Abstract
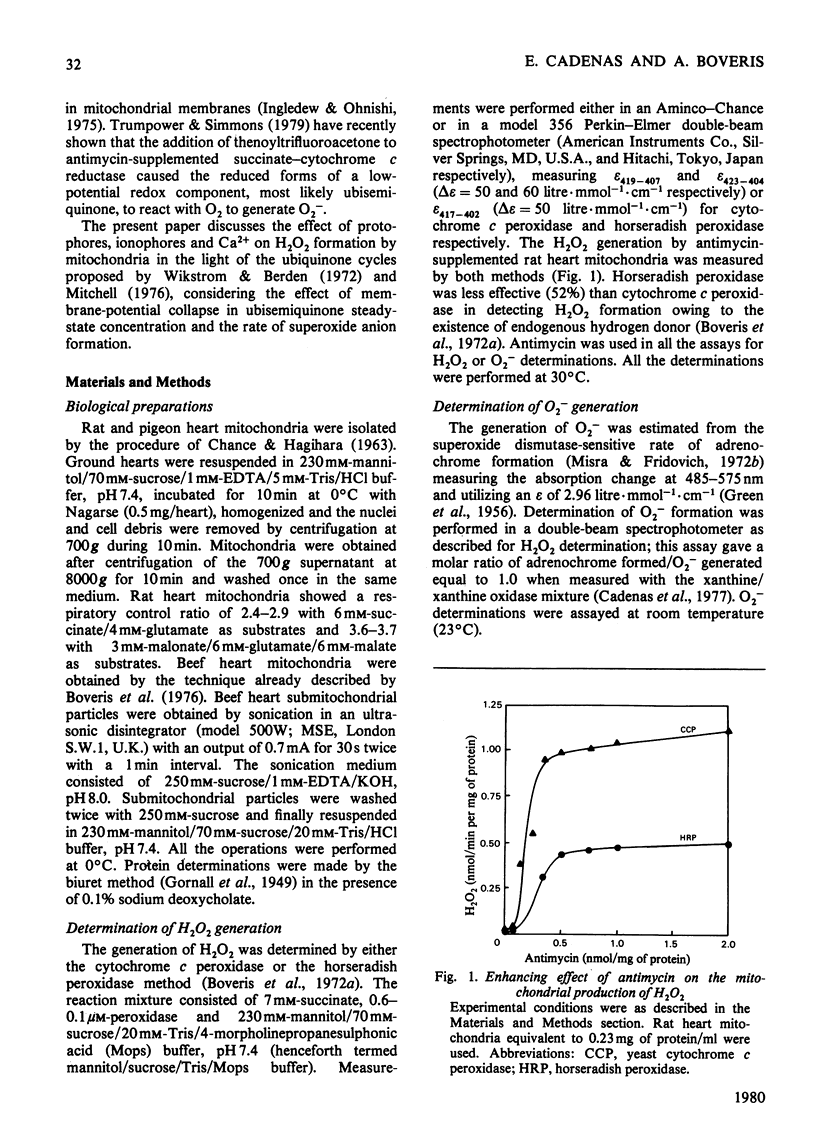
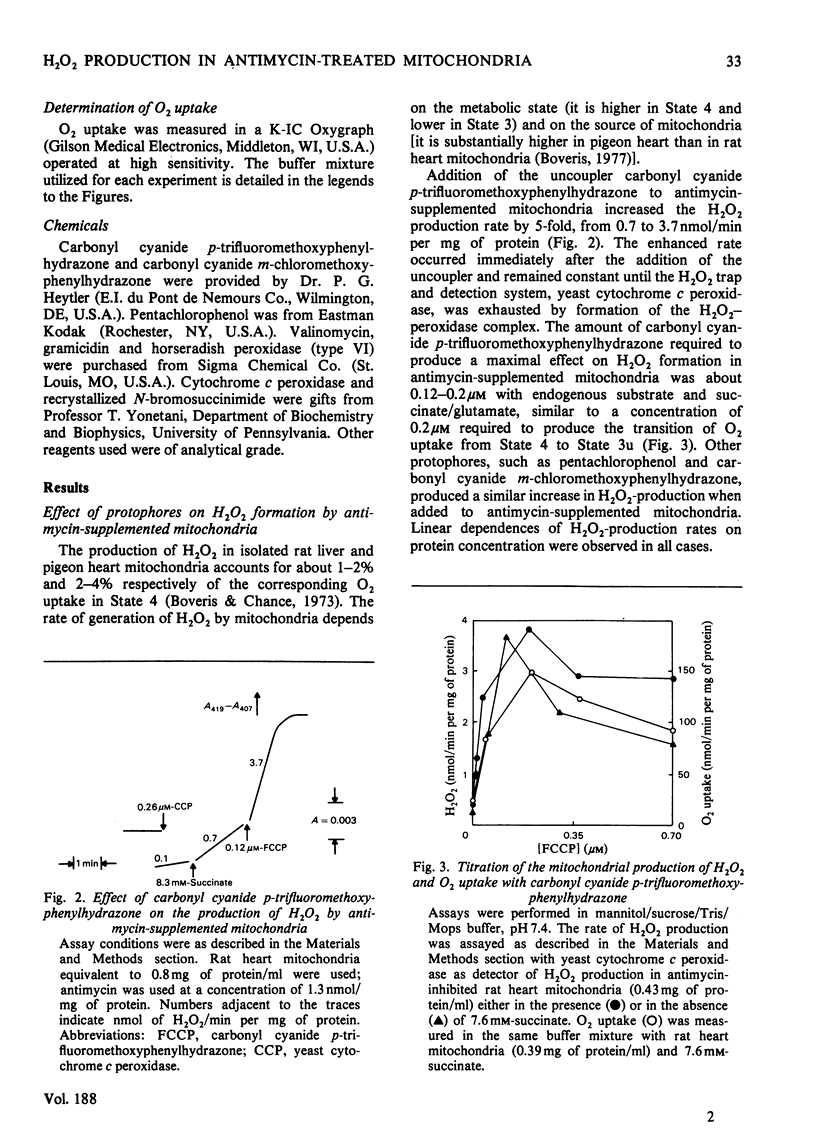
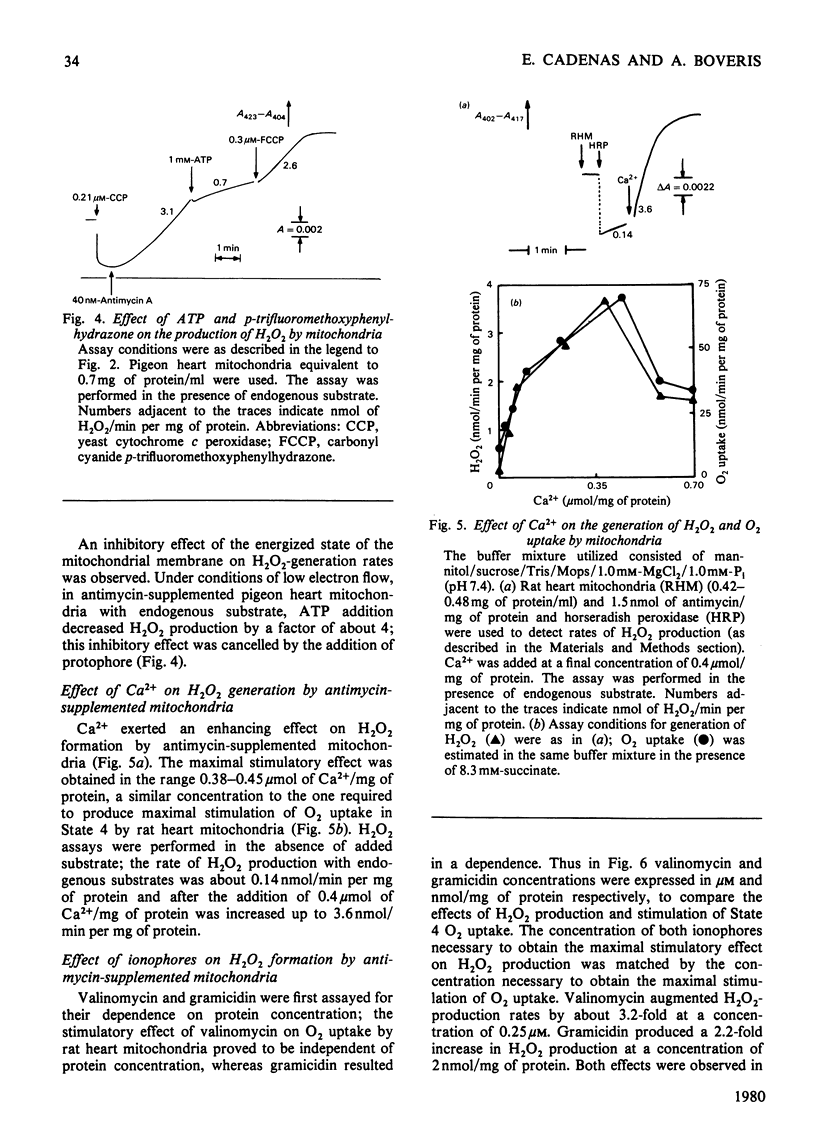
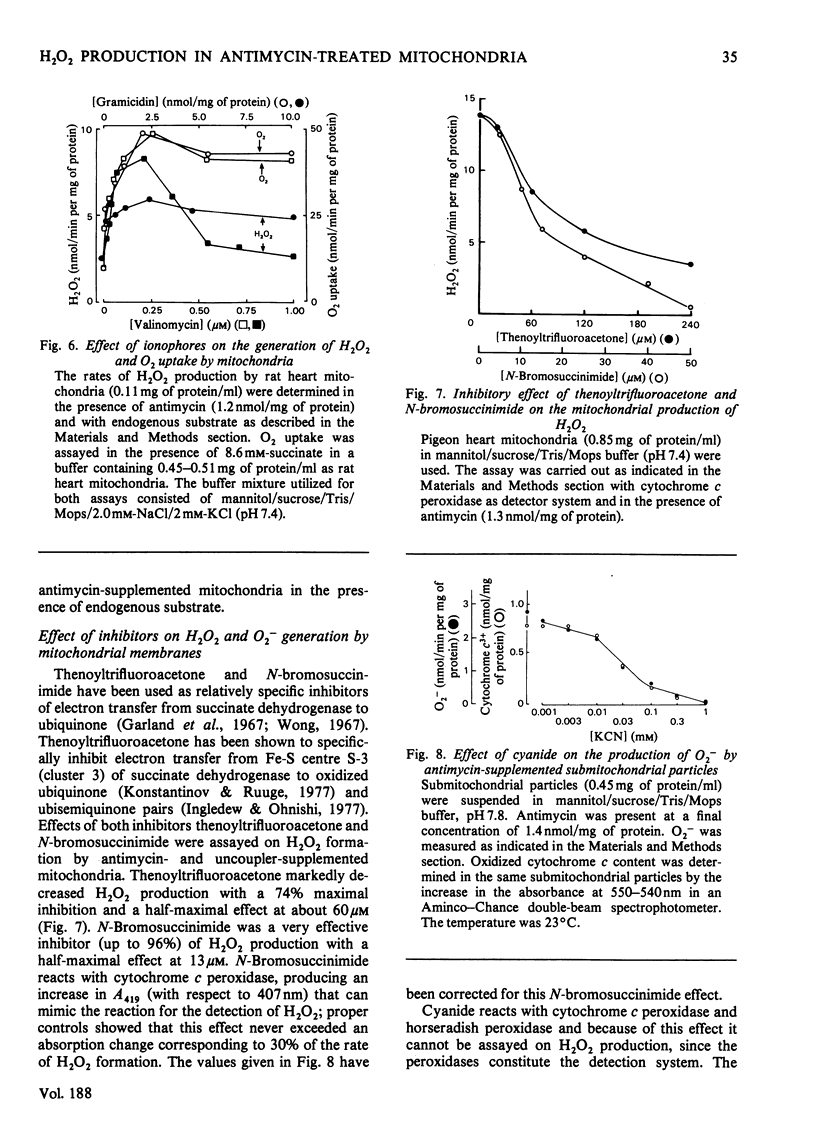
Rat and pigeon heart mitochondria supplemented with antimycin produce 0.3–1.0nmol of H2O2/min per mg of protein. These rates are stimulated up to 13-fold by addition of protophores (carbonyl cyanide p-trifluoromethoxyphenylhydrazone, carbonyl cyanide m-chloromethoxyphenylhydrazone and pentachlorophenol). Ionophores, such as valinomycin and gramicidin, and Ca2+ also markedly stimulated H2O2 production by rat heart mitochondria. The enhancement of H2O2 generation in antimycin-supplemented mitochondria and the increased O2 uptake of the State 4-to-State 3 transition showed similar protophore, ionophore and Ca2+ concentration dependencies. Thenoyltrifluoroacetone and N-bromosuccinimide, which inhibit succinate–ubiquinone reductase activity, also decreased mitochondrial H2O2 production. Addition of cyanide to antimycin-supplemented beef heart submitochondrial particles inhibited the generation of O2−, the precursor of mitochondrial H2O2. This effect was parallel to the increase in cytochrome c reduction and it is interpreted as indicating the necessity of cytochrome c13+ to oxidize ubiquinol to ubisemiquinone, whose autoxidation yields O2−. The effect of protophores, ionophores and Ca2+ is analysed in relation to the propositions of a cyclic mechanism for the interaction of ubiquinone with succinate dehydrogenase and cytochromes b and c1 [Wikstrom & Berden (1972) Biochim. Biophys. Acta 283, 403–420; Mitchell (1976) J. Theor. Biol. 62, 337–367]. A collapse in membrane potential, increasing the rate of ubisemiquinone formation and O2− production, is proposed as the molecular mechanism for the enhancement of H2O2 formation rates observed on addition of protophores, ionophores and Ca2+.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boveris A., Cadenas E. Mitochondrial production of superoxide anions and its relationship to the antimycin insensitive respiration. FEBS Lett. 1975 Jul 1;54(3):311–314. doi: 10.1016/0014-5793(75)80928-8. [DOI] [PubMed] [Google Scholar]
- Boveris A., Cadenas E., Stoppani A. O. Role of ubiquinone in the mitochondrial generation of hydrogen peroxide. Biochem J. 1976 May 15;156(2):435–444. doi: 10.1042/bj1560435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A., Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973 Jul;134(3):707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A., Erecińska M., Wagner M. Reduction kinetics of cytochromes b. Biochim Biophys Acta. 1972 Feb 28;256(2):223–242. doi: 10.1016/0005-2728(72)90055-2. [DOI] [PubMed] [Google Scholar]
- Boveris A. Mitochondrial production of superoxide radical and hydrogen peroxide. Adv Exp Med Biol. 1977;78:67–82. doi: 10.1007/978-1-4615-9035-4_5. [DOI] [PubMed] [Google Scholar]
- Boveris A., Oshino N., Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972 Jul;128(3):617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas E., Boveris A., Ragan C. I., Stoppani A. O. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch Biochem Biophys. 1977 Apr 30;180(2):248–257. doi: 10.1016/0003-9861(77)90035-2. [DOI] [PubMed] [Google Scholar]
- Dionisi O., Galeotti T., Terranova T., Azzi A. Superoxide radicals and hydrogen peroxide formation in mitochondria from normal and neoplastic tissues. Biochim Biophys Acta. 1975 Oct 22;403(2):292–300. doi: 10.1016/0005-2744(75)90059-5. [DOI] [PubMed] [Google Scholar]
- GREEN S., MAZUR A., SHORR E. Mechanism of the catalytic oxidation of adrenaline by ferritin. J Biol Chem. 1956 May;220(1):237–255. [PubMed] [Google Scholar]
- Garland P. B., Chance B., Ernster L., Lee C. P., Wong D. Flavoproteins of mitochondrial fatty acid oxidation. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1696–1702. doi: 10.1073/pnas.58.4.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingledew W. J., Ohnishi T. Properties of the S-3 iron-sulphur centre of succinate dehydrogenase in the intact respiratory chain of beef heart mitochondria. FEBS Lett. 1975 Jun 15;54(2):167–171. doi: 10.1016/0014-5793(75)80067-6. [DOI] [PubMed] [Google Scholar]
- Ingledew W. J., Ohnishi T. The probable site of action of thenolytrifluoracetone on the respiratory chain. Biochem J. 1977 Jun 15;164(3):617–620. doi: 10.1042/bj1640617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingledew W. J., Salerno J. C., Ohnishi T. Studies on electron paramagnetic resonance spectra manifested by a respiratory chain hydrogen carrier. Arch Biochem Biophys. 1976 Nov;177(1):176–184. doi: 10.1016/0003-9861(76)90427-6. [DOI] [PubMed] [Google Scholar]
- Klingenberg M., Rottenberg H. Relation between the gradient of the ATP/ADP ratio and the membrane potential across the mitochondrial membrane. Eur J Biochem. 1977 Feb 15;73(1):125–130. doi: 10.1111/j.1432-1033.1977.tb11298.x. [DOI] [PubMed] [Google Scholar]
- Konstantinov A. A., Ruuge E. K. Semiquinone Q in the respiratory chain of electron transport particles: electron spin resonance studies. FEBS Lett. 1977 Sep 1;81(1):137–141. doi: 10.1016/0014-5793(77)80946-0. [DOI] [PubMed] [Google Scholar]
- Loschen G., Flohé L., Chance B. Respiratory chain linked H(2)O(2) production in pigeon heart mitochondria. FEBS Lett. 1971 Nov 1;18(2):261–264. doi: 10.1016/0014-5793(71)80459-3. [DOI] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972 May 25;247(10):3170–3175. [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The univalent reduction of oxygen by reduced flavins and quinones. J Biol Chem. 1972 Jan 10;247(1):188–192. [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Respiration-driven proton translocation in rat liver mitochondria. Biochem J. 1967 Dec;105(3):1147–1162. doi: 10.1042/bj1051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Possible molecular mechanisms of the protonmotive function of cytochrome systems. J Theor Biol. 1976 Oct 21;62(2):327–367. doi: 10.1016/0022-5193(76)90124-7. [DOI] [PubMed] [Google Scholar]
- Trumpower B. L., Simmons Z. Diminished inhibition of mitochondrial electron transfer from succinate to cytochrome c by thenoyltrifluoroacetone induced by antimycin. J Biol Chem. 1979 Jun 10;254(11):4608–4616. [PubMed] [Google Scholar]
- Wikström M. K., Berden J. A. Oxidoreduction of cytochrome b in the presence of antimycin. Biochim Biophys Acta. 1972 Dec 14;283(3):403–420. doi: 10.1016/0005-2728(72)90258-7. [DOI] [PubMed] [Google Scholar]
- Yu C. A., Yu L., King T. E. Reconstitution of succinate-Q reductase. Biochem Biophys Res Commun. 1977 Dec 7;79(3):939–946. doi: 10.1016/0006-291x(77)91201-3. [DOI] [PubMed] [Google Scholar]


