Abstract
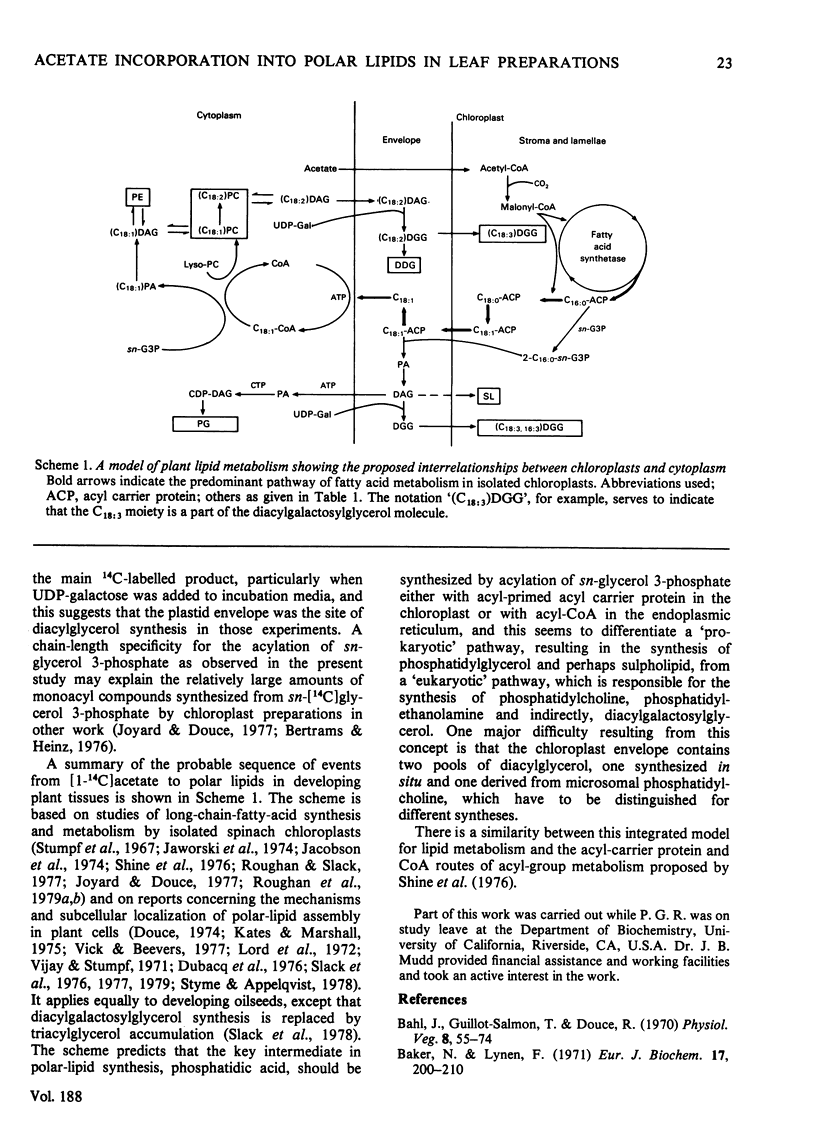
1. Isolated spinach (Spinacia oleracea) chloroplasts were incapable of accumulating polar lipids when incubated with [1-14C]acetate in a cofactor-free medium. When CoA, ATP and glycerol 3-phosphate were added to incubation media, the accumulated products were non-esterified fatty acids, acyl-CoA and 1,2-diacylglycerol, all intermediates of lipid metabolism. 2. Chloroplast acyl-CoA was used to synthesize phosphatidylcholine only when a microsomal fraction was added back to the incubation medium. 3. The 1,2-diacylglycerol synthesized by isolated chloroplasts was converted almost quantitatively into diacylgalactosylglycerol when exogenous UDP-galactose was available. 4. Stereospecific analyses of the isolated lipids suggested that the diacylglycerol synthesized by isolated chloroplasts may be an important precursor for the synthesis in vivo of diacylgalactosylglycerol and phosphatidylglycerol but was unlikely to be a precursor of phosphatidylcholine. 5. A scheme for plant-lipid biosynthesis is presented that integrates the functions of chloroplasts, the cytoplasm and the endoplasmic reticulum.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADBEER C., STUMPF P. K. Fat metabolism in higher plants. XIII. Phosphatidic acid synthesis and diglyceride phosphokinase activity in mitochondria from peanut cotyledons. J Lipid Res. 1960 Apr;1:214–220. [PubMed] [Google Scholar]
- Baker N., Lynen F. Factors involved in fatty acyl CoA desaturation by fungal microsomes. The relative roles of acyl CoA and phospholipids as substrates. Eur J Biochem. 1971 Mar 11;19(2):200–210. doi: 10.1111/j.1432-1033.1971.tb01305.x. [DOI] [PubMed] [Google Scholar]
- Bartels C. T., James A. T., Nichols B. W. Metabolism of trans-3-hexadecenoic acid by Chlorella vulgaris and by lettuce leaf. Eur J Biochem. 1967 Dec;3(1):7–10. doi: 10.1111/j.1432-1033.1967.tb19492.x. [DOI] [PubMed] [Google Scholar]
- CHENIAE G. M. PHOSPHATIDIC ACID AND GLYCERIDE SYNTHESIS BY PARTICLES FROM SPINACH LEAVES. Plant Physiol. 1965 Mar;40:235–243. doi: 10.1104/pp.40.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor K. A., Mudd J. B. Biosynthesis of phosphatidylcholine by enzyme preparations from spinach leaves. J Lipid Res. 1971 Jul;12(4):403–411. [PubMed] [Google Scholar]
- Douce R., Guillot-Salomon T. Sur l'incorporation de la radioactivite du sn-glycerol-3-phosphate-(14)C dans le monogalactosyldiglyceride des plastes isoles. FEBS Lett. 1970 Nov 18;11(2):121–124. doi: 10.1016/0014-5793(70)80507-5. [DOI] [PubMed] [Google Scholar]
- Douce R. Site of biosynthesis of galactolipids in spinach chloroplasts. Science. 1974 Mar 1;183(4127):852–853. doi: 10.1126/science.183.4127.852. [DOI] [PubMed] [Google Scholar]
- Gurr M. I., Brawn P. The biosynthesis of polyunsaturated fatty acids by photosynthetic tissue. The composition of phosphatidyl choline species in Chlorella vulgaris during the formation of linoleic acid. Eur J Biochem. 1970 Nov;17(1):19–22. doi: 10.1111/j.1432-1033.1970.tb01126.x. [DOI] [PubMed] [Google Scholar]
- Haverkate F., van Deenen L. L. Isolation and chemical characterization of phosphatidyl glycerol from spinach leaves. Biochim Biophys Acta. 1965 Jul 7;106(1):78–92. doi: 10.1016/0005-2760(65)90097-4. [DOI] [PubMed] [Google Scholar]
- Jacobson B. S., Jaworski J. G., Stumpf P. K. Fat Metabolism in Higher Plants: LXII. Stearl-acyl Carrier Protein Desaturase from Spinach Chloroplasts. Plant Physiol. 1974 Oct;54(4):484–486. doi: 10.1104/pp.54.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J. G., Goldschmidt E. E., Stumpf P. K. Fat metabolism in higher plants. Properties of the palmityl acyl carrier protein: stearyl acyl carrier protein elongation system in maturing safflower seed extracts. Arch Biochem Biophys. 1974 Aug;163(2):769–776. doi: 10.1016/0003-9861(74)90539-6. [DOI] [PubMed] [Google Scholar]
- Joyard J., Douce R. Site of synthesis of phosphatidic acid and diacyglycerol in spinach chloroplasts. Biochim Biophys Acta. 1977 Feb 23;486(2):273–285. doi: 10.1016/0005-2760(77)90023-6. [DOI] [PubMed] [Google Scholar]
- Kannangara C. G., Stumpf P. K. Fat metabolism in higher plants. I. The biosynthesis of polyunsaturated fatty acids by isolated spinach chloroplasts. Arch Biochem Biophys. 1972 Feb;148(2):414–424. doi: 10.1016/0003-9861(72)90159-2. [DOI] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Beevers H. Intracellular distribution of enzymes of the cytidine diphosphate choline pathway in castor bean endosperm. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2429–2432. doi: 10.1073/pnas.69.9.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall M. O., Kates M. Biosynthesis of phosphatidylglycerol by cell-free preparations from spinach leaves. Biochim Biophys Acta. 1972 Apr 18;260(4):558–570. doi: 10.1016/0005-2760(72)90005-7. [DOI] [PubMed] [Google Scholar]
- Moore T. S., Lord J. M., Kagawa T., Beevers H. Enzymes of phospholipid metabolism in the endoplasmic reticulum of castor bean endosperm. Plant Physiol. 1973 Jul;52(1):50–53. doi: 10.1104/pp.52.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani H. Y., Barber J. An improved method for isolating chloroplasts retaining their outer membranes. Biochim Biophys Acta. 1977 Sep 14;461(3):500–512. [PubMed] [Google Scholar]
- Noda M., Fujiwara N. Positional distribution of fatty acids in galactolipids of Artemisia princeps leaves. Biochim Biophys Acta. 1967 Feb 14;137(1):199–201. [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panter R. A., Boardman N. K. Lipid biosynthesis by isolated plastids from greening pea, Pisum sativum. J Lipid Res. 1973 Nov;14(6):664–671. [PubMed] [Google Scholar]
- Renkonen O., Bloch K. Biosynthesis of monogalactosyl diglycerides in photoauxotrophic Euglena gracilis. J Biol Chem. 1969 Sep 25;244(18):4899–4903. [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. On the control of long-chain-fatty acid synthesis in isolated intact spinach (Spinacia oleracea) chloroplasts. Biochem J. 1979 Nov 15;184(2):193–202. doi: 10.1042/bj1840193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Mudd J. B., McManus T. T., Slack C. R. Linoleate and alpha-linolenate synthesis by isolated spinach (Spinacia oleracea) chloroplasts. Biochem J. 1979 Dec 15;184(3):571–574. doi: 10.1042/bj1840571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G. Phosphatidyl choline: Donor of 18-carbon unsaturated fatty acids for glycerolipid biosynthesis. Lipids. 1975 Oct;10(10):609–614. doi: 10.1007/BF02532725. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Slack C. R., Holland R. High rates of [1-14C]acetate incorporation into the lipid of isolated spinach chloroplasts. Biochem J. 1976 Sep 15;158(3):593–601. doi: 10.1042/bj1580593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Slack C. R. Long-chain acyl-coenzyme A synthetase activity of spinach chloroplasts is concentrated in the envelope. Biochem J. 1977 Feb 15;162(2):457–459. doi: 10.1042/bj1620457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G. Turnover of the glycerolipids of pumpkin leaves. The importence of phosphatidylcholine. Biochem J. 1970 Mar;117(1):1–8. doi: 10.1042/bj1170001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine W. E., Mancha M., Stumpf P. K. Fat metabolism in higher plants. The function of acyl thioesterases in the metabolism of acyl-coenzymes A and acyl-acyl carrier proteins. Arch Biochem Biophys. 1976 Jan;172(1):110–116. doi: 10.1016/0003-9861(76)90054-0. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Balasingham N. Labelling of glycerolipids in the cotyledons of developing oilseeds by [1-14C] acetate and [2-3H] glycerol. Biochem J. 1978 Feb 15;170(2):421–433. doi: 10.1042/bj1700421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Balasingham N. Labelling studies in vivo on the metabolism of the acyl and glycerol moieties of the glycerolipids in the developing maize leaf. Biochem J. 1977 Feb 15;162(2):289–296. doi: 10.1042/bj1620289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Browse J. Evidence for an oleoyl phosphatidylcholine desaturase in microsomal preparations from cotyledons of safflower (Carthamus tinctorius) seed. Biochem J. 1979 Jun 1;179(3):649–656. doi: 10.1042/bj1790649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Terpstra J. Some properties of a microsomal oleate desaturase from leaves. Biochem J. 1976 Apr 1;155(1):71–80. doi: 10.1042/bj1550071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G. The kinetics of incorporation in vivo of (14C)acetate and (14C)carbon dioxide into the fatty acids of glycerolipids in developing leaves. Biochem J. 1975 Nov;152(2):217–228. doi: 10.1042/bj1520217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stymne S., Appelqvist L. A. The biosynthesis of linoleate from oleoyl-CoA via oleoyl-phosphatidylcholine in microsomes of developing safflower seeds. Eur J Biochem. 1978 Oct;90(2):223–229. doi: 10.1111/j.1432-1033.1978.tb12594.x. [DOI] [PubMed] [Google Scholar]
- Sumida S., Mudd J. B. Biosynthesis of cytidine diphosphate diglyceride by enzyme preparations from cauliflower. Plant Physiol. 1970 Jun;45(6):719–722. doi: 10.1104/pp.45.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B., Beevers H. Fatty Acid synthesis in endosperm of young castor bean seedlings. Plant Physiol. 1978 Aug;62(2):173–178. doi: 10.1104/pp.62.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B., Beevers H. Phosphatidic Acid synthesis in castor bean endosperm. Plant Physiol. 1977 Mar;59(3):459–463. doi: 10.1104/pp.59.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay I. K., Stumpf P. K. Fat metabolism in higher plants. XLVI. Nature of the substrate and the product of oleyl coenzyme A desaturase from Carthamus tinctorius. J Biol Chem. 1971 May 10;246(9):2910–2917. [PubMed] [Google Scholar]
- Weaire P. J., Kekwick R. G. The synthesis of fatty acids in avocado mesocarp and cauliflower bud tissue. Biochem J. 1975 Feb;146(2):425–437. doi: 10.1042/bj1460425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. P., Watson G. R., Leung S. P. Galactolipid Synthesis in Vicia faba Leaves: II. Formation and Desaturation of Long Chain Fatty Acids in Phosphatidylcholine, Phosphatidylglycerol, and the Galactolipids. Plant Physiol. 1976 Feb;57(2):179–184. doi: 10.1104/pp.57.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. F., Rinne R. W. Studies on lipid synthesis and degradation in developing soybean cotyledons. Plant Physiol. 1976 Mar;57(3):375–381. doi: 10.1104/pp.57.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]


