common complementary alternative therapy, played an important role in the treatment of diabetes, such as lowering blood glucose, improving symptoms and signs, preventing and treating complications, and improving the quality of life.7
Gegen Qinlian Decoction (GQD) originated from the Treatise on Febrile Diseases written by Zhongjing Zhang in the Han Dynasty in China about 1800 years ago. It consisted of Puerariae Lobatae Radix (Gegen in Chinese, Pueraria montana var. lobata (Willd). Maesen & S.M.Almeida ex Sanjappa & Predeep [Fabaceae]), Scutellariae Radix (Huangqin in Chinese, Scutellaria baicalensis Georgi [Lamiaceae]), Coptidis Rhizoma (Huanglian in Chinese, Coptis chinensis Franch. [Ranunculaceae]), and Glycyrrhizae Radix et Rhizoma (Gancao in Chinese, Glycyrrhiza glabra L. [Fabaceae]) in a mass ratio of 8:3:3:2, and is a classic TCM formula for treating acute diarrhea and dysentery related to damp-heat syndrome.8–10
Professor Xiaolin Tong applied GQD to treat T2D with dampness-heat syndrome according to the theory of “same treatment for different diseases”, and it was effective.11,12 Studies have shown that GQD has a good anti-diabetic effect in diabetic rats and 3T3-L1 adipocytes.13
However, when GQD is used to treat type 2 diabetes, whether Puerariae Lobatae Radix or Coptidis Rhizoma is the main drug for hypoglycemic effect has always been a controversial issue among many clinicians. The main drug is also known as the monarch drug. In a formula, the monarch drug targets the disease or main symptoms suffered. It reflects the main direction of the prescription treatment.14 The dose-effect relationship of the main drug in one formula is particularly important for clinical medication guidance.15
There is currently no standardized study to confirm whether using Puerariae Lobatae Radix as the main drug or Coptidis Rhizoma as the main drug of GQD is more effective in the treatment of T2D. The uncertainty of the main drug and the unclear dose-effect relationship makes it difficult for GQD to achieve the best benefit in T2D. Therefore, we designed three clinical studies to determine the main drug of GQD in treating T2D and observe the relationship between the main drug dose and efficacy.
Materials and Methods
The three studies were conducted in Guang’anmen Hospital, Jishuitan Hospital of Peking University, China-Japan Friendship Hospital, and Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine. All studies adhered to the tenets of the Declaration of Helsinki. The Ethics Committee of Guang’anmen Hospital approved the protocol and informed consent form. The ethics number was Guang’anmen Hospital Ethics Committee 2010 No.035, and the Study 1 has been registered on ClinicalTrials.gov (http://clinicaltrials.gov/show/NCT01219803).
Study Design and Participants
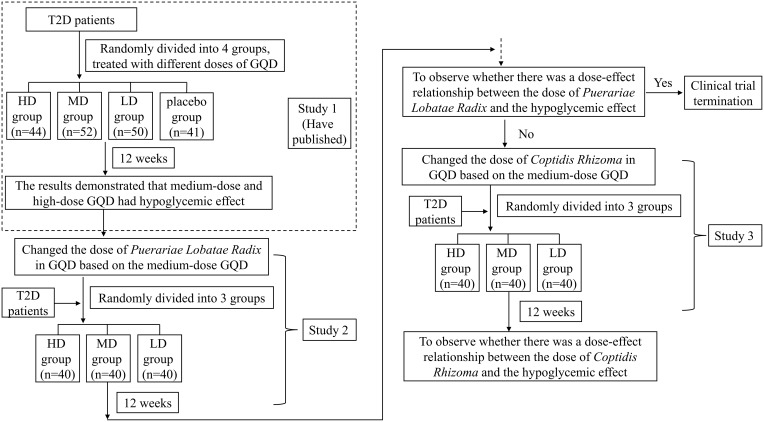
In Study 1, directly divided GQD into low, medium, and high dose groups, and placebo control groups, to observe the varying efficacy among these groups. The results of study 1 have been published, and the results showed that diabetic patients receiving high and medium doses of GQD had significantly lower fasting blood glucose (FBG) and glycated hemoglobin (HbA1c) compared with those receiving low dose of GQD and placebo.16 On this basis, we conducted Study 2 and Study 3, only changing the dose of Puerariae Lobatae Radix or Coptidis Rhizoma in the medium dose of GQD, and set up three dose groups of high, medium, and low to observe the relationship between the dose of main drug and clinical efficacy.
Study 2 and Study 3 were all randomized, double-blind, dose parallel controlled, and multicenter clinical trials. Regarding the sample size of the studies, we employed adaptive design. Each study aimed to recruit 120 eligible patients according to the inclusion criteria, with 40 cases in each group. Depending on the statistical results, the number of cases could be increased as deemed appropriate. Each study included a 2-week screening period and a 12-week treatment period. The flowchart was shown in Figure 1.
Figure 1.
The flowchart of the study.
The inclusion criteria were as follows. 1. Patients with new-onset T2D diagnosed in accordance with the 1999 World Health Organization criteria;17 2. TCM syndrome differentiation is the dampness-heat syndrome;18 3. Aged 30–65 years; 4. After the screening period (diet control + exercise for 2 weeks), HbA1c ≥7.0%, FBG 7.0–13.9 mmol/L or 2 hours postprandial blood glucose (2hPG) ≥11.1mmol/L; 5. Sign the informed consent.
The definition of damp-heat syndrome and the exclusion criteria were described in Additional File 1.
Randomization and Masking
Randomization and blinding were conducted by third-party personnel who did not participate in study design, data acquisition, and clinical evaluation. The stratified block randomization method was used. Stratified according to the centers. With the help of the SAS statistical software PROC PLAN process, a random code table was generated. The eligible participants were randomly divided into three groups (1:1:1), receiving the GQD with high, medium and low doses of main drugs. The envelope method was used for allocation concealment. Paste the corresponding drug number on the conspicuous position of the external package of the decoction according to the formed random code. Each subject used a unique drug number throughout the trial. The participants, clinical investigators, and statisticians were blinded to treatment allocation. Unblinding was conducted only after all the study data were recorded.
Interventions
All patients involved in the study received standardized diet, exercise, and lifestyle guidance provided by professional diabetes doctors. According to different groups, patients were given different doses of GQD for treatment. One unit of GQD decocted 300 mL of decoction. The patient took it orally twice in the morning and evening, 150 mL each time. The treatment course was 12 weeks.
In study 2, the three groups of patients received GQD with different doses of Puerariae Lobatae Radix. The specific doses for each group were as follows.
Low dose (LD) group: Puerariae Lobatae Radix 24g, Scutellariae Radix 27g, Coptidis Rhizoma 27g and Glycyrrhizae Radix et Rhizoma 18g.
Medium dose (MD) group: Puerariae Lobatae Radix 72g, Scutellariae Radix 27g, Coptidis Rhizoma 27g and Glycyrrhizae Radix et Rhizoma 18g.
High dose (HD) group: Puerariae Lobatae Radix 120g, Scutellariae Radix 27g, Coptidis Rhizoma 27g and Glycyrrhizae Radix et Rhizoma 18g.
In study 3, the three groups of patients received GQD with different doses of Coptidis Rhizoma. The specific doses for each group were as follows.
LD group: Puerariae Lobatae Radix 72g, Scutellariae Radix 27g, Coptidis Rhizoma 9g and Glycyrrhizae Radix et Rhizoma 18g.
MD group: Puerariae Lobatae Radix 72g, Scutellariae Radix 27g, Coptidis Rhizoma 27g and Glycyrrhizae Radix et Rhizoma 18g.
HD group: Puerariae Lobatae Radix 72g, Scutellariae Radix 27g, Coptidis Rhizoma 45g and Glycyrrhizae Radix et Rhizoma 18g.
The dose of each herb of GQD in different groups in the Study 2 and Study 3 also can be found in the Additional File 2 Table S1-S2.
Quality Control and Safety Experiments
Herbs were all provided by Beijing Shuangqiaoyanjing Chinese herb manufacturer. We determined the content of the purchased herbs according to the standards set by the “China Pharmacopoeia (2005)” to decide which herbal materials to use in the clinical study. The content of GQD was determined by high-performance liquid chromatography (HPLC). Specific fingerprint peaks were identified, including puerarin (PubChem CID: 5281807), baicalin (PubChem CID: 64982), and berberine (PubChem CID: 2353). Before the clinical studies, we conducted an acute toxicity test of GQD in mice. The results showed that no toxic reaction was observed at the maximum single oral dose equivalent to 44.7 times the daily dose of human beings. The decoction was prepared and quality controlled by Beijing Jiulong Pharmaceutical Factory according to the standard production process. The detailed description of drug quality control and safety experiments can be found in the Additional File 2. The HPLC graphs and main chemicals in the decoction of GQD can be found in the Additional File 2 Figure S1.
Outcomes Measurement
The primary outcomes of our study were the changes in HbA1c relative to baseline after 12 weeks of treatment. The secondary outcomes were the following indicators at 12 weeks and their changes from baseline. (1) FBG and 2hPG. (2) Blood lipids, including cholesterol (CHO), triglyceride (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL). (3) Weight, body mass index (BMI), waist circumference (WC), hip circumference (HC), and waist-hip ratio (WHR). (4) Fasting insulin (FINS), Homeostasis Model Assessment-β (HOMA-β), Homeostasis Model Assessment of Insulin Sensitivity (HOMA-IS), and Homeostasis Model Assessment of Insulin Resistance (HOMA-IR). (5) Total symptom score. The TCM symptoms scoring standard can be found in the Additional File 1.
Study assessments were conducted at 0, 4, 8, and 12 weeks. Measurements of HbA1c, FINS, and blood lipids were taken at 0 and 12 weeks. Measurements of FBG, 2hPG, weight, WC, HC, and total symptom score were performed at 0, 4, 8, and 12 weeks.
Safety indicators included vital signs (blood pressure, respiration, and heart rate), blood routine, urine routine, stool routine, electrocardiogram (ECG), liver function (alanine aminotransferase and aspartate transaminase), and renal function (creatinine and blood urea nitrogen). Vital signs were monitored at 0, 4, 8, and 12 weeks. Laboratory examinations were monitored at 0 and 12 weeks. Adverse events, including hypoglycemic events, were recorded in detail at any time throughout the trial and classified as treatment-related or non-treatment-related by experts based on their potential relevance to the intervention.
The correlation analysis between the main drug dose and the change value of HbA1c was evaluated based on the data from three clinical trials.
Statistical Analysis
Software SAS 9.2 was used to perform statistical analysis. All statistical tests were conducted by two-sided tests. The difference was statistically significant when the P value was less than 0.05. Quantitative variables were described as mean (standard deviation), compared between groups using analysis of variance/rank-sum test, and changes in each group before and after treatment were compared using paired t-test. Qualitative variables were described as numbers and percentages and compared between groups using the chi-square test/Fisher’s exact test. Analysis of covariance was used to compare the primary outcomes among the three groups.
Results
Subject Characteristics
In Study 2, a total of 210 subjects were assessed for eligibility, 120 eligible subjects were randomized, and 97 subjects accomplished the study (32 in the HD group, 30 in the MD group, and 35 in the LD group). A total of 23 subjects (8 in the HD group, 10 in the MD group, and 5 in the LD group) were removed from the study because of loss of follow-up, adverse events, withdrawal, poor efficacy, or other reason. The Consolidated Standards of Reporting Trials (CONSORT) flow chart can be found in the Additional File 3 Figure S2.
In Study 3, a total of 192 subjects were assessed for eligibility, 122 eligible subjects were randomized, and 106 subjects accomplished the study (34 in the HD group, 36 in the MD group, and 36 in the LD group). A total of 16 subjects (7 in the HD group, 5 in the MD group, and 4 in the LD group) were removed from the study because of loss of follow-up, adverse events, withdrawal, violation of protocol, poor efficacy, or other reason. The CONSORT flow chart can be found in the Additional File 3 Figure S3.
The baseline characteristics of the subjects who accomplished the study were shown in Additional File 3 Table S3-S4. There were no statistically significant differences in patient demographics and primary outcomes (p > 0.05).
Outcomes
HbA1c
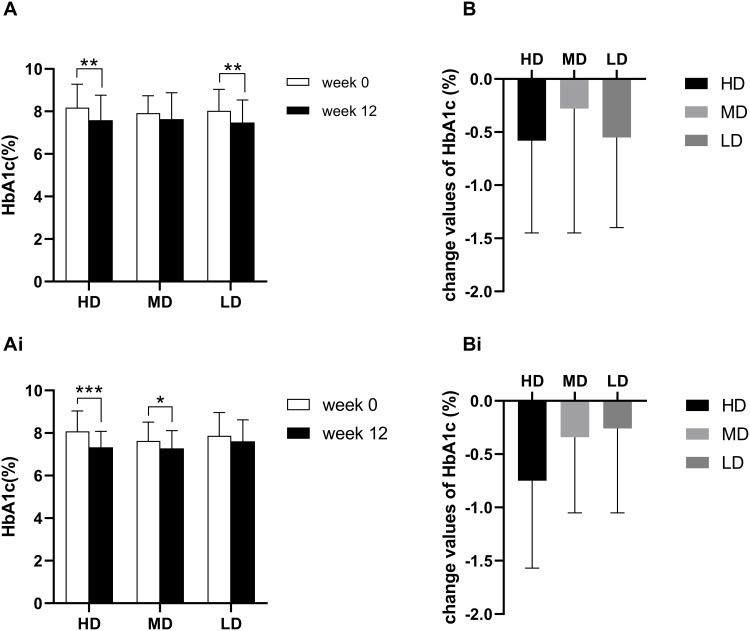
In Study 2, the HbA1c in the HD group was 7.59 (1.17) at week 12, with a mean change of −0.58 from baseline (P<0.001). The HbA1c in the MD group was 7.64 (1.24) at week 12, with a mean change of −0.28 from baseline (P=0.2003). The HbA1c in the LD group was 7.48 (1.06) at week 12, with a mean change of −0.55 from baseline (P<0.001). The analysis of the covariance model and two-by-two comparison showed that the difference between the three groups was not statistically significant (F=0.66, P=0.5206) (Figure 2A-B).
Figure 2.
(A) The HbA1c in Study 2. (B) The change values of HbA1c in Study 2. (Ai), The HbA1c in Study 3. (Bi), The change values of HbA1c in Study 3. The paired t-test was used to compare the difference between week 0 and week 12, *** P < 0.0001, ** P < 0.001, and * P < 0.01.
However, in Study 3, the HbA1c in the HD group was 7.33 (0.75) at week 12, with a mean change of −0.75 from baseline (P<0.0001). The HbA1c in the MD group was 7.28 (0.83) at week 12, with a mean change of −0.34 from baseline (P=0.0093). The HbA1c in the LD group was 7.61 (1.01) at week 12, with a mean change of −0.26 from baseline (P=0.0595). According to the covariance analysis model and pairwise comparison results, the change values of HbA1c were significantly different among the three groups (F=3.11, P=0.0492) (Figure 2Ai-Bi).
The results showed that the change value of HbA1c was not significantly correlated with the dose of Puerariae Lobatae Radix. The effect of different doses of Coptidis Rhizoma on reducing HbA1c was significantly different, and the effect was positively correlated with the dose.
FBG and 2hPG
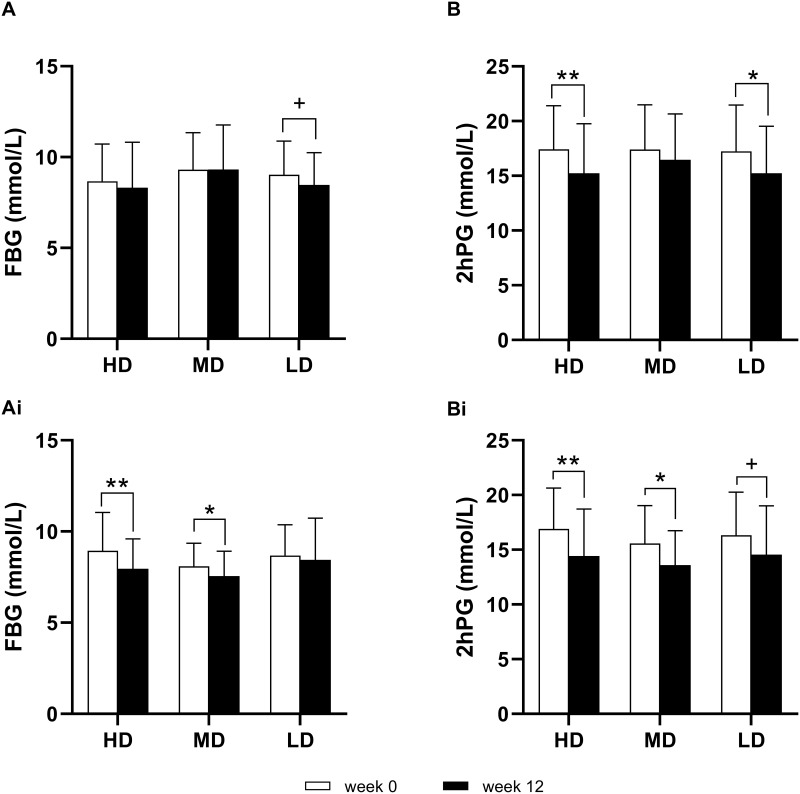
In Study 2, the FBG in the HD group was 8.32 (2.50) at week 12, with a mean change of −0.35 from baseline (P=0.3115). The FBG in the MD group was 9.32 (2.45) at week 12, with a mean change of 0.01 from baseline (P=0.9628). The FBG in the LD group was 8.47 (1.77) at week 12, with a mean change of −0.55 from baseline (P=0.0377). By analysis of covariance, the difference among the three groups was not significant (F=1.27, P=0.2850) (Figure 3A).
Figure 3.
(A) The FBG in Study 2. (B) The 2hPG in Study 2. (Ai), The FBG in Study 3. (Bi), The 2hPG in Study 3. The paired t-test was used to compare the difference between week 0 and week 12, ** P < 0.001, * P < 0.01, and + P < 0.05.
The 2hPG in the HD group was 15.23 (4.52) at week 12, with a mean change of −2.19 from baseline (P < 0.001). The 2hPG in the MD group was 16.47 (4.19) at week 12, with a mean change of −0.94 from baseline (P=0.1488). The 2hPG in the LD group was 15.23 (4.31) at week 12, with a mean change of −2.00 from baseline (P=0.0089). By analysis of covariance, the difference among the three groups was not significant (F=1.30, P=0.2781) (Figure 3B).
In Study 3, the FBG in the HD group was 7.96 (1.63) at week 12, with a mean change of −0.99 from baseline (P=0.0002). The FBG in the MD group was 7.56 (1.36) at week 12, with a mean change of −0.50 from baseline (P=0.0094). The FBG in the LD group was 8.45 (2.28) at week 12, with a mean change of −0.23 from baseline (P=0.4522). By analysis of covariance, the difference among the three groups was not significant (F=2.24, P=0.1116) (Figure 3Ai).
The 2hPG in the HD group was 14.42 (4.30) at week 12, with a mean change of −2.48 from baseline (P=0.0002). The 2hPG in the MD group was 13.59 (3.14) at week 12, with a mean change of −1.94 from baseline (P=0.0079). The 2hPG in the LD group was 14.56 (4.45) at week 12, with a mean change of −1.72 from baseline (P=0.0255). By analysis of covariance, the difference among the three groups was not significant (F=0.26, P=0.7732) (Figure 3Bi).
The results showed that the change values of FBG and 2hPG were not significantly correlated with the dose of Puerariae Lobatae Radix, but tended to be positively correlated with the dose of Coptidis Rhizoma.
The other secondary outcomes were shown in Additional File 4 Figure S4-S7.
Safety Analysis
The adverse events in Study 2 and Study 3 were summarized in Additional File 5 Table S5-S6.
In Study 2, among 120 randomly assigned patients, one patient in the LD group was not included in the safety analysis set due to not taking the study drugs and withdrawing informed consent. The most common adverse event was urinary tract infection, followed by abnormal ECG. In Study 3, all 122 randomly assigned patients entered the safety analysis set. The most common adverse event was urinary protein-positive, followed by urinary leukocyte elevation.
Correlation Analysis
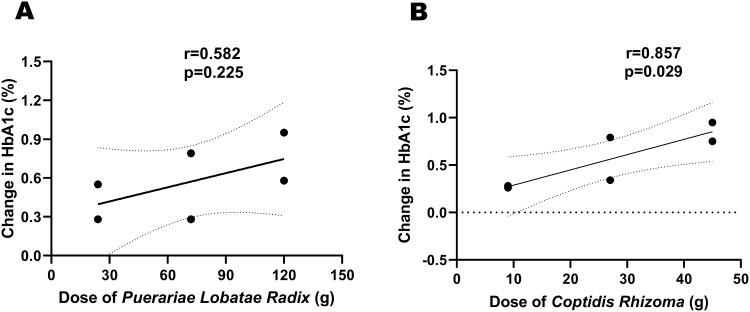
The Pearson correlation was used to analyze the correlation between the main drug dose and the change in HbA1c. The results showed that the correlation coefficient between the dose of Puerariae Lobatae Radix and the change in HbA1c was 0.582, with a p-value of 0.225 (Figure 4A). However, the correlation coefficient between the dose of Coptidis Rhizoma and the change in HbA1c was 0.857, with a p-value of 0.029 (Figure 4B). There was a significant correlation between the dose of Coptidis Rhizoma and the change in HbA1c.
Figure 4.
(A) The correlation between the dose of Puerariae Lobatae Radix and the change in HbA1c. (B) The correlation between the dose of Coptidis Rhizoma and the change in HbA1c.
Discussion
GQD has been used in China for thousands of years as a classical TCM formula for treating acute diarrhea and dysentery related to damp-heat syndrome. With the development of modern medicine, GQD has been used to treat many diseases except diarrhea, among which T2D is one of the most common indications.19 The hypoglycemic effect of GQD is achieved through multiple mechanisms, including improving insulin resistance, inhibiting inflammation, and regulating the structure of the intestinal microbiota.20–23
However, the uncertainty of its main drug makes it difficult for GQD to achieve the best efficacy in the intervention of T2D. We explored the main drug of GQD for treating T2D through three randomized, double-blind, dose-parallel controlled clinical trials.
In the trial to verify whether Puerariae Lobatae Radix is the main drug of GQD in treating T2D, we chose the validated and effective medium-dose GQD as the basic formula, kept the dose of other herbs unchanged, increased and decreased the dose of Puerariae Lobatae Radix, and observed the relationship between the dose of Puerariae Lobatae Radix and the hypoglycemic effect of GQD. The results showed that only changing the dose of Puerariae Lobatae Radix did not show significant differences in hypoglycemic efficacy among the three groups, indicating that Puerariae Lobatae Radix may not be the main drug of GQD in treating T2D. In the trial to validate whether Coptidis Rhizoma is the main drug, we also used the basic formula as the reference standard, fixed the dose of other herbs, and increased and decreased the dose of Coptidis Rhizoma. The results showed that with the change in Coptidis Rhizoma dose, the hypoglycemic effect of GQD was different, and the difference was statistically significant, indicating that Coptidis Rhizoma may be the main drug of GQD in treating T2D. In addition, the results of correlation analysis based on three clinical trials also confirmed the conclusion.
Berberine is the main component of Coptidis Rhizoma. Studies have shown that the potential mechanism of Coptidis Rhizoma in the treatment of diabetes was the glucose-dependent promotion of insulin secretion by berberine.24 In addition, studies have confirmed that berberine could reduce intestinal glucose absorption by inhibiting the translocation of intestinal glucose transporter 2, enhance insulin secretion and prevent β cell dysfunction through miR-204/ SIRT1 signaling pathway, and improve glucocorticoid receptor-mediated insulin resistance by reducing the association of glucocorticoid receptors with phosphatidylinositol-3-kinase.25–27 A clinical trial showed that glycosylated hemoglobin was significantly reduced in patients with newly diagnosed T2D after 12 weeks of treatment with berberine, and further metagenomics and metabolomics studies revealed that the hypoglycemic effect of berberine was mediated through the inhibition of deoxycholic acid biotransformation by Ruminococcus bromii.28
Our studies provided a theoretical basis for adjusting the dose of GQD in the treatment of T2D. It also suggested that the main drug should be re-identified when the main disease treated by the formula changed. Changes in the dose of the main drug could affect the efficacy of the whole formula. The studies also established a method to identify the main drug and demonstrated that the main drug in the traditional formula had a dose-effect relationship with disease.
However, the studies also have some limitations. Firstly, Study 2 and Study 3 only used dose parallel controls and did not include a placebo control group, which may limit our accurate evaluation of the effectiveness of the intervention measures. Therefore, in further research, priority should be given to setting up a placebo control group to more accurately evaluate the efficacy of treatment. Secondly, since three treatment groups were set in the trials, the sample size of subjects in a single treatment group was relatively small. Therefore, in further research, the sample size should be increased. Thirdly, 12 weeks course of treatment was relatively short, and there was no follow-up after the study, which means that we ignored the long-term efficacy of the study drug. Therefore, in further research, the course of medication should be extended and patient follow-up should be increased.
Conclusion
When GQD was used to treat T2D, the dose of Coptidis Rhizoma in the decoction was positively correlated with the hypoglycemic efficacy. It was proved from a clinical point of view that Coptidis Rhizoma in GQD played a major role in the treatment of T2D patients with the dampness-heat syndrome. Furthermore, we showed the efficacy and safety of GQD with Coptidis Rhizoma as the main drug in T2D treatment, as well as a method to determine the main drug for TCM formulas.
Funding Statement
This study was supported by the National key basic research development plan (973) project (2010CB530601).
Abbreviations
2hPG, 2 hours postprandial blood glucose; BMI, body mass index; CHO, cholesterol; CONSORT, Consolidated Standards of Reporting Trials; ECG, electrocardiogram; FBG, fasting blood glucose; FINS, fasting insulin; GQD, Gegen Qinlian Decoction; HbA1c, glycated hemoglobin; HC, hip circumference; HD, High dose; HDL, high-density lipoprotein; HOMA-IR, Homeostasis Model Assessment of Insulin Resistance; HOMA-IS, Homeostasis Model Assessment of Insulin Sensitivity; HOMA-β, Homeostasis Model Assessment-β; HPLC, high-performance liquid chromatography; LD, Low dose; LDL, low-density lipoprotein; MD, Medium dose; T2D, type 2 diabetes; TCM, Traditional Chinese medicine; TG, triglyceride; WC, waist circumference; WHR, waist-hip ratio.
Data Sharing Statement
We intend to share the raw data and detailed research protocol. Interested researchers can apply for data access by contacting the corresponding author of this study, Fengmei Lian. The data will be made available to the public within 12 months after the publication of the article and will be retained for at least 5 years for use by the research community.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Tremblay J, Hamet P. Environmental and genetic contributions to diabetes. Metabolism. 2019;100S:153952. [DOI] [PubMed] [Google Scholar]
- 2.Association AD. 10. cardiovascular disease and risk management: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S125–S150. doi: 10.2337/dc21-S010 [DOI] [PubMed] [Google Scholar]
- 3.Association AD. 11. microvascular complications and foot care: standards of medical care in diabetes-2021. Diab Care. 2021;44(Suppl 1):S151–S167. doi: 10.2337/dc21-S011 [DOI] [PubMed] [Google Scholar]
- 4.Sun H, Saeedi P, Karuranga S, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabet Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabet Res Clin Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023 [DOI] [PubMed] [Google Scholar]
- 6.Khan MAB, Hashim MJ, King JK, et al. Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10:107–111. doi: 10.2991/jegh.k.191028.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Society CD. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 Edition). Chin J Endocrinol Metab. 2021;37(04):311–398. doi: 10.3760/cma.j.cn311282-20210304-00142 [DOI] [Google Scholar]
- 8.Qiu L. Table and explanation of unit values of length, capacity and weight in China’s past dynasties. China Metrol. 2006;(10):46–48+76. doi: 10.16569/j.cnki.cn11-3720/t.2006.10.026 [DOI] [Google Scholar]
- 9.Tong XL, Mu LC, Ji HY, et al. Study on drug dosage of treatise on febrile diseases. J Trad Chin Med. 2009;50(04):368–372. doi: 10.13288/j.11-2166/r.2009.04.020 [DOI] [Google Scholar]
- 10.Xie M. Formulaology. 3rd ed. Bei Jing: People ‘s Medical Publishing House; 2016. [Google Scholar]
- 11.Zhao LH, Lian FM, Ji HY, et al. Clinical examples of treatment for type 2 diabetes by professor Tong Xiao-lin using Ge-Gen-Qin-Lian decoction. Chin J Exp Tradit Med Formul. 2011;17:249–251. [Google Scholar]
- 12.Tong XL, Zhao LH, Lian FM, et al. Clinical observations on the dose-effect relationship of gegen qin lian decoction on 54 out-patients with type 2 diabetes. J Tradit Chin Med. 2011;31:56–59. doi: 10.1016/S0254-6272(11)60013-7 [DOI] [PubMed] [Google Scholar]
- 13.Zhang CH, Xu GL, Liu YH, et al. Anti-diabetic activities of Gegen Qinlian Decoction in high-fat diet combined with streptozotocin-induced diabetic rats and in 3T3-L1 adipocytes. Phytomedicine. 2013;20:221–229. doi: 10.1016/j.phymed.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 14.He Y, Gai Y, Wu X, et al. Quantitatively analyze composition principle of Ma Huang Tang by structural equation modeling. J Ethnopharmacol. 2012;143:851–858. doi: 10.1016/j.jep.2012.08.010 [DOI] [PubMed] [Google Scholar]
- 15.Zhu CS, Nie AZ, Wang X, et al. Research progress on dose- effect relationship of Chinese materia medica. Chin Trad Herbal Drugs. 2019;50(07):1708–1712. [Google Scholar]
- 16.Xu J, Lian F, Zhao L, et al. Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula. ISME J. 2015;9:552–562. doi: 10.1038/ismej.2014.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO Consultation. Definition, Diagnosis, and Classification of Diabetes Mellitus and Its Complications. Geneva, Switzerland: World Health Organization; 1999. [Google Scholar]
- 18.Zheng XY. The Guidelines for Clinical Research of New Drugs of Traditional Chinese Medicine. Bei Jing: China Medical Science and Technology Press; 2002. [Google Scholar]
- 19.Lu JZ, Ye D, Ma BL. Constituents, pharmacokinetics, and pharmacology of Gegen-Qinlian Decoction. Front Pharmacol. 2021;12:668418. doi: 10.3389/fphar.2021.668418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tu J, Zhu S, Li B, et al. Gegen Qinlian Decoction coordinately regulates PPARgamma and PPARalpha to improve glucose and lipid homeostasis in diabetic rats and insulin resistance 3T3-L1 adipocytes. Front Pharmacol. 2020;11:811. doi: 10.3389/fphar.2020.00811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Y, Huang J, Wang N, et al. Network pharmacology-based analysis and experimental exploration of antidiabetic mechanisms of Gegen Qinlian Decoction. Front Pharmacol. 2021;12:649606. doi: 10.3389/fphar.2021.649606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian J, Bai B, Gao Z, et al. Alleviation effects of GQD, a traditional Chinese medicine formula, on diabetes rats linked to modulation of the gut microbiome. Front Cell Infect Microbiol. 2021;11:740236. doi: 10.3389/fcimb.2021.740236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao Z, Zhang W, He L, et al. Double-blinded, randomized clinical trial of Gegen Qinlian decoction pinpoints Faecalibacterium as key gut bacteria in alleviating hyperglycemia. Precis Clin Med. 2024;7(1):pbae003. doi: 10.1093/pcmedi/pbae003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao MM, Lu J, Li S, et al. Berberine is an insulin secretagogue targeting the KCNH6 potassium channel. Nat Commun. 2021;12:5616. doi: 10.1038/s41467-021-25952-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lv X, Zhao Y, Yang X, et al. Berberine potentiates insulin secretion and prevents beta-cell dysfunction through the miR-204/SIRT1 signaling pathway. Front Pharmacol. 2021;12:720866. doi: 10.3389/fphar.2021.720866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng Z, Yu Y, Zhang Y, et al. Highly bioavailable Berberine formulation improves glucocorticoid receptor-mediated Insulin Resistance via reduction in association of the glucocorticoid receptor with phosphatidylinositol-3-kinase. Int J Biol Sci. 2020;16:2527–2541. doi: 10.7150/ijbs.39508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang M, Yang H, Yang E, et al. Berberine decreases intestinal GLUT2 translocation and reduces intestinal glucose absorption in mice. Int J Mol Sci. 2021;23:327. doi: 10.3390/ijms23010327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Gu Y, Ren H, et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat Commun. 2020;11:5015. doi: 10.1038/s41467-020-18414-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We intend to share the raw data and detailed research protocol. Interested researchers can apply for data access by contacting the corresponding author of this study, Fengmei Lian. The data will be made available to the public within 12 months after the publication of the article and will be retained for at least 5 years for use by the research community.