Abstract
Heat stress (HS) has become a major concern for the poultry industry in many countries. HS impacts gut health by causing damaged mucosal microstructures, increased oxidative stress, weakened immunity, and heightened permeability to toxins and poultry pathogens. We investigated the potential benefits to broiler chickens subjected to HS of dietary supplementation with Bacillus subtilis 14823. Growth performance, gut barrier integrity, and expressions of inflammatory cytokines were analyzed. The results indicated that dietary supplementation with B. subtilis spores at concentrations of 1 × 106 CFU/g of feed (BS6 group) and 1 × 107 CFU/g of feed (BS7 group) improved body weight and body weight gain during d 0–42 (P < 0.05), while the feed intake of the BS7 group was highest (P < 0.05). Additionally, the BS6 group showed a better feed conversion ratio than the control (CON) group (P < 0.05). The BS7 group showed the lowest serum fluorescein isothiocyanate-dextran levels (P < 0.05), and both the BS6 and BS7 groups showed lower corticosterone levels than the CON group (P < 0.05). Additionally, both the BS6 and BS7 groups demonstrated increased villi height and villus height/crypt depth ratio, along with decreased crypt depth in the duodenum and ileum (P < 0.05). However, only the BS7 group exhibited greater improvements than the CON group in the jejunum at d 35. Furthermore, at d 14 and 35, mRNA expressions of occludin, claudin-1, and tight junction protein-1 in the jejunum were upregulated (P < 0.05), and expression levels of five inflammatory cytokine genes were downregulated in the ileum (P < 0.05). Our findings provide new insights and evidence supporting the application of B. subtilis 14823 for enhancing growth performance, gut barrier integrity, and modulating inflammatory cytokines in broilers.
Keywords: B. subtilis 14823, Broilers, Performance, Gut barrier integrity, Inflammatory cytokines
Introduction
Global warming is projected to continue. In the poultry industry, heat stress (HS) has become a major concern in many countries (Ahmad et al., 2008). HS poses a significant challenge for broiler producers due to high economic losses resulting from impaired animal welfare and productivity (Ringseis and Eder, 2022). In tropical countries, such as Thailand, temperatures between 36 °C and 40 °C are considered hazardous for broilers (Aengwanich, 2007) but summer temperatures between 38 °C and 44 °C are not uncommon (Phanprasit et al., 2021). For broiler producers, gut health is a particularly problematic issue (Tsiouris et al., 2018; Liu et al., 2020) and HS has a detrimental impact on the gut health of broilers. The birds can exhibit damaged mucosal microstructures, increased oxidative stress, weakened immunity and heightened gut permeability to toxins and pathogens (Qiu et al., 2021). Nutrient digestion may suffer due to reduced enzymatic activity, decreased absorptive surface area and mucosal injury. Furthermore, altered gene and protein expression can increase intestinal permeability, in the form of leaky gut and related neuroinflammation (Jiang et al., 2021).
Along with these alterations in immune and inflammatory responses, systemic hormonal changes resulting from HS often lead to reduced feed intake and decreased production performance in modern fast-growing broilers (Patra and Kar, 2021). HS activates the hypothalamic-pituitary-adrenal (HPA) axis, leading to the synthesis of corticosterone in the adrenal glands. This process induces the release of cytokines and glucocorticoids, resulting in protein breakdown, stunted growth, and elevated mortality rates in chickens subjected to HS (Wang et al., 2018; Mohammed et al., 2021). HS has also been shown to adversely affect chicken meat quality (Gholamreza et al., 2019). In response to these challenges, the use of probiotics, particularly Bacillus species, has gained traction as a viable nutritional strategy to mitigate the adverse effects of HS in poultry (Wang et al., 2019; Ahmad et al., 2008). B. subtilis can be found in diverse environments, including the gastrointestinal tracts of animals and humans (Williams and Weir, 2024) and is notable for its widespread presence and potential health benefits. Its capacity to form spores that are resistant to bile and gastrointestinal enzymes, and high temperatures and pH, allows B. subtilis to withstand harsh conditions and confer health benefits to the host (Memon et al., 2022).
The adaptability of B. subtilis has proved useful in various food and feed applications (Jiang et al., 2021). Supplementation with Bacillus-based probiotics has demonstrated positive impacts on the growth performance, nutrient digestibility, intestinal morphology and immune response of young pigs (Mun et al., 2021). These probiotics have been reported to strengthen tight junctions, reduce inflammatory responses under stress, and influence intestinal microbiota composition (Memon et al., 2022; Rhayat et al., 2019). More specifically, broilers given B. subtilis probiotics and exposed to daily HS at 32 °C from day 15 to day 42 exhibited reduced stress indicators and hepatic IL-6 levels, showing an enhanced capacity to manage HS by regulating the microbiota-gut-immune axis and alleviating heat-induced behavioral and inflammatory responses (Wang et al., 2018; Jiang et al., 2021).
While the potential health benefits of B. subtilis are recognized, it is essential to note that probiotic effects can be strain-specific (Williams, 2010; Bermudez-Brito et al., 2017; Plaza-Diaz et al., 2014). In particular, the effects of B. subtilis 14823, isolated from the jejunum of Ross 308 broilers, have not been previously investigated. Therefore, the primary objective of this study is to evaluate the effectiveness of B. subtilis 14823 in promoting growth performance, enhancing gut barrier integrity, and modulating inflammatory cytokines in broilers exposed to HS. This research is significant as it may provide a foundation for developing targeted probiotic interventions tailored for specific strains, thereby improving poultry health and productivity under HS conditions.
Materials and methods
Preparation of B. subtilis spores
B. subtilis (isolate code 14823), a strain isolated from the jejunum of healthy 35-day-old Ross 308 broiler chicks, was cultured in LB medium to produce antimicrobial peptides. These peptides were then quantified and their concentrations enhanced using Fast Protein Liquid Chromatography. The proteins were characterized through Mass Spectrometry and High-Performance Liquid Chromatography (HPLC). The Bacillus species was identified by sequencing the 16S ribosomal RNA-encoding DNA, following the method described by Piewngam et al. (2018). Subsequently, B. subtilis cultures at concentrations of 1 × 106 and 1 × 107 CFU/ml were formulated into a dry powder.
Animal ethics
All procedures in the current study followed the guidelines and rules for animal experiments of Rajamangala University of Technology Srivijaya, Thailand (Code IAC 05-01-2023) and conformed to the Ethical Principles and Guidelines for the Use of Animals for Scientific Purposes, published by the National Research Council of Thailand (Application No. U1-01641-2558).
Experimental animals and treatments
A total of 660 male Ross 308 broiler chicks were obtained from a commercial hatchery. The chicks were randomly assigned to four groups: 1) the CON group (receiving a basal diet); 2) the CPB group (receiving a basal diet with a commercial probiotic Bacillus supplementation of 1 × 106 CFU/g of feed); 3) the BS6 group (receiving a basal diet with a cultured B. subtilis spore supplementation of 1 × 106 CFU/g of feed), and 4) the BS7 group (receiving a basal diet with a cultured B. subtilis spore supplementation of 1 × 107 CFU/g of feed). Each group had five replicates, with thirty-three chicks per replicate. The commercial probiotic Bacillus supplementation given to the CPB group was made from a unique spore-forming B. subtilis strain with a product concentration of 1 × 108 CFU/g. The recommended dosage is 10 mg/kg of feed, resulting in a final concentration of 1 × 106 CFU/kg in the feed. The BS6 group received cultured B. subtilis spores at the same concentration. The BS7 group was supplemented with a higher level of cultured B. subtilis spores (1 × 10⁷ CFU/g of feed) to evaluate the potential benefits of increased probiotic exposure. The composition and nutrient levels of the basal diet met the nutritional requirements for both starter and grower-finisher phases of birds raised in tropical climates, formulated to be similar to the requirements outlined in the NRC (1994) recommendations, as shown in Table 1. The chicks were housed in an open production house in the Southern Region of Thailand during the summer months of April and May 2023. The ambient temperature ranged from 33.0 to 38.0 °C, averaging 35.0 °C, with relative humidity consistently between 70 and 75%. Each experimental group was allocated a 2.5 m² ground enclosure equipped with a self-feeder and a waterer. Bedding material was composed of rice hulls, and the lighting schedule included 23 h of light from days 1 to 4, with a gradual decrease to 18 h of light per day starting from day 7. All birds had ad-libitum access to feed and water during the experiment.
Table 1.
Feed ingredients and calculated analysis of basal diet.
| Basal diet | ||||
|---|---|---|---|---|
| Items (as fed) | Starter phase | Grower-Finisher phase | ||
| Feed ingredients (g/kg) | ||||
| Corn | 606.4 | 626.1 | ||
| Fish meal (580 g CP/kg) | 60 | 60 | ||
| Soybean meal (480 g CP/kg) | 271.2 | 234 | ||
| Palm oil | 38.8 | 54.7 | ||
| Dicalciumphosphate (180 g P/kg) | 13.4 | 16.4 | ||
| DL-Methionine | 2.2 | 0.8 | ||
| Salt | 3 | 3 | ||
| Broiler premix 1 | 5 | 5 | ||
| Calculated analysis (g/kg) | ||||
| Metabolizable energy (MJ/kg) | 12.9 | 13.3 | ||
| Dry matter | 884.6 | 885.8 | ||
| Crude protein (CP) | 210 | 195 | ||
| Crude fat | 70.3 | 85.9 | ||
| Crude fiber | 30.2 | 30.3 | ||
| Lysine | 11.5 | 10.4 | ||
| Methionine + Cystine | 9 | 7.2 | ||
| Methionine | 5.3 | 4.3 | ||
| Threonine | 8 | 7.4 | ||
| Valine | 10.1 | 9.4 | ||
| Iso-leucine | 8.8 | 8.1 | ||
| Arginine | 13.5 | 12.3 | ||
| Tryptophan | 2.1 | 1.9 | ||
| Calcium | 8.7 | 9 | ||
| Available phosphorus | 4.6 | 4 |
1 The broiler trace mineral premix, per kilogram of diet, comprised 149.6 mg of manganese, 125.1 mg of zinc, 16.5 mg of iron, 1.7 mg of copper, 1.05 mg of iodine, 0.25 mg of selenium, and calcium levels ranging from 6.27 mg to 8.69 mg. The calcium carbonate base of the premix contained less than 1% mineral oil. The vitamin premix, also per kilogram of diet, supplied 11,023 IU of vitamin A, 3,858 IU of vitamin D3, 46 IU of vitamin E, 0.0165 mg of vitamin B12, 5.845 mg of riboflavin, 45.39 mg of niacin, 20.21 mg of D-pantothenic acid, 477.67 mg of choline, 1.47 mg of menadione, 1.75 mg of folic acid, 7.17 mg of pyridoxine, 2.94 mg of thiamine, and 0.55 mg of biotin, with ground rice hulls serving as the carrier.
Determination of growth performance
Body weight (BW), body weight gain (BWG), and feed intake (FI) were recorded at days (d) 0, 21, and 42. Data were analyzed for three periods: d 0–21, d 22–42, and the entire trial (d 0–42). Feed conversion ratio (FCR) was calculated as the ratio of total FI to total BWG for each period. Mortality was monitored daily.
Intestinal barrier function
Intestinal barrier function was assessed using an indicator of fluorescein isothiocyanate dextran (FITC-d). Increased serum FITC-d indicated barrier dysfunction in the form of permeability. The method was adapted from Vicuña et al. (2015) and Kikusato et al. (2021), with modifications. On d 14 and d 35, all chicks were fasted for 5 h. Subsequently, one randomly chosen chicken from each replicate (n = 5 per group), based on average BW, was orally administered FITC-d (3-5 kDa; Sigma Aldrich Co., St. Louis, MO) at a dose of 2.2 mg/kg BW. One hour later, blood samples were collected using a wing vein puncher, allowed to clot for 3 h at room temperature (20-25 °C), and then centrifuged at 1,500 × g for 15 min at 4 °C. The serum was diluted with PBS at a 1: 1 ratio, and the FITC-d serum concentration was determined using a spectrofluorometer at 485 nm excitation and 528 nm emission wavelengths, based on a standard curve.
Serum corticosterone concentration
The samples from serum FITC-d measurement were utilized for corticosterone (COR) analysis. The analysis was conducted using a liquid chromatography system equipped with a photodiode array detector 2996, 1525 binary HPLC pumps, in-line degasser AF, and a 717 plus autosampler, controlled by Empower software (all from Waters Corporation). To extract corticosterone from plasma, 1 mL of serum spiked with an internal standard of flumethasone was mixed with 0.6 mL of 0.25 M NaOH and left at room temperature for 10 min. Subsequently, 15 mL of dichloromethane was added to facilitate hormone extraction. The organic layer obtained by centrifugation at 6,000 g for 5 min was transferred to a clean flask and dried under rotary evaporation at 40 °C. The dried hormone was dissolved in a 60: 40 v/v methanol–water mobile phase and filtered through a 0.45 µm nylon syringe filter. A 20 µL sample was injected onto a Purospher® STAR RP-18 encapped column (5 µm, Merck KGaA, Darmstadt, Germany), with a mobile phase flow rate set at 1.2 mL/min. Detection was performed at 248 nm. COR concentration was determined using a standard curve constructed from each standard and the peak area ratio of the internal standard (Theapparat et al., 2017; Khongthong et al., 2023).
Total RNA extraction and mRNA expression of inflammatory cytokines and tight junctions
Chicks were selected as described for serum FITC-d measurement, fasted for 6 h, and euthanized. Tissue samples from the jejunum, approximately 2 cm in length, were collected, rinsed with chilled sterile PBS, treated with liquid nitrogen, and preserved in 2 mL cryotubes at −80 °C for RNA extraction. Total RNA was extracted from the jejunum to analyse mRNA expressions of inflammatory cytokines and tight junction proteins using quantitative real-time polymerase chain reaction (real-time PCR). RNA was extracted with the RNeasy Mini Kit (Qiagen, Hilden, Germany), following the manufacturer's protocol. RNA concentration was determined using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA) at 260 nm and 280 nm. Samples with an A260/280 ratio of 1.8–2.0 were retained, and RNA integrity was confirmed via 1% agarose gel electrophoresis. Subsequently, 2 μg of total RNA per sample were diluted 1: 10 with nuclease-free water and stored at −80 °C. Real-time PCR was conducted using a Bio-Rad CFX machine (Biorad, Temecula, CA) and a SYBR Green-based mix (biotechrabbit GmbH, Berlin, Germany) in a 20 µL reaction volume. Cycling parameters included reverse transcription at 50 °C, denaturation at 95 °C for 3 min, followed by 40 cycles of denaturation at 95 °C for 10 s, and primer-specific annealing and extension at 60 °C for 30 s each. A melt curve analysis was performed for each gene post-PCR. Nucleotide sequences for the genes of interest are listed in Table 2, with primers designed using Primer Blast software (NCBI-NIH, Bethesda, MD). Duplicate samples with a ≤ 5% difference were considered acceptable. Relative gene expressions were determined using the 2−ΔΔCT method (Livak and Schmittgen, 2001; Khongthong et al., 2023), normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH), comparing the cycle threshold values of the target genes to that of the reference gene, GAPDH.
Table 2.
Primers used for real-time PCR amplification of target genes.
| Gene | GenBank Gene accession no. | Primer sequence (5′-3′) | Product size (bp) | Annealing temp |
|---|---|---|---|---|
| IFN-γ | NM_205149.1 | F:AGCTGACGGTGGACCTATTATT R:GGCTTTGCGCTGGATTC |
105 | 58 |
| IL-4 | AJ621249 | F:GTGCCCACGCTGTGCTTA R:AGGAAACCTCTCCCTGGATGTC |
167 | 58 |
| TNF-α | NM_204267 | F:GAGCGTTGACTTGGCTGTC R:AAGCAACAACCAGCTATGCAC |
190 | 58 |
| iNOS | D85422 | F:CCTGTACTGAAGGTGGCTATTGG R:AGGCCTGTGAGAGTGTGCAA |
134 | 58 |
| NF-κB | NM_205129 | F:GTGTGAAGAAACGGGAACTG R:GGCACGGTTGTCATAGATGG |
143 | 58 |
| CLDN-1 | NM_001013611.2 | F:GGCCACGTCATGGTATGG R:AACGGGTGTGAAAGGGTCATAG |
62 | 58 |
| OCLD | NM_205128.1 | F:ACGGCAGCACCTACCTCAA R:GGGCGAAGAAGCAGATGAG |
123 | 60 |
| TJP-1 | XM_413773.4 | F:GGATGTTTATTTGGGCGGC R:GTCACCGTGTGTTGTTCCCAT |
187 | 60 |
| MUC-2 | XM001234581.3 | F:CCCTGGAAGTAGAGGTGACTG R:TGACAAGCCATTGAAGGACA |
143 | 60 |
| GAPDH | NM_204305 | F:ACATGGCATCCAAGGAGTGAG R:GGGGAGACAGAAGGGAACAGA |
260 | 60 |
F, Forward primer; R, Reverse primer.
IFN-γ, cytokines interferon gamma; IL-4, interleukin-4; TNF-α, tumor necrosis factor alpha; iNOS, nitric oxide synthase; NF-κB, nuclear factor; CLDN-1, claudin-1; OCLD, occluding; TJP-1, tight junction proteins-1; MUC-2, mucin-2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Intestinal histomorphology
On d 35 of age, 2-cm segments from the midpoints of duodenum, jejunum and ileum were collected as described for mRNA expression measurement, rinsed in saline, fixed in 10% formalin and Bouin solution, dehydrated via an alcohol-toluene sequence, embedded in paraffin, and sliced into 5 μm-thick sections. These were stained with haematoxylin and eosin according to Awad et al. (2011), and examined under an Olympus BX43 optical microscope. Digital histology images were analyzed using Photoshop CS following Rodjan et al. (2021) to calculate the villus height to crypt depth ratios for each intestinal segment.
Statistical analysis
Experimental data were analyzed using a completely randomized design, featuring four treatments and five replications each, using the General Linear Model in SAS software (SAS, 2004). The Shapiro–Wilk and Levene's tests were employed to assess normal distribution and variance homogeneity, respectively. Treatment differences were evaluated using Tukey's test at P < 0.05, with results presented as means ± SEM (n = 5). P-values below 0.10 were considered to indicate trends. The experimental model was Yij = μ + Ti + eij, where Yij represents the observed value, μ the mean, Ti the treatment effect, and eij the random error. Pearson's correlation analysis (GraphPad Prism version 9.5.0, La Jolla, CA, USA) was used to assess relationships between and among body weight, intestinal health parameters, blood biochemistry parameters, and mRNA expressions of tight junction proteins and inflammatory cytokines. Correlation strengths were interpreted as follows: ≥ 0.20 = weak, ≥ 0.40 = moderate, ≥ 0.70 = strong, and ≥ 0.90 = very strong (Rowntree, 1981). The significance level was set at 5%.
Results
Quantification of fengycin production
The quantification of fengycin production by cultured B. subtilis isolated (isolate Code 14823) from jejunum sections of Ross 308 broilers revealed distinct concentrations of various fengycin compounds (Table 3). The hydroxylated compound B-OH-C16-FenA was present at the highest concentration of 3.63 μM, highlighting its predominance among the fengycin variants. Other significant compounds included B-OH-C17-FenA at 2.77 μM and B-OH-C16-FenB at 1.24 μM. Non-hydroxylated forms such as C16-Fen A and C17-Fen A were present in lower concentrations, at 0.36 μM and 0.46 μM, respectively. The cumulative concentration of all measured fengycin compounds was 11.50 μM.
Table 3.
Quantification of fengycin production by Bacillus subtilis (isolate code 14823) isolated from jejunum.
| Fengycin compound | Concentration (μM) |
|---|---|
| B-OH-C16-FenA | 3.63 |
| C16-Fen A | 0.36 |
| B-OH-C17-FenA | 2.77 |
| C17-Fen A | 0.46 |
| B-OH-C16-FenB | 1.24 |
| C16-Fen B | 1.03 |
| B-OH-C17-FenB | 1.13 |
| C17-Fen B | 0.89 |
| Total fengycin concentration | 11.50 |
Note: The measurements represent intensity values from the integration of m/z peaks associated with specific fengycin species, obtained via RP-HPLC/MS. Values are in μM, calibrated using weighed and diluted aliquots of the Bacillus lipopeptide surfactin.
Growth parameters and viability
The growth performance of broiler chicks across each growth phase showed that chicks supplemented with dietary probiotics showed significantly improved performances compared to the CON group (P < 0.05) from d 0 to 42. The improvement was particularly significant for the overall phase. The BS6 and BS7 groups (supplemented with cultured B. subtilis 14823) exhibited superior growth performances compared to the CPB group (supplemented with the commercial B. subtilis probiotic). Specifically, broilers in the BS7 group, which received a higher dose of B. subtilis 14823, had the highest BW and BWG, highlighting a dose-dependent response within the B. subtilis treatments. All probiotic-supplemented groups (CPB, BS6, BS7) showed significantly higher BW and BWG compared to the CON group (P < 0.05, Table 4). The BS7 group also demonstrated a more pronounced improvement in FCR compared to the CON group, further distinguishing the efficacy of the treatment. Despite these differences in growth performances and feed efficiency, viability did not differ significantly among the groups.
Table 4.
Changes in growth performance from d 0 to 42 of age of broiler chickens supplemented with dietary probiotic.
| Dietary probiotic groups |
|||||||
|---|---|---|---|---|---|---|---|
| Parameters | CON | CPB | BS6 | BS7 | SEM | P - value | |
| d 0 | IBW, g | 42.78 | 43.38 | 42.71 | 43.11 | 0.298 | 0.385 |
| d 21 | BW, g | 965.60b | 1024.59a | 983.82b | 994.63ab | 10.00 | 0.006 |
| d 42 | BW, g | 3170.30b | 3374.94a | 3307.29a | 3399.65a | 28.40 | 0.001 |
| d 0-21 | BWG, g | 922.82b | 981.21a | 941.11ab | 951.52ab | 9.94 | 0.006 |
| FI, g | 1159.73 | 1187.41 | 1159.48 | 1168.36 | 8.98 | 0.136 | |
| FCR, g/g | 1.26a | 1.21b | 1.23ab | 1.22ab | 0.01 | 0.008 | |
| d 22-42 | BWG, g | 2204.70b | 2381.77a | 2310.70ab | 2405.03a | 29.42 | 0.001 |
| FI, g | 3203.35ab | 3241.37ab | 3194.47b | 3351.43a | 36.79 | 0.030 | |
| FCR, g/g | 1.45a | 1.36b | 1.38ab | 1.39ab | 0.019 | 0.018 | |
| d 0-42 | BWG, g | 3127.52b | 3331.56a | 3264.57a | 3356.54a | 32.66 | 0.001 |
| FI, g | 4363.07b | 4428.78ab | 4353.94b | 4519.78a | 38.29 | 0.027 | |
| FCR, g/g | 1.39a | 1.33b | 1.33b | 1.35ab | 0.01 | 0.021 | |
| Viability, % | 98.70 | 98.75 | 99.38 | 99.35 | 0.71 | 0.847 | |
In rows, means followed by the same letter are not significantly different (P > 0.05) by Tukey's HSD test.
CON, control group (basal diet); CPB, commercial probiotic Bacillus (1 × 106 CFU/g of feed); BS6, probiotic B. subtilis spores (1 × 106 CFU/g of feed), BS7, probiotic B. subtilis spores (1 × 107 CFU/g of feed); SEM, standard error of the mean (n = 5).
IBW, Initial body weight; BW, body weight; BWG, body weight gain; FI, feed intake; FCR, feed conversion ratio.
Intestinal barrier function
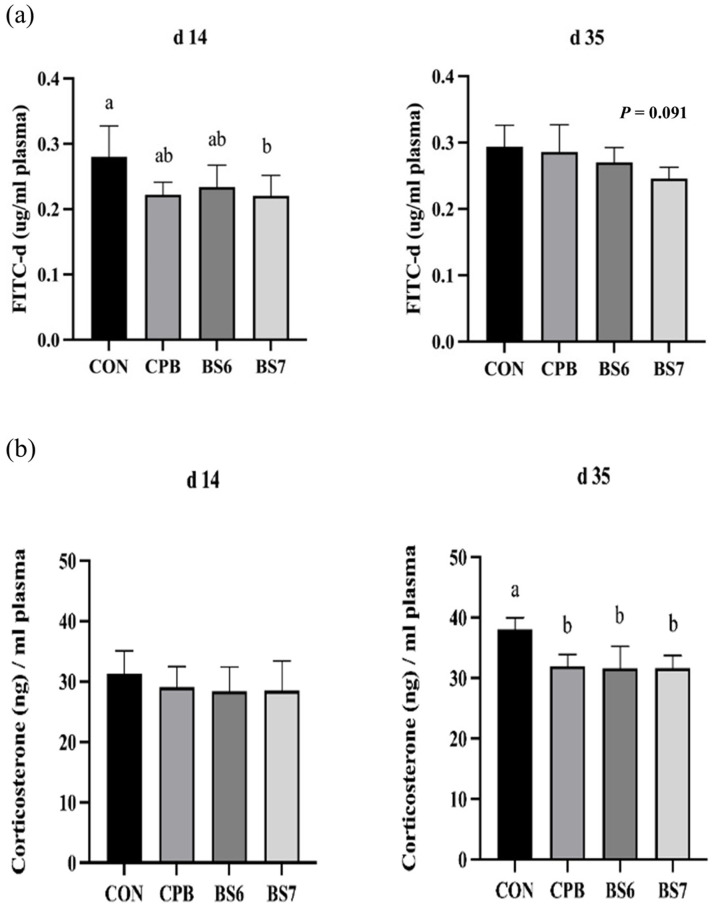
On d 14, significant differences in FITC-d levels were observed across the groups (P < 0.05). The BS7 group had the lowest FITC-d level, followed by the BS6 and CPB groups, while the CON group showed the highest value. No significant differences in FITC-d levels were observed among the groups on d 35, although a downward trend was noted (P = 0.091, Fig. 1a). COR levels on d 35 were significantly higher in the CON group compared to the probiotic-supplemented groups (P < 0.05). Among the probiotic-supplemented groups, the BS6 and BS7 groups had the lowest COR levels, with the CPB group showing intermediate values. No significant differences in COR levels were observed on d 14 (P < 0.05, Fig. 1b).
Fig. 1.
Serum fluorescein isothiocyanate dextran (FITC-d) (a) and corticosterone concentration levels (b) of heat-stressed broiler chickens at days 14 and 35 of age from four dietary treatments: control basal diet (CON); basal diet with commercial probiotic Bacillus included at 1 × 10⁶ CFU/g of feed (CPB); basal diet with separated B. subtilis spores included at 1 × 10⁶ CFU/g of feed (BS6); and basal diet with separated B. subtilis spores included at 1 × 10⁷ CFU/g of feed (BS7). Error bars represent the standard error of the mean. Statistically significant differences among groups were determined using Tukey's HSD test (n = 5, P < 0.05), with different letters indicating significance.
Intestinal histomorphology
The results on d 35 showed significant improvements in duodenal villus heights (DVH), duodenal crypt depth (DCD), and the DVH/CD ratio across all probiotic-supplemented groups compared to the CON group (P < 0.05, Table 5). Among the probiotic groups, the BS6 and BS7 groups demonstrated greater enhancements in DVH and the DVH/CD ratio than the CPB group, with the BS7 group showing the highest values. The CPB and BS7 groups both exhibited significantly higher jejunal villus heights (JVH) and jejunal villus height to crypt depth ratios (JVH/CD ratio) compared to the CON group (P < 0.05). The BS7 group also had a significantly lower jejunal crypt depth (JCD) compared to all other groups. The BS6 group showed moderate improvements in JVH and JVH/CD ratios, indicating a dose-dependent effect between the BS6 and BS7 treatments. Regarding the ileum, the BS6 group displayed the highest ileum villus heights (IVH) and ileum villus height to crypt depth ratios (IVH/CD ratio), followed by the BS7 and CPB groups, which both showed significantly improved values compared to the CON group (P < 0.05). The CON group consistently showed the lowest values across all intestinal sections.
Table 5.
Intestinal morphology at d 35 of age of broiler chickens supplemented with dietary probiotics.
| Dietary probiotic groups |
|||||||
|---|---|---|---|---|---|---|---|
| Parameters | CON | CPB | BS6 | BS7 | SEM | P - value | |
| d 35 | |||||||
| DVH, µm | 1056.98b | 1361.48a | 1407.90a | 1429.46a | 70.08 | 0.006 | |
| DCD, µm | 213.59a | 161.57b | 136.15b | 143.65b | 10.31 | < 0.001 | |
| DVH/CD ratio | 5.05b | 8.54a | 10.80a | 9.94a | 0.77 | < 0.001 | |
| d 35 | |||||||
| JVH, µm | 933.36b | 1236.67a | 1075.60ab | 1308.62a | 67.18 | 0.005 | |
| JCD, µm | 196.04b | 191.64b | 196.31b | 91.55a | 8.13 | < 0.001 | |
| JVH/CD ratio | 4.78b | 6.43b | 5.49b | 14.47a | 0.60 | < 0.001 | |
| d 35 | |||||||
| IVH, µm | 1038.81c | 896.28d | 1560.99a | 1196.35b | 33.84 | < 0.001 | |
| ICD, µm | 316.29a | 158.82b | 158.09b | 148.22b | 14.05 | < 0.001 | |
| IVH/CD ratio | 3.35d | 5.87c | 9.91a | 8.06b | 0.42 | < 0.001 | |
In rows, means followed by the same letter are not significantly different (P > 0.05) by Tukey's HSD test.
CON, control group (basal diet); CPB, commercial probiotic Bacillus (1 × 106 CFU/g of feed); BS6, probiotic B. subtilis spores (1 × 106 CFU/g of feed), BS7, probiotic B. subtilis spores (1 × 107 CFU/g of feed); SEM, standard error of the mean (n = 5).
DVH, duodenal villus height; DCD, duodenal crypt depth; DVH/CD ratio, duodenal villus height to crypt depth ratio.
JVH, Jejunal villus heights; JCD, Jejunal crypt depth; JVH/CD ratio, Jejunal villus height to crypt depth ratio.
IVH, ileal villus height; ICD, ileal crypt depth; IVH/CD ratio, ileal villus height to crypt depth ratio.
mRNA expression of intestinal tight junctions and inflammatory cytokines
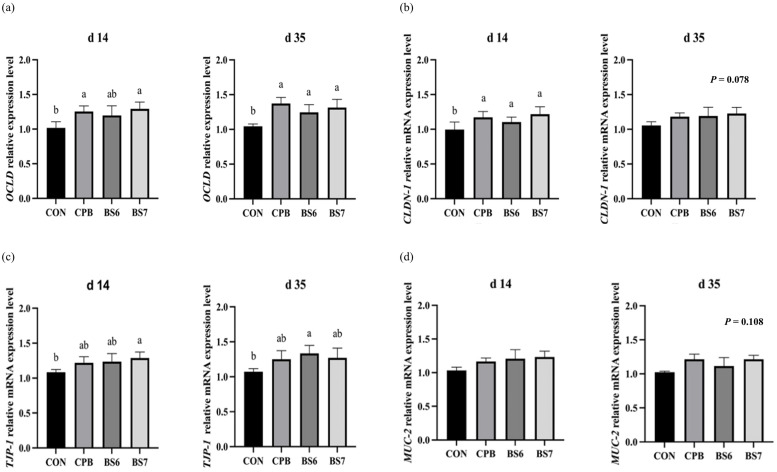
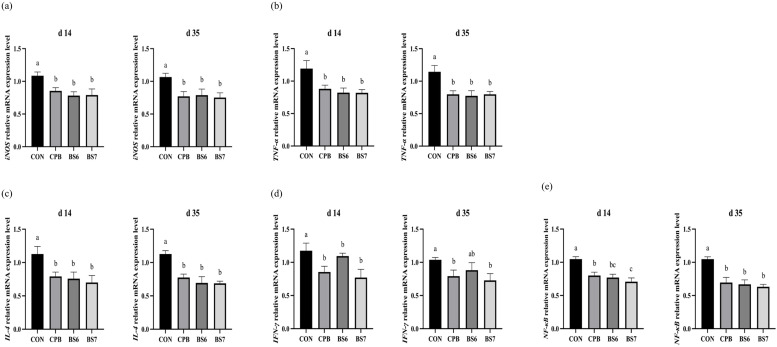
The analysis of mRNA expression levels revealed significant improvements in the expression of intestinal tight junction proteins (Fig. 2). The expression levels of occludin (OCLD) (Fig. 2a) exhibited statistically significant increases in the probiotic-supplemented groups. The increases were higher in the BS6 and BS7 groups than the CPB group on both d 14 and d 35 (P < 0.05). Claudin-1 (CLDN-1) (Fig. 2b) and tight junction protein-1 (TJP-1) (Fig. 2c) also showed statistically significant increases in expression in the treated groups on both d 14 and 35 (P < 0.05). In contrast, the expression of mucin-2 (MUC-2) (Fig. 2d) did not show statistically significant changes, although increasing trends were observed on d 35. In terms of intestinal inflammatory cytokines, the expression levels of nitric oxide synthase (iNOS) (Fig. 3a), tumor necrosis factor alpha (TNF-α) (Fig. 3b), interleukin-4 (IL-4) (Fig. 3c), interferon gamma (IFN-γ) (Fig. 3d), and nuclear factor (NF-κB) (Fig. 3e) were significantly lower in the probiotic-treated groups compared to the CON group across all measured cytokines at both d 14 and d 35. Inflammatory cytokine levels were consistently lower in the B. subtilis 14823-supplemented groups than the commercial B. subtilis probiotic-supplemented group, with all experimental groups showing varying degrees of response.
Fig. 2.
Tight junction gene expression levels of occludin (OCLD) (a), claudin-1 (CLDN-1) (b), tight junction protein-1 (TJP-1) (c), and mucin-2 (MUC-2) (d) in the jejunal segment of heat-stressed broiler chickens at days 14 and 35 of age from four dietary treatments: control basal diet (CON); basal diet with commercial probiotic Bacillus included at 1 × 10⁶ CFU/g of feed (CPB); basal diet with separated B. subtilis spores included at 1 × 10⁶ CFU/g of feed (BS6); and basal diet with separated B. subtilis spores included at 1 × 10⁷ CFU/g of feed (BS7). Error bars represent the standard error of the mean. Statistically significant differences among groups were determined using Tukey's HSD test (n = 5, P < 0.05), with different letters indicating significance.
Fig. 3.
The inflammatory cytokine gene expression levels of nitric oxide synthase (iNOS) (a), tumor necrosis factor alpha (TNF-α) (b), interleukin-4 (IL-4) (c), cytokines interferon gamma (IFN-γ) (d), and nuclear factor (NF-κB) (e) in the ileal segment of heat-stressed broiler chickens at days 14 and 35 of age from four treatments: Baseline diet (CON, control group); commercial probiotic Bacillus with 1 × 10⁶ CFU/g of feed (CPB); separated B. subtilis spores with 1 × 10⁶ CFU/g of feed (BS6); and separated B. subtilis spores with 1 × 10⁷ CFU/g of feed (BS7). Error bars represent the standard error of the mean. Statistically significant differences among groups were determined using Tukey's HSD test (n = 5, P<0.05). Different letters indicate significance.
Correlations between growth, intestinal health, blood biochemistry, and mRNA expressions of tight junctions and inflammatory cytokines
Analysis of BW, DVH, DCD and JVH, FITC-d and COR, and mRNA expression levels of tight junction proteins (OCLD, CLDN-1, TJP-1) and inflammatory cytokines (iNOS, TNF-α, IL-4, IFN-γ, NF-κB) revealed some significantly notable correlations (Table 6). BW was positively correlated with JVH and OCLD, but negatively correlated with COR, iNOS, IFN-γ, and NF-κB. DVH showed a negative correlation with DCD and positive correlations with OCLD and TJP-1. DCD was negatively correlated with OCLD, CLDN-1, TJP-1, and all cytokines. Overall, the results indicated that increased levels of tight junction proteins were associated with decreased levels of inflammatory cytokines.
Table 6.
Correlation of body weight with intestinal morphology, serum concentrations, and gene expression at day 35 of broiler age (n = 5/group).
| Parameters | DVH | DCD | JVH | FITC-d | COR | OCLD | CLDN-1 | TJP-1 | iNOS | TNF-α | IL-4 | IFN-γ | NF-κB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW | 0.26 | -0.28 | 0.63** | -0.24 | -0.52* | 0.50* | 0.19 | 0.03 | -0.57** | -0.31 | -0.33 | -0.60** | -0.50* |
| DVH | 1 | -0.64** | 0.21 | -0.12 | -0.30 | 0.54* | 0.32 | 0.58** | -0.49* | -0.57** | -0.62** | -0.65** | -0.71** |
| DCD | 1 | -0.40 | 0.15 | 0.52* | -0.58** | -0.58** | -0.49* | 0.62** | 0.72** | 0.85** | 0.44* | 0.74** | |
| JVH | 1 | -0.30 | -0.56** | 0.61** | 0.34 | 0.04 | -0.69** | -0.54* | -0.58** | -0.55* | -0.68** | ||
| FITC-d | 1 | 0.60** | -0.16 | -0.23 | -0.16 | 0.45* | 0.35 | 0.36 | 0.37 | 0.43 | |||
| COR | 1 | -0.58** | -0.57** | -0.33 | 0.78** | 0.76** | 0.71** | 0.60** | 0.72** | ||||
| OCLD | 1 | 0.35 | 0.34 | -0.75** | -0.77** | -0.69** | -0.65** | -0.74** | |||||
| CLDN-1 | 1 | 0.59** | -0.35 | -0.59** | -0.50* | -0.40 | -0.44* | ||||||
| TJP-1 | 1 | -0.39 | -0.56* | -0.51* | -0.40 | -0.48* | |||||||
| iNOS | 1 | 0.84** | 0.82** | 0.67** | 0.89** | ||||||||
| TNF-α | 1 | 0.85** | 0.63** | 0.87** | |||||||||
| IL-4 | 1 | 0.55* | 0.90** | ||||||||||
| IFN-γ | 1 | 0.65** |
BW, body weight; DVH, duodenal villus height; DCD, duodenal crypt depth, JVH, jejunal villus height; FITC-d, fluorescein isothiocyanate dextran; COR, corticosterone; OCLD, occluding; CLDN-1, claudin-1; TJP-1, tight junction proteins-1; iNOS, nitric oxide synthase; TNF-α, tumor necrosis factor alpha; IL-4, interleukin-4; IFN-γ, cytokines interferon gamma; NF-κB, nuclear factor.
*correlation is significant at the 0.05 level (2-tailed), **correlation is significant at the 0.01 level (2-tailed).
Discussion
In this study, we demonstrated that supplementation with B. subtilis 14823 significantly improved growth performance in broiler chicks raised under HS conditions, as evidenced by increased BW, BWG, and FI. HS is known to negatively affect broilers by impairing gut health, weakening the intestinal barrier, and promoting inflammation, all of which contribute to poor growth performance and higher mortality. However, the specific mechanisms by which B. subtilis 14823 enhances growth performance under these conditions remain to be fully understood.
Our results suggest that B. subtilis 14823 could serve as an effective nutritional strategy to mitigate the adverse effects of HS. Notably, the BS7 group (supplemented with 1 × 107 CFU/g) showed significant increases in BW, BWG, and FI compared to other groups throughout the experimental period. Furthermore, the BS6 group (supplemented with 1 × 106 CFU/g) demonstrated a significantly improved FCR compared to the CON group. These performance improvements align with the findings of Wang et al. (2018), who also observed enhanced BW, average daily gain, and FCR in broilers supplemented with commercial B. subtilis. Similarly, Zhang et al. (2021) reported increased BW and improved FCR in broilers fed a basal diet supplemented with B. subtilis.
The significant increase in FI observed in the BS7 group may be a result of prolonged or more frequent feeding due to reduced stress levels. Previous studies have suggested that B. subtilis supplementation increases FI (Zhang et al., 2021) and improves antioxidant capacity while optimizing the inflammatory state in broilers (Zhang et al., 2024). However, it is important to note that this study did not measure oxidative stress markers. Although previous studies have proposed that B. subtilis supplementation may reduce oxidative stress (Zhang et al., 2024), we cannot attribute the improved growth performance in our study to reduced oxidative stress without direct measurements of the relevant indices. Therefore, further studies are needed to investigate the underlying mechanisms of B. subtilis 14823 and confirm these results.
However, besides enhancing FI, B. subtilis 14823 supplementation may have other effects, such as improving nutrient absorption, supporting gut integrity, and potentially modulating immune responses under stress conditions. While our findings contribute to the growing body of evidence supporting the use of B. subtilis in broiler diets, our results indicate that the cultured B. subtilis probiotic (BS6 and BS7 treatments), as well as the commercial probiotic (CPB treatment), enhanced growth in broiler chicks under heat stress. These probiotics likely mitigate oxidative stress, protein breakdown, and inflammation caused by heat stress, thereby reducing muscle loss, BW decline, and, potentially, economic impacts in broiler farming. This is consistent with findings from Wang et al. (2018), who found that B. subtilis-based probiotics reduced heat stress behaviors and inflammation in broiler chicks. Quinteiro-Filho et al. (2010) linked heat stress to oxidative damage, protein breakdown, and weakened intestinal barriers, which exacerbated inflammation and stress, and harmed the organism. Zmrhal et al. (2023) also highlighted the significant impact on inflammation and oxidative stress in the chicken immune system.
High environmental temperatures alter the neuroendocrine system of poultry (Lara & Rostagno, 2013). HS stimulates the HPA axis, resulting in increased levels of corticotropin-releasing hormone and adrenocorticotropic hormone, leading to elevated corticosteroid levels (Abo-Al-Ela et al., 2021) that negatively impact production performance and health as levels increase (Brown et al., 2021). The impact on health causes subtle changes in the intestinal mucosa and triggers inflammatory processes in broiler chicks that impair the health of the birds (Khongthong et al., 2023). This stress affects the physiological and behavioral status of the animals, increasing their susceptibility to stress and immunosuppression, ultimately leading to heightened intestinal permeability, often referred to as leaky gut (Jiang et al., 2021). In the current study, chicks in the BS7 group exhibited significantly lower FITC-d levels compared to the control group at d 14, with a similar trend observed at d 35. Moreover, COR levels in chicks receiving all three dietary probiotic treatments were significantly reduced at d 35. These data suggest that dietary probiotic supplementation can significantly influence physiological markers such as FITC-d and COR levels in serum. Typically, in response to elevated COR levels, chicks increase their water intake and decrease feed consumption. Over the course of days 0 to 42, the BS7 group showed greater improvements in productive performance compared to the other groups, while the BS6 group significantly improved FCR compared to the CON group. This indicates that both concentrations of B. subtilis 14823 can be included in chicken diets without negative effects and may be as effective as, or perhaps even more effective than, some commercial probiotics. Furthermore, B. subtilis 14823 can reduce COR levels and mitigate stress-associated adverse effects, such as increased intestinal permeability, while also decreasing FITC-d levels. While hormonal responses to stress from the HPA axis are thought to influence FI, potentially reducing both FI and BWG (George et al., 2010), in our study, the BS7 group exhibited higher FI and BW than all other groups, suggesting that the BS7 supplementation was better able to reduce stress hormone levels in the chicks.
While both the commercial probiotic strains and B. subtilis 14823 had beneficial effects, key differences emerged in our study. The targeted strain, B. subtilis 14823, demonstrated superior results in terms of BW, FI, and FCR, especially in the BS7 group. These differences may stem from the higher concentration of spores (1 × 10⁷ CFU/g) in the BS7 group, which likely contributed to better gut health and stress reduction compared to commercial strains used in other studies (Wang et al., 2018; El-Banhawy et al., 2021).
The unique strain characteristics of B. subtilis 14823, such as the production of hydroxylated fengycins, could be responsible for the improved gut integrity, and stronger anti-inflammatory effects observed in this study. These properties may explain why the BS7 group showed greater improvements in BW and intestinal morphology than the CPB and CON groups, especially under HS conditions. However, the mechanisms governing the production of hydroxylated fengycins by this Bacillus strain still require further research for a clearer understanding and for developing the application of this strain as a feed additive in animals raised in tropical regions.
Significant correlations between various physiological markers, growth performance, and intestinal health were revealed in this study of broiler chickens under HS. For instance, a positive association was found between BW and intestinal morphology, specifically with JVH and the expression of tight junction genes like OCLD. This indicates that birds with better gut health, as indicated by taller villi and enhanced gut barrier function, tended to exhibit higher BW. Conversely, negative correlations were observed between BW and inflammatory markers such as iNOS, TNF-α, and NF-κB, suggesting that birds with higher BW were better able to mitigate inflammation. This reduction in the expression of inflammatory cytokines, particularly in the BS7 group, aligns with the ability of Bacillus strains to improve growth performance under HS conditions by reducing oxidative damage and promoting gut health. Although our study did not measure oxidative damage directly, focusing instead on an analysis of stress hormones, these hormones are nonetheless significant endpoints. Our findings suggest that the improvement in growth performance observed with B. subtilis 14823 supplementation may primarily be attributed to a mitigation of oxidative stress. This aligns with prior research that highlighted the role of probiotics in enhancing redox status and reducing oxidative damage in broiler chicks. Therefore, future studies should measure specific redox status indices, as their absence in this trial limits our ability to conclusively attribute the observed improvements to oxidative stress reduction. The relationship between COR levels and intestinal permeability (as measured by FITC-d) further underscores the role of probiotics in reducing stress-induced gut damage. The reduction in COR levels observed in the BS7 group suggests a protective effect against HS-induced physiological stress, allowing for better nutrient absorption and improved growth.
Gut health is a crucial factor in overall animal productivity. When the gut is not functioning properly, it can result in reduced nutrient absorption and energy loss in animals. Previous findings indicated that B. subtilis, as a probiotic, plays a key role not only in enhancing the growth of broiler chicks, but also in improving gut health, especially under HS, by promoting better gut morphology (Mushtaq et al., 2024), correcting an imbalance in gut bacteria (Ducatelle et al., 2023), strengthening gut integrity (Qiu et al., 2021), and supporting immune response and associated neuroinflammation (Jiang et al., 2021; Yaqoob et al., 2022). Impairment of the intestinal barrier, also known as leaky gut or intestinal wall leakage syndrome, primarily arises from bacterial infections, oxidative stress, exposure to chronic allergens, and dysbiosis. This condition is implicated in various pathological states (Di Vincenzo et al., 2024). Research in poultry production has demonstrated that compromised barrier integrity leads to increased intestinal permeability, which triggers both local and systemic inflammatory responses, predominantly due to heat-induced oxidative stress and pathogenic infections (Liu et al., 2021; Jiang et al., 2021; Qiu et al., 2021; Mohammed et al., 2021). Oxidative stress and inflammation significantly alter the structure of the gut and damage the gastrointestinal mucosa. Furthermore, HS compromises blood flow to the intestine, leading to ischemic damage and oxidative stress, ultimately resulting in a decrease in feed efficiency and growth performance. The present results align with Liu et al. (2021), who suggested that B. subtilis and similar probiotics can bolster gut health and support immune responses, particularly under HS. The enhanced integrity of the intestinal barrier further promotes nutrient absorption and growth performance. Therefore, these findings demonstrate that dietary supplementation with B. subtilis 14823 can enhance gut health and overall performance in broiler chicks under HS while reinforcing the need for further investigation into the underlying mechanisms of action and long-term effects of probiotic supplementation in commercial poultry operations.
Conclusion
For broiler chicks under heat stress conditions, the inclusion of cultured Bacillus subtilis 14823 in the basal diet at supplementations of 1 × 106 and 1 × 10⁷ CFU/g of feed improved growth performance as much as a commercial probiotic, without adverse effects. In particular, Bacillus subtilis 14823 at a concentration of 1 × 10⁷ CFU/g of feed enhanced gut barrier integrity, reduced expressions of inflammatory cytokines, and mitigated heat stress, leading to higher body weight. These findings highlight the potential of this targeted dietary supplementation to improve growth performance and intestinal health in broiler chicks under challenging environmental conditions.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
This work was supported by the Fundamental Fund (grant number 181006, 2023). The funders played no role in the design of the study, data collection and analysis, decision to publish, or manuscript preparation. The authors would like to thank the Faculty of Agriculture, Rajamangala University of Technology Srivijaya, for providing the experimental workspace in their poultry farm. We are also grateful to Miss Pornpawee Wongkul and Mr. Jakkrapong Muangsub from the Faculty of Veterinary Science, Rajamangala University of Technology Srivijaya, for their assistance with specimen collection and for extending all necessary help to carry out the animal trial.
References
- Abo-Al-Ela H.G., El-Kassas S., El-Naggar K., Abdo S.E., Jahejo A.R., Al Wakeel R.A. Stress and immunity in poultry: light management and nanotechnology as effective immune enhancers to fight stress. Cell Stress Chaperones. 2021;26:457–472. doi: 10.1007/s12192-021-01204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aengwanich W. Effects of high environmental temperature on the productive performance of Thai indigenous, Thai indigenous crossbred and broiler chickens. Int. J. Poult. Sci. 2007;6:349–353. doi: 10.3923/pjbs.2007.2736.2739. [DOI] [PubMed] [Google Scholar]
- Ahmad T., Khalid T., Mushtaq T., Mirza M.A., Nadeem A., Babar M.E., Ahmad G. Effect of potassium chloride supplementation in drinking water on broiler performance under heat stress conditions. Poult. Sci. 2008;87:1276–1280. doi: 10.3382/ps.2007-00299. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Hess M., Twarużek M., Grajewski J., Kosicki R., Böhm J., Zentek J. The impact of the fusarium mycotoxin deoxynivalenol on the health and performance of broiler chickens. Int. J. Mol. Sci. 2011;12:7996–8012. doi: 10.3390/ijms12117996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez-Brito M., Lehtinen M.J., Forssten S.D., Nurminen P. Importance of the gastrointestinal life cycle of Bacillus for probiotic functionality. J. Food Sci. Technol. 2017;54:2570–2584. doi: 10.1007/s13197-017-2688-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C.L.J., Zaytsoff S.J.M., Montina T., Inglis G.D. Corticosterone-mediated physiological stress alters liver, kidney, and breast muscle metabolomic profiles in chickens. Animals. 2021;11:3056. doi: 10.3390/ani11113056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Vincenzo F., Del Gaudio A., Petito V., Lopetuso L.R., Scaldaferri F. Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern. Emerg. Med. 2024;19:275–293. doi: 10.1007/s11739-023-03374-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducatelle R., Goossens E., Eeckhaut V., Immerseel F.V. Poultry gut health and beyond. Anim. Nutr. 2023;13:240–248. doi: 10.1016/j.aninu.2023.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Banhawy N., Basha H.A., Abo-Samaha M.I., Mandour M.A., Sharaf M.M. Effects of Bacillus subtilis supplementation in Ovo or water on hatchability and growth performances of broilers. Alex. J. Vet. Sci. 2021;70:106–113. [Google Scholar]
- George S.A., Khan S., Briggs H., Abelson J.L. CRH-stimulated cortisol release and food intake in healthy, non-obese adults. Psychoneuroendocrinology. 2010;35:607–612. doi: 10.1016/j.psyneuen.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholamreza Z., Xi H., Xi F., Dong U.A. How can heat stress affect chicken meat quality? – a review. Poult. Sci. 2019;98:1551–1556. doi: 10.3382/ps/pey399. [DOI] [PubMed] [Google Scholar]
- Jiang S., Yan F.F., Hu J.Y., Mohammed A., Cheng H.W. Bacillus subtilis-based probiotic improves skeletal health and immunity in broiler chickens exposed to heat stress. Animals (Basel) 2021;11:1494. doi: 10.3390/ani11061494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusato M., Xue G., Pastor A., Niewold T.A., Toyomizu M. Effects of plant-derived isoquinoline alkaloids on growth performance and intestinal function of broiler chickens under heat stress. Poult. Sci. 2021;100:957–963. doi: 10.1016/j.psj.2020.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khongthong S., Faroongsarng D., Roekngam N., Nopparat J., Kraitavin W., Pastor A., Steiner T., Theapparat Y. Sanguinarine-based isoquinoline alkaloids modulated the gut-brain axis and enhanced growth performance and gut integrity in natural heat stress broiler chickens. Livest. Sci. 2023;275 [Google Scholar]
- Lara L.J., Rostagno M.H. Impact of heat stress on poultry production. Animals (Basel) 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Teng P.Y., Kim W.U., Applegate T.J. Assay considerations for fluorescein isothiocyanate-dextran (FITC-d): an indicator of intestinal permeability in broiler chickens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Ren M., Ren K., Jin Y., Yan M. Heat stress impacts on broiler performance: a systematic review and meta-analysis. Poult. Sci. 2020;99:6205–6211. doi: 10.1016/j.psj.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Memon F.U., Yang Y., Zhang G., Leghari I.H., Lev F., Wang Y., Laghari F., Khushk F.A., Si H. Chicken gut microbiota responses to dietary Bacillus subtilis probiotic in the presence and absence of Eimeria infection. Microorganisms. 2022;10:1548. doi: 10.3390/microorganisms10081548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed A.A., Zaki R.S., Negm E.A., Mahmoud M.A., Cheng H.W. Effects of dietary supplementation of a probiotic (Bacillus subtilis) on bone mass and meat quality of broiler chickens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun D., Kyoung H., Kong M., Ryu S., Jang K.B., Baek J., Park K.I., Song M., Kim Y. Effects of Bacillus-based probiotics on growth performance, nutrient digestibility, and intestinal health of weaned pigs. J. Anim. Sci. Technol. 2021;63:1314–1327. doi: 10.5187/jast.2021.e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushtaq M., Ali B., Ali M., BiBi N., Raut R., Suliman G.M., Swelum A.A. Different levels of single-strain probiotic (Bacillus subtilis) with proteolytic enzyme (serratiopeptidase) can be used as an alternative to antibiotic growth promoters in broiler. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2023.103400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC. Nutrient Requirement of Poultry. 1994 [Google Scholar]
- Patra A.K., Kar I. Heat stress on microbiota composition, barrier integrity, and nutrient transport in gut, production performance, and its amelioration in farm animals. J. Anim. Sci. Technol. 2021;63:211–247. doi: 10.5187/jast.2021.e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Diaz J., Gomez-Llorente C., Fontana L., Gil A. Modulation of immunity and inflammatory gene expression in the gut, in inflammatory diseases of the gut and in the liver by probiotics. World J Gastroenterol. 2014;20:15632–15649. doi: 10.3748/wjg.v20.i42.15632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phanprasit W., Rittaprom K., Dokkem S., Meeyai A.C., Boonyayothin V., Jaakkola J.J.K., Näyhä S. Climate warming and occupational heat and hot environment standards in Thailand. Saf. Health. Work. 2021;12:119–126. doi: 10.1016/j.shaw.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piewngam P., Zheng Y., Nguyen T.H., Dickey S.W., Joo H.S., Villaruz A.E., Glose K.A., Fisher E.L., Hunt R.L., Li B., Chiou J., Pharkjaksu S., Khongthong S., Cheung G.Y.C., Kiratisin P., Otto M. Pathogen elimination by probiotic Bacillus via signalling interference. Nature. 2018;562:532–537. doi: 10.1038/s41586-018-0616-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu K., Li C.L., Wang J., Qi G.H., Gao J., Zhang H.J., Wu S.G. Effects of dietary supplementation with Bacillus subtilis, as an alternative to antibiotics, on growth performance, serum immunity, and intestinal health in broiler chickens. Front. Nutr. 2021;29 doi: 10.3389/fnut.2021.786878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M.L., Sakai M., Sa L.R., Ferreira A.J., Palermo-Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010;89:1905–1914. doi: 10.3382/ps.2010-00812. [DOI] [PubMed] [Google Scholar]
- Rhayat L., Maresca M., Nicoletti C., Perrier J., Brinch K.S., Christian S., Devillard E., Eckhardt E. Effect of Bacillus subtilis strains on intestinal barrier function and inflammatory response. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringseis R., Eder K. Heat stress in pigs and broilers: role of gut dysbiosis in the impairment of the gut-liver axis and restoration of these effects by probiotics, prebiotics and synbiotics. J Anim Sci Biotechnol. 2022;13:126. doi: 10.1186/s40104-022-00783-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodjan P., Wattanasit S., Thongprajukaew K., Faroongsarng D. Effect of dietary coated granules containing garlic oil diallyl disulphide and diallyl trisulphide on performance, in vitro digestibility and gastrointestinal functionality in laying hens. J. Anim. Physiol. Anim. Nutr. 2021;106:118–131. doi: 10.1111/jpn.13554. [DOI] [PubMed] [Google Scholar]
- Rowntree D. Charles Scribner's Sons; New York, NY, USA: 1981. Statistics Without Tears: A Primer for Non-Mathematicians. [Google Scholar]
- SAS. SAS institute., Inc Cary; Nc. USA: 2004. SAS/STAT users gulde: statistics. Version 9.2. Ed. [Google Scholar]
- Theapparat Y., Rodjan P., Boonyoung P., Reakham N., Khongthong S., Lamai J. Effect of yeast cell wall on the corticosterone and intestinal villi of crossbred native Thai chickens. 21st European Symposium on Poultry Nutrition. Salou/Vila-Secam; Tarragona, Spain: 2017. (ESPN 2017) [Google Scholar]
- Tsiouris V., Georgopoulou I., Batzios C., Pappaioannou N., Ducatelle R., Fortomaris P. Heat stress as a predisposing factor for necrotic enteritis in broiler chicks. Avian Pathol. 2018;47:616–624. doi: 10.1080/03079457.2018.1524574. [DOI] [PubMed] [Google Scholar]
- Vicuña E.A., Kuttappan V.A., Tellez G., Hernandez-Velasco X., Seeber-Galarza R., Latorre J.D., Faulkner O.B., Wolfenden A.D., Hargis B.M., Bielke L.R. Dose titration of FITC-D for optimal measurement of enteric inflammation in broiler chicks. Poult. Sci. 2015;94:1353–1359. doi: 10.3382/ps/pev111. [DOI] [PubMed] [Google Scholar]
- Wang X., Farnell Y.Z., Kiess A.S., Peebles E.D., Wamsley K.G., Zhai W. Effects of Bacillus subtilis and coccidial vaccination on cecal microbial diversity and composition of Eimeria-challenged male broilers. Poult. Sci. 2019;98:3839–3849. doi: 10.3382/ps/pez096. [DOI] [PubMed] [Google Scholar]
- Wang W.C., Yan F.F., Hu J.Y., Amen O.A., Cheng H.W. Supplementation of Bacillus subtilis-based probiotic reduces heat stress-related behaviors and inflammatory response in broiler chickens. Anim. Sci. J. 2018;96:1654–1666. doi: 10.1093/jas/sky092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N.T. Probiotics. Am. J. Health Syst. Pharm. 2010;67:449–458. doi: 10.2146/ajhp090168. [DOI] [PubMed] [Google Scholar]
- Williams N., Weir T.L. Spore-based probiotic Bacillus subtilis: Current applications in humans and future perspectives. Fermentation. 2024;10:78. doi: 10.3390/fermentation10020078. [DOI] [Google Scholar]
- Yaqoob M.U., Wang G., Wang M. An updated review on probiotics as an alternative of antibiotics in poultry — A review. Anim. Biosci. 2022;35:1109–1120. doi: 10.5713/ab.21.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Zhong G., Shao D., Wang Q., Hu Y., Wu T., Ji C., Shi S. Dietary supplementation with Bacillus subtilis promotes growth performance of broilers by altering the dominant microbial community. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhou J., Ji L., Zhang L., Zhao L., Guo Y., Wei H., Lu L. Bacillus subtilis improves antioxidant capacity and optimizes inflammatory state in broilers. Anim Biosci. 2024;37:1041–1052. doi: 10.5713/ab.23.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmrhal V., Svoradova A., Venusova E., Slama P. The influence of heat stress on chicken immune system and mitigation of negative impacts by baicalin and baicalein. Animals (Basel) 2023;13:2564. doi: 10.3390/ani13162564. [DOI] [PMC free article] [PubMed] [Google Scholar]