Abstract
Aquatic environments, including marine and freshwater ecosystems, are vital for ecological balance and biodiversity. The rising global demand for aquaculture products necessitates increased production, with intensified aquaculture practices posing significant environmental risks. This review explores the pathways through which chemical pollutants, heavy metals, pharmaceuticals, and environmental stressors induce teratogenic effects in aquatic species. The review highlights the impact of pesticide include triazine herbicides, organophosphate and organochlorine insecticides, and carbamates on aquatic life, emphasizing their interference with endocrine systems and developmental processes. Heavy metals like mercury, lead, cadmium, arsenic, and chromium are noted for their persistence and bioaccumulative properties, disrupting cellular and hormonal functions. Pharmaceuticals, including NSAIDs, antibiotics, and chemotherapeutic agents, exert teratogenic effects by disrupting physiological and developmental pathways. Environmental stressors includes temperature fluctuations, salinity variations, pH changes, and oxygen level imbalances exacerbate the teratogenic impact of pollutants. This review highlights the importance of comprehensive environmental management and understanding these complex interactions is essential for formulating efficient strategies to safeguard the effective measures to protect aquatic ecosystems and the biodiversity.
Keywords: Aquatic ecosystem, Teratogens, Endocrine disruption, Oxidative damage, Pollutants
Graphical Abstract
Highlights
-
•
Chemical pollutants and environmental stress are primary teratogens on aquatic species.
-
•
Pesticides disrupt endocrine systems and neural functions, causing teratogens.
-
•
Heavy metals leading to oxidative stress and hormonal imbalances.
-
•
Pharmaceutical wastes interfere with aquatic system, exacerbating teratogenic effects.
-
•
There is an urgency to mitigate these impacts and protect aquatic ecosystems.
1. Introduction
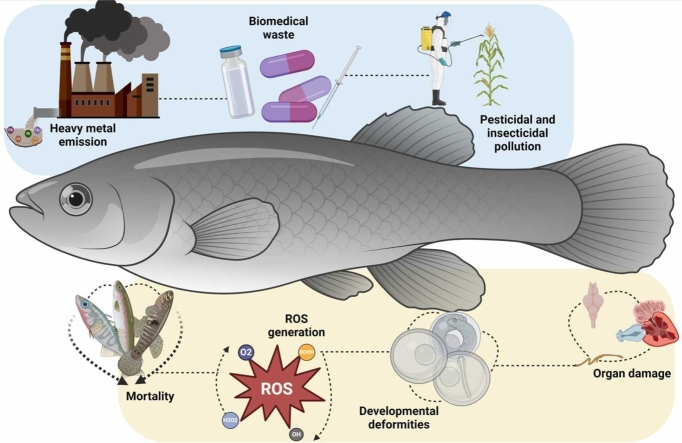
Aquatic environments, encompassing both marine and freshwater ecosystems, are integral to the planet's ecological balance. The global demand for aquaculture products is rising, necessitating an increase in production to 102 million tons by 2025 to sustain current consumption levels (FAO, 2016). [1] Aquatic species are crucial to human survival, offering nutritional resources, economic benefits, and ecosystem services. [2] They contribute to global biodiversity, regulate climate by sequestering carbon, and maintain ecological balance. The preservation and sustainable management of these species are essential for ensuring food security, economic stability, and environmental health. [3], [4] Aquaculture intensification, which involves the excessive use of proteinaceous feeds, nitrogenous fertilizers, and higher fish stocking densities, is a potential strategy to meet this demand, especially as land resources for expansion are limited. However, such intensified practices can result in increased concentrations of nitrogenous and other toxic metabolites in aquatic environments, posing significant risks to aquatic organisms. [5]
The occurrence of toxic compounds in aquatic system has profound implications for aquatic organisms, impacting their development and overall health. [6] Aquatic organisms are consistently exposed to these pollutants through direct contact with contaminated water, ingestion of polluted sediments, and bioaccumulation through the food chain. [7] Numerous studies have documented the pervasive presence of chemical pollutants in aquatic environments and their detrimental effects on aquatic life. Furthermore, previous studies mentioned the use of biological tools to support legislative objectives aimed at maintaining high aquatic environmental quality. However, bioindicators and biomarkers serve as early-warning indicators of environmental stressors, their applications are limited in certain contexts. [8], [9] Embryotoxicological and teratotoxicological studies are critical for understanding the effects of these pollutants at early developmental stages. [1], [7] Six teratogenic mechanisms associated with pollutant exposure in aquatic species include folate antagonism, neural crest cell disruption, endocrine disruption, oxidative stress, vascular disruption, and receptor- or enzyme-mediated teratogenesis. [10] Understanding these mechanisms is essential for assessing the impacts of environmental pollutants on aquatic organisms.
Heavy metal pollution in aquatic environments has garnered significant attention due to its persistence and bioaccumulative properties. These metals can disrupt the endocrine system of aquatic species, leading to developmental anomalies. [11], [12] On other side, pharmaceuticals, particularly those that are not fully metabolized and are excreted into wastewater, represent another significant source of aquatic pollution. These substances can disrupt the endocrine and reproductive systems of aquatic organisms, leading to teratogenic effects [13]. For example, exposure to synthetic estrogen used in contraceptives has been linked to the feminization of male fish and intersex conditions, severely impacting population dynamics and reproductive success. [14], [15] Furthermore, pesticides, widely used in agriculture to control pests, also contribute to aquatic pollution and teratogenicity. For instance, organophosphate exposure has been associated with craniofacial deformities and impaired swimming behavior in fish. [16]
Moreover, environmental stressors, such as temperature fluctuations, hypoxia, and salinity changes, can exacerbate the teratogenic effects of pollutants. These stressors impair the ability of aquatic organisms to detoxify and repair damage caused by contaminants, increasing their susceptibility to developmental abnormalities. [17], [18] Additionally, the combined effects of multiple stressors can lead to complex interactions that are difficult to predict and manage. Aquatic pollution represents a significant environmental challenge that adversely impacts aquatic ecosystems and poses a threat to biodiversity. The proliferation of contaminants, compounded by environmental stressors and aquaculture practices, has led to a dramatic increase in teratogenic effects among aquatic species. [18] This review aims to elucidate the pathways through which various pollutants cause teratogenicity in aquatic species, highlighting the critical need for comprehensive environmental management and pollution mitigation strategies.
2. Chemical pollutants
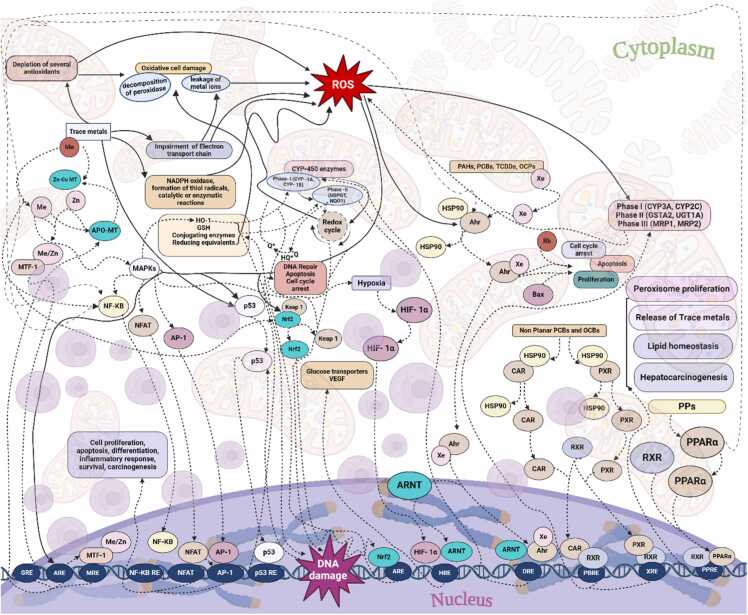
Chemical pollutants are a significant environmental concern, especially regarding their impact on aquatic ecosystems. The increasing industrialization, chemical employed agricultural activities, and urbanization have led to elevated levels of these contaminants in water bodies, posing a threat to aquatic species and the broader ecological balance. [19], [20] According to a report by the United Nations Food and Agriculture Organization (FAO), approximately 2.3 million tonnes of pesticides are used worldwide annually, with significant portions reaching aquatic ecosystems through runoff. [19] On other hands factories and industrial plants discharge various chemicals, including heavy metals and organic pollutants, into nearby water bodies. The World Bank estimates that industries release around 300–500 million tonnes of heavy metals, solvents, toxic sludge, and other waste into the world's water annually. [21] The Environmental Protection Agency (EPA) reports that urban runoff is a leading cause of water quality impairment in the developed countries. [22] Furthermore, medications consumed by humans and animals often end up in water bodies through sewage systems. A study published in the Journal Environmental Health Perspectives found that over 80 % of the rivers and streams in the United States contained traces of pharmaceuticals, including antibiotics, antidepressants, and hormones. [23] Generally, chemical exposure generate reactive oxygen species (ROS) which activate signaling pathways such as nf-κb, mapks, and ap-1, regulating genes involved in inflammation, apoptosis, and cell survival. ROS-induced oxidative stress causes DNA damage, activating p53, which can induce cell cycle arrest, apoptosis, or DNA repair. Additionally, it interacting with nuclear receptors like AhR, PPARα, and CAR disrupt cellular homeostasis by modulating genes involved in detoxification, lipid metabolism, and proliferation. The exposure also induces detoxification enzymes like GST and UGTs to mitigate toxicity. However, chronic exposure overwhelms these systems, leading to persistent oxidative stress, inflammation, and disrupted metabolic and immune responses in aquatic species (Fig. 1). [24]
Fig. 1.
Main oxidative interactions of chemicals include activation of receptors (AhR, CAR, PXR), transcription factors (Nrf2, NF-κB), and enzymes (CYP450, GST), leading to ROS production and cellular oxidative stress responses.
2.1. Pesticides
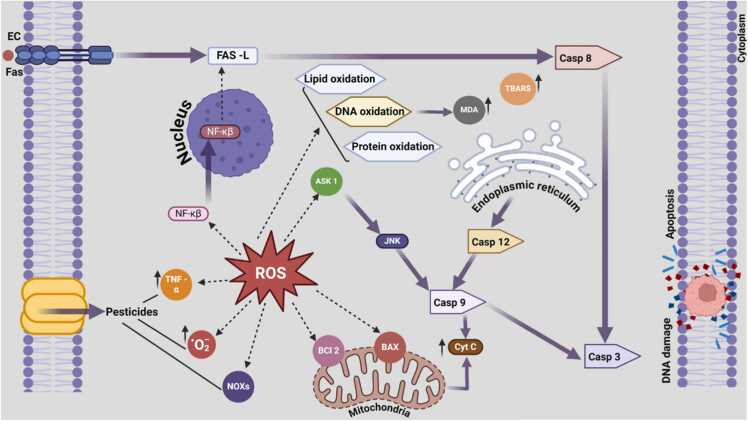
Pesticides are widely employed in agriculture to control pests and enhance crop productivity. However, their extensive use has resulted in the contamination of aquatic environments, posing significant risks to aquatic species (Table 1). Pesticide toxicity in aquatic species primarily involves the generation of ROS, leading to oxidative stress. Pesticides interact with cellular receptors, activating enzymes like NADPH oxidases (NOXs), which produce ROS. This oxidative stress damages lipids, proteins, and DNA, resulting in lipid peroxidation, protein oxidation, and DNA oxidation. ROS activate signaling pathways, including NF-κB and ASK1, promoting inflammatory responses and further ROS production. Mitochondrial dysfunction follows, releasing cytochrome c, activating caspase-9 and caspase-3, leading to apoptosis. Additionally, ER stress activates caspase-12 and JNK pathways, while the FAS pathway activates caspase-8, culminating in programmed cell death and significant impact on aquatic health (Fig. 2). [25]
Table 1.
Classes of pesticides with examples, and their potential harmful effects on aquatic species.
| Pesticide class | Examples | Damage Caused |
|---|---|---|
| Insecticide | DDT, Malathion | Neurological damage, cancer risk, reproductive harm |
| Herbicide | Glyphosate, Atrazine | Potential carcinogen, endocrine disruptor, aquatic toxicity |
| Fungicide | Mancozeb, Chlorothalonil | Dermal irritation, respiratory issues, potential carcinogen |
| Rodenticide | Warfarin, Brodifacoum | Internal bleeding, kidney and liver damage, secondary poisoning of non-target species |
| Bactericide | Streptomycin, Copper compounds | Antibiotic resistance, disruption of microbial communities |
| Nematicide | Aldicarb, Oxamyl | Reproductive and developmental toxicity, neurotoxicity |
| Acaricide | Abamectin, Fenpyroximate | Skin irritation, respiratory problems, potential carcinogen |
| Molluscicide | Metaldehyde, Ferric phosphate | Respiratory issues, toxic to aquatic life |
| Avicide | 4-Aminopyridine, DRC−1339 | Neurological damage, respiratory issues |
| Antimicrobial | Triclosan, Chlorine dioxide | Endocrine disruptor, antibiotic resistance |
| Algicide | Copper sulfate, Simazine | Skin irritation, respiratory problems |
| Defoliant | Calcium cyanamide, Thidiazuron | Dermal toxicity, respiratory issues |
| Desiccant | Calcium carbide, Magnesium sulfate | Respiratory problems, skin irritation |
| Miticide | Tetradifon, Amitraz | Skin and eye irritation, potential carcinogen |
| Virucide | Glutaraldehyde, Sodium hypochlorite | Respiratory issues, skin irritation |
| Larvicide | Temephos, Methoprene | Developmental toxicity, potential endocrine disruptor |
| Repellent | DEET, Picaridin | Skin irritation, respiratory issues |
| Biopesticide | Bacillus thuringiensis, Neem oil | Allergic reactions, potential toxicity to beneficial insects |
| Fumigant | Methyl bromide, Phosphine | Respiratory issues, potential carcinogen |
| Plant growth regulator | Gibberellins, Auxins | Hormonal imbalances, reproductive issues |
Fig. 2.
Mechanism of ROS-induced teratogenicity in aquatic species, highlighting pesticide impact, oxidative stress, and apoptosis pathways via caspase activation.
2.1.1. Triazine herbicides
Triazine herbicides, including atrazine, simazine, and cyanazine, are extensively used to manage broadleaf and grassy weeds in crops such as corn and sugarcane. These chemicals act as endocrine disruptors, interfering with hormonal regulation and increasing protein degradation rates during the development of aquatic organisms. [26] Furthermore, it disrupted the hypothalamic-pituitary-gonadal (HPG) axis, altering steroidogenesis and these herbicides and their metabolites inhibit the expression of key enzymes in the steroidogenic pathway, leading to reduced levels of testosterone and other sex hormones. [27] In zebrafish (Danio rerio), this disruption can result in various developmental abnormalities, such as edema, craniofacial abnormalities, heart defects, and impaired fin development. [28] In Gilt-head bream (Sparus aurata), triazine exposure has been linked to hepatic lesions and hepatocyte damage, characterized by lipid inclusion and extensive necrosis. [29] Amphibians are also significantly affected by triazine herbicides. In species like the Northern leopard frog (Rana pipiens) and the African clawed frog (Xenopus laevis), atrazine exposure can lead to the feminization of male frogs and the induction of intersex conditions, resulting in reduced fertility and reproductive success. Additionally, delayed metamorphosis has been observed in the Argentinian frog (Rhinella arenarum) upon exposure to these herbicides. [30], [31] Exposure to atrazine at 4 mg/L disrupts normal developmental processes in zebrafish, and even concentrations between 10 and 20 mg/L further impair organogenesis, reduce motility, and cause functional disturbances in cardiac and circulatory systems. Previous studies have also documented deformities, including edema, yolk sac malformation, spinal curvature, and body shortening in approximately 25 % of early life stages exposed to atrazine and its degradation products. [32], [33]
2.1.2. Organophosphate insecticides
Organophosphate insecticides, including chlorpyrifos, malathion, diazinon, fenitrothion, and guthion 2S, are primarily used to control a variety of insect pests in crops such as corn, soybeans, fruit trees, and ornamental plants. These chemicals inhibit acetylcholinesterase (AChE), an enzyme crucial for nerve function, by binding irreversibly to its active site. This inhibition causes an accumulation of acetylcholine in synapses, leading to continuous stimulation of postsynaptic receptors can lead to excessive neuronal firing, which can cause neurodevelopmental toxicity and various developmental disruptions. In fish like rainbow trout (Oncorhynchus mykiss) and zebrafish, AChE inhibition results in neurotoxicity, evidenced by reduced hatching success, edema, skeletal deformities, and impaired swimming behavior characterized by paused, jerky movements. [34], [35] This occurs due to disrupted cholinergic signaling and increased oxidative stress, leading to cellular damage and developmental abnormalities. Studies on amphibians, such as African clawed frog and the Northern leopard frog, organophosphate exposure causes neuromuscular defects, including abnormal tail flexure and distorted myocytes, by interfering with cholinergic transmission and inducing oxidative stress. These disruptions lead to skeletal deformities, such as notochord thinning, and increased mortality, as observed in Western narrow-mouthed toad (Gastrophryne olivacea) and Axolotl (Ambystoma mexicanum). The oxidative stress and mitochondrial dysfunction further exacerbate these developmental issues, leading to delayed metamorphosis and reproductive impairments. [36], [37]
2.1.3. Organochlorine insecticides
Organochlorine insecticides, including benzene hexachloride, methoxychlor, endosulfan, lindane, dieldrin, heptachlor, and thiacloprid, are commonly used to control a wide range of insects on crops such as tea, coffee, and vegetables. In fish species such as medaka (Oryzias latipes) and zebrafish, organochlorine insecticides interfere with the endocrine system by mimicking natural hormones, disrupting hormonal regulation. These chemicals bind to estrogen receptors, leading to altered gene expression and hormonal imbalances. This disruption can cause skeletal deformities, such as trunk curvature, reduced growth, and abnormal behaviour. [38] Additionally, in Gilt-head bream and rainbow trout, endosulfan exposure in these fish has been linked to hepatocytic alterations, myoskeletal deformities, and exophthalmia due to oxidative stress and mitochondrial dysfunction, which impair normal cellular processes and energy production. [39], [40] In amphibians, organochlorines cause developmental delays and defects through similar endocrine-disrupting mechanisms. These chemicals interfere with the HPG axis, leading to altered hormone levels and disrupted signalling pathways essential for normal development. [40] For example, exposure to these insecticides can result in poor blood circulation, body axis curvature, and cardiac dysfunction in species like sea snail (Marginella oranata). Additionally, behavioral alterations such as increased predation susceptibility are observed in species long-toed salamander (Ambystoma macrodactylum), linked to disruptions in neuromuscular activity and oxidative stress that damage neural tissues and impair normal development. [41]
2.1.4. Carbamate insecticides/ nematicides
Carbamates, including carbaryl, aldicarb, thiobencarb, thiuram, and methomyl, are widely used in agriculture to control pests. These compounds, derived from carbamic acid, are known to have detrimental effects on non-target organisms, including aquatic species. Exposure to carbamates has been shown to cause skeletal deformities such as twisted notochords, distortions, shortened anterior-posterior axes, disorganized somites, reduced hatching success, impaired growth, lack of nutritional absorption (yolk sac edema), RBC accumulation, and impaired reproductive performance in fish species such as zebrafish, medaka, and fathead minnow (Pimephales promelas). [42] These may attribute due to the induction of oxidative stress and disruption of mitochondrial function by disrupting normal cellular processes and metabolic functions. This oxidative stress leading to lipid peroxidation results from oxygen free radicals interacting with polyunsaturated fatty acids in cell membranes, leading to cellular damage, structural damage, and DNA damage. The ROS, including superoxide, hydrogen peroxide, hydroxyl radicals, singlet oxygen, peroxyl and alkoxyl radicals, lipid hydroperoxide, peroxynitrite, hypochlorous acid, and ozone. [43] These reactive intermediates interact with cellular macromolecules, causing cytotoxic effects. Additionally, N-nitrosocarbofuran, a gastric metabolite of carbofuran, may form an O⁶-methylguanine DNA adduct, exhibiting mutagenic and carcinogenic properties. Mitochondrial dysfunction impairs energy production and promotes apoptosis. [44] In amphibians, carbamate exposure can result in abnormal limb development, craniofacial deformities, increased susceptibility to predation due to impaired mobility, disrupted metamorphosis, respiratory dysfunctions, and increased mortality in amphibian larvae, especially in A. barbourin and R. sphenocephala. In Rana perezi, carbamates cause organ morphology damage, mainly in gills and liver. [45], [46] Carbamates inhibit AChE, leading to the accumulation of acetylcholine at synapses, causing neurotoxicity. This disruption in neural signaling can interfere with normal development, resulting in teratogenic effects in common carp (Cyprinus carpio) and Asian common toad (Duttaphrynus melanostictus). [47], [48]
2.1.5. Combined pesticides
Pesticides are often used in combinations to enhance their efficacy against pests and weeds. However, the combined use of different classes of pesticides, such as organosulfurs, methyl isothiocyanate, terbutryn (a triazine), and triasulfuron (a sulfonylurea), can have complex and potentially harmful effects on non-target aquatic species. Exposure to combined pesticides has been shown to cause developmental abnormalities such as spinal deformities, craniofacial malformations, impaired fin development, and larval growth in fish species like zebrafish and medaka. [44] Methyl isothiocyanate, released from organosulfurs like dazomet and metam sodium, acts by inhibiting cellular respiration by disrupting the electron transport chain in mitochondria. It also interferes with mitotic spindle formation, leading to cell cycle arrest and teratogenic effects such as spinal deformities and craniofacial malformations in fish species. [39] Additionally, in Gilt-head bream, these chemicals can cause liver damage characterized by the loss of cellular shape and the formation of loosely arranged cords in hepatocytes with lipid inclusions. [49] In amphibians, combined pesticide exposure can lead to limb malformations, disrupted metamorphosis, increased mortality, and increased susceptibility to predation due to impaired mobility. In Northern leopard frog, exposure has been linked to altered growth development, sex differentiation, and behavioral activity. [50] Terbutryn disrupts photosynthesis in plants but can act as an endocrine disruptor in aquatic species, affecting hormonal regulation necessary for normal development. Triasulfuron inhibits acetolactate synthase, an enzyme critical for branched-chain amino acid synthesis in plants, but in aquatic species, it can disrupt protein synthesis and cellular division, leading to teratogenic effects. [39], [44], [50]
2.2. Heavy metals
Heavy metals, a group of elements exhibiting metallic properties and high densities, are naturally occurring but have become concentrated in aquatic environments due to human activities such as mining, industrial processes, and agricultural runoff. [51] Notable heavy metals, including mercury, lead, cadmium, arsenic, and chromium, are present at significant concentrations in aquatic system known to cause teratogenic effects in aquatic species due to their high toxicity and ability to interfere with cellular processes (Table 2).
Table 2.
Heavy metal classes, examples, and their potential toxicological effects on aquatic species.
| Heavy Metal | Examples | Species Affected | Damage Caused |
|---|---|---|---|
| Mercury | Methylmercury | Salmo salar | Neurological damage, reproductive issues |
| Cadmium | Cadmium chloride | Mussels (Mytilus edulis) | Kidney damage, respiratory problems |
| Lead | Lead nitrate | Cyprinus carpio | Neurological damage, hematological effects |
| Arsenic | Arsenate | Shrimp (Penaeus monodon) | Carcinogenic effects, DNA damage |
| Chromium | Chromium(VI) | Oncorhynchus mykiss | Oxidative stress, respiratory issues |
| Nickel | Nickel sulfate | Oreochromis niloticus | Reproductive toxicity, growth inhibition |
| Copper | Copper sulfate | Algae (Chlorella vulgaris) | Photosynthesis inhibition, oxidative stress |
| Zinc | Zinc chloride | Danio rerio | Growth inhibition, reproductive issues |
| Selenium | Selenite | Oncorhynchus tshawytscha | Reproductive toxicity, oxidative stress |
| Aluminum | Aluminum sulfate | Salvelinus fontinalis | Respiratory issues, gill damage |
2.2.1. Mercury (Hg)
MeHg easily crosses cell membranes due to its lipophilic nature, binding to sulfhydryl groups in proteins. This disrupts protein function and induces oxidative stress by generating ROS, which leads to cellular damage and apoptosis. Furthermore, it disrupts neurotransmitter functions, particularly glutamate signaling, resulting in neurological damage. [52] This can cause spinal deformities, craniofacial abnormalities, and impaired fin development in species like zebrafish and fathead minnow. In species such as Siberian sturgeon (Acipenser baerii), rainbow trout, and goldfish (Carassius auratus), Hg exposure inhibits sperm mobility, arrests spermination, and causes testicular atrophy by interfering with hormonal regulation and cellular integrity. [53] In amphibians like the African clawed frog and American bullfrog (Lithobates catesbeianus), it can induces oxidative stress, leading to DNA damage and cellular apoptosis. This results in limb malformations, disrupted metamorphosis, and increased mortality. Additionally it interferes with calcium homeostasis and neurotransmitter release, causing developmental and behavioral abnormalities. [54] This includes impaired motor functions and increased susceptibility to predation due to compromised mobility and coordination. Further, at 18.5 μg/L Hg, Meretrix meretrix embryos experience developmental arrest at the gastrula or blastula stages, and at 187 μg/L, only morula-stage embryos are observed. Toxicant exposure initially impacts behavior, such as swimming, and physiological processes, like growth, eventually leading to increased mortality. [55]
2.2.2. Lead (Pb)
Pb mimics calcium due to its similar ionic radius, interfering with calcium-dependent processes. This can disrupt cellular signaling and enzyme functions, leading to skeletal deformities such as scoliosis and craniofacial malformations in zebrafish and rainbow trout. It can induces oxidative stress by generating ROS and reducing antioxidant defenses. This results in cellular damage, impaired growth, and neurobehavioral changes. It also leads to reduced hatching success and developmental issues like yolk-sac edema and spinal curvatures in catfish (Clarias gariepinus). In fish like Oryzias melastigma and Carassius gibelio, Pb exposure causes irregular oocytes, follicular atresia, and altered ovarian steroidogenesis. [56], [57] This disrupts gametogenesis, resulting in reproductive damage. Furthermore, Pb exposure in amphibians such as Xenopus larvae results in the accumulation of lead in the liver, causing DNA damage and genotoxic effects. This leads to limb deformities and disrupted development. Additionally it can interferes with the endocrine system, altering hormonal secretions necessary for normal development. This results in developmental delays and increased mortality rates, particularly in species like Bufo arenarum and Bufo bufo. Similar to its effects in fish, it disrupts neurotransmitter release and neuronal function in amphibians, contributing to neurobehavioral abnormalities and increased susceptibility to predation due to impaired mobility. [58], [59]
2.2.3. Cadmium (Cd)
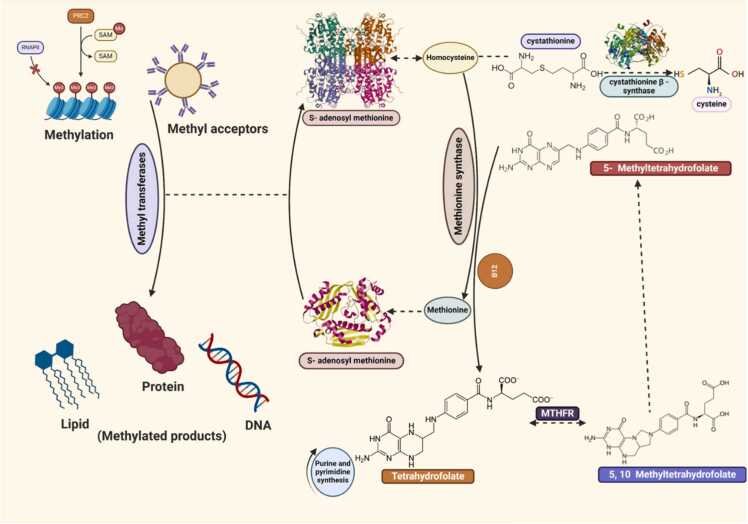
Cd competes with zinc for binding sites on zinc-dependent enzymes and proteins, leading to a disruption in their normal functions. Zinc is essential for the structural stability and activity of numerous enzymes, including those involved in DNA synthesis and repair, antioxidant defense, and metabolic processes. This competition leads to impaired cellular metabolism and developmental processes, resulting in skeletal deformities such as kyphosis, lordosis, and C-shaped larvae in zebrafish, medaka, and mosquitofish (Gambusia affinis). Disrupted enzyme activity also impairs growth and hatching success. [60], [61] It further induces oxidative stress by generating ROS and interfering with the antioxidant defense mechanisms, such as glutathione peroxidase and superoxide dismutase. Cd can also replace iron and copper in various cellular processes, further contributing to ROS production. Ferritin is a protein that stores iron and releases it in a controlled fashion. Cd can displace iron from ferritin, releasing free iron into the cytoplasm and catalyzes the formation of hydroxyl radicals (•OH) through Fenton reactions. Additionally, it can also interfere with iron-sulfur clusters in mitochondrial enzymes, such as aconitase. This displacement impairs the function of the electron transport chain leads to electron leakage and the subsequent formation of superoxide radicals (O2•−). This contributes to mitochondrial dysfunction and further oxidative stress. [62] Copper is a crucial cofactor for superoxide dismutase, an enzyme that catalyzes the dismutation of superoxide radicals into oxygen and hydrogen peroxide. Cd can displace copper from SOD, reducing its activity leads to the accumulation of superoxide radicals, increasing oxidative stress within the cell. Elevated ROS levels cause damage to lipids, proteins, and DNA repair mechanisms leads to epigenetic changes, such as DNA methylation and histone modifications, altering gene expression, result in cell death (apoptosis). Cadmium inhibits methionine synthase, leading to homocysteine accumulation and methionine depletion, reducing S-adenosyl methionine (SAM) levels, a key methyl donor. This impairment results in decreased methylation of DNA, proteins, and lipids, affecting gene expression and cellular functions (Fig. 3). [63] This oxidative damage manifests as developmental abnormalities, including cardiac edema, blastodermal lesions, and bent spines in fish species like Pagrus major. [64] Cd disrupts calcium homeostasis by competing with calcium ions (Ca²⁺) for transport and binding sites in cellular processes. It affects calcium channels, pumps, and binding proteins, leading to altered intracellular calcium levels. Disruption of calcium homeostasis affects bone development and muscle function, causing spinal deformities and impaired locomotion. In fish, this results in shortened body length, swim bladder issues, and neurobehavioral changes. [65] In amphibians, it leads to limb malformations and delayed metamorphosis. In amphibians like African clawed frog and common toad (Bufo bufo), Cd exposure results in limb malformations and increased mortality rates due to impaired DNA repair and altered gene expression. [66], [67] Cd disrupts endocrine function by interfering with hormone synthesis, secretion, and receptor function. It can mimic or block the action of hormones, disrupting hormonal signaling pathways essential for growth and development. In amphibians, hormonal imbalances cause issues such as shortened limbs, extra digits, and fused digits, affecting their survival and reproductive success. [62], [68]
Fig. 3.
Methionine cycle and methylation pathway in aquatic species, showing the roles of homocysteine, S-adenosyl methionine, and methionine synthase in DNA, protein, and lipid methylation processes.
2.2.4. Arsenic (As)
The compounds, particularly arsenite (As³⁺), disrupt cellular redox homeostasis by interfering with thiol-containing proteins and enzymes. As binds to thiol groups in glutathione (GSH) and other proteins, depleting antioxidant reserves and impairing the function of thiol-dependent enzymes. This disruption leads to an increase in oxidative stress and a decrease in the cell’s ability to neutralize ROS. This oxidative stress disrupts mitochondrial integrity, reducing membrane potential and causing uncontrolled ROS production. The mitochondria, being a primary site for arsenic-induced oxidative damage, become dysfunctional, leading to impaired ATP production and cellular energy metabolism. [69], [70] In fish species like zebrafish, rainbow trout, and medaka, this results in craniofacial malformations, impaired fin development, and growth retardation. As inhibits key enzymes in the Krebs cycle, such as succinate dehydrogenase, which impairs cellular respiration. [71], [72] This inhibition leads to decreased ATP production and increased ROS generation result in Anguilla japonica exhibit inhibited spermatogenesis, reduced egg production rates, and testicular necrosis. Moreover, it inhibits DNA repair pathways, including base excision repair and nucleotide excision repair. This inhibition is due to arsenic's ability to bind with zinc finger motifs in DNA repair proteins, impairing their function and leads to the accumulation of DNA damage, increasing the risk of mutations and genomic instability. [73] Similar to its effects in fish, arsenic exposure in amphibians leads to the production of ROS, which triggers oxidative stress and inflammation. The oxidative stress activates transcription factors like NF-κB, leading to the expression of pro-inflammatory cytokines. In amphibians such as African clawed frog and Bufo bufo, this results in limb deformities, disrupted development, and increased susceptibility to infections due to immune system impairment. [74], [75] Additionally, it interferes with endocrine functions by disrupting the synthesis, secretion, and receptor activity of hormones. This interference affects hormonal regulation critical for normal growth and development includes delayed metamorphosis, limb malformations, and increased mortality rates. [75] Further it can bio accumulates in tissues, particularly in the liver and nervous system, causing neurotoxic effects. It disrupts neurotransmitter functions and induces oxidative damage in neuronal cells. [76] In leopard frogs, this metal exposure reduces swimming ability and causes neurological impairments due to the accumulation of arsenic in neural tissues and associated oxidative damage. [77] As interferes with signal transduction pathways, including the MAPK (Mitogen-Activated Protein Kinase) pathway, PI3K/Akt pathway, and JNK (c-Jun N-terminal Kinase) pathway through altering the phosphorylation status of key signaling proteins. [78] This disruption can affects cell proliferation, apoptosis, and differentiation. In fish and amphibians, this leads to disrupted development, abnormal cell growth, and increased apoptosis, contributing to deformities and reduced survival rates. In aquatic species, the impairment of immune system results in increased susceptibility to infections and impaired immune responses. For example, in Scylla serrata (mud crab), this exposure leads to oxidative stress and immune impairment, making them more susceptible to pathogens. [68], [74]
2.2.5. Chromium (Cr)
Cr(VI) primarily exists as chromate (CrO₄²⁻) and dichromate (Cr₂O₇²⁻) anions, which mimic sulfate (SO₄²⁻) and phosphate (PO₄³⁻) ions. These anions utilize sulfate and phosphate transporters to penetrate cell membranes to overcome the cellular defenses due to its high solubility and strong oxidizing properties. Inside the cell, Cr(VI) is reduced to trivalent chromium (Cr(III)), generating ROS and leading to extensive DNA damage, including strand breaks, crosslinks, and mutations. [79] Cr(VI) can also directly bind to DNA and proteins, causing structural alterations and interfering with cellular functions. In fish species like zebrafish and medaka, this results in developmental abnormalities such as spinal curvatures, craniofacial malformations, and fin erosion. The oxidative damage also impairs growth and swimming abilities in other species like Odontesthes bonariensis and Clarias gariepinus. [80] Additionally, Cr(VI) inhibits several critical cellular enzymes, including those involved in the detoxification processes and DNA repair such as NADPH-cytochrome P450 reductase, leading to impaired detoxification of reactive intermediates. Inhibition of these enzymes results in the accumulation of toxic intermediates and extensive DNA damage, contributing to developmental defects, such as spinal curvatures and craniofacial malformations in species like zebrafish and medaka. [81] Furthermore, it leads to impaired reproductive functions through mitochondrial disruptions in fish includes testicular fibrosis, spermatic lobule shrinkage, and oocyte immaturity. [82] Additionally, Cr(VI) disrupts intracellular calcium signaling by interacting with calcium channels and binding proteins. This interference affects calcium-dependent processes, including muscle contraction and neurotransmitter release. In amphibians like Hypsiboas pulchellus and Rana tigrina, this disruption leads to limb deformities, abnormal development, and increased mortality [83], [84]. The altered calcium homeostasis also affects swimming abilities and overall motor functions in these species. Epigenetic alterations through DNA methylation and endocrine disruption through hormonal imbalance due to Cr exposure contribute to developmental abnormalities and impaired immune responses in both fish and amphibians. This includes delayed hatching, reduced body size, skeletal malformations, and increased susceptibility to infections observed in species like Hypsiboas pulchellus. [85] In fish species like Odontesthes bonariensis and Clarias gariepinus, endocrine disruption results in impaired spawning capabilities, reduced egg production, and sperm immobility. [81]
2.3. Pharmaceuticals
Pharmaceuticals encompass a wide range of medicinal compounds classified by their therapeutic purposes, such as antibiotics, analgesics, and antidepressants, etc. The increased consumption of these pharmaceuticals inevitably leads to higher levels of their discharge into the environment [13]. Over the past two decades, numerous studies have reported the presence of trace levels of various prescription and over-the-counter pharmaceuticals in aquatic ecosystems. Unlike most other environmental contaminants, pharmaceuticals are specifically designed to alter physiological functions. Consequently, they have a high potential to affect non-target organisms because the mechanisms through which many pharmaceuticals exert their effects are conserved across different animal phyla (Table 3). [86]
Table 3.
Pharmaceutical classes and their potential toxicological effects on aquatic species.
| Parmaceutical | Class | Species Affected | Damage Caused |
|---|---|---|---|
| Ibuprofen | NSAID | Pimephales promelas | Reproductive issues, altered hormone levels |
| Diclofenac | NSAID | Oncorhynchus mykiss | Kidney damage, gill damage |
| Carbamazepine | Antiepileptic | Danio rerio | Neurological effects, behavioral changes |
| Fluoxetine | Antidepressant | Oncorhynchus mykiss | Behavioral changes, reproductive issues |
| Sulfamethoxazole | Antibiotic | Algae (Pseudokirchneriella subcapitata) | Growth inhibition |
| Trimethoprim | Antibiotic | Pimephales promelas | Reproductive issues, growth inhibition |
| Metoprolol | Beta-blocker | Oncorhynchus mykiss | Cardiotoxicity, altered behavior |
| Propranolol | Beta-blocker | Pimephales promelas | Cardiotoxicity, reproductive issues |
| Atenolol | Beta-blocker | Danio rerio | Cardiotoxicity, growth inhibition |
| Estradiol | Hormone | Pimephales promelas | Endocrine disruption, reproductive issues |
| Ethinylestradiol | Hormone | Oncorhynchus mykiss | Endocrine disruption, reproductive impairements |
| Venlafaxine | Antidepressant | Pimephales promelas | Behavioral changes, reproductive issues |
| Ciprofloxacin | Antibiotic | Oncorhynchus mykiss | Growth inhibition, reproductive damage |
| Ofloxacin | Antibiotic | Algae (Chlorella vulgaris) | Growth inhibition, photosynthesis inhibition |
| Paracetamol | Analgesic | Pimephales promelas | Liver toxicity, kidney damage |
| Amoxicillin | Antibiotic | Danio rerio | Growth restriction, reproductive issues |
| Erythromycin | Antibiotic | Oncorhynchus mykiss | Growth inhibition, reproductive impairment |
| Clarithromycin | Antibiotic | Algae (Pseudokirchneriella subcapitata) | Growth inhibition, photosynthesis inhibition |
| Azithromycin | Antibiotic | Pimephales promelas | Growth retardation, reproductive issues |
| Doxycycline | Antibiotic | Oncorhynchus mykiss | Growth inhibition, reproductive issues |
| Sertraline | Antidepressant | Danio rerio | Behavioral changes, reproductive issues |
| Atenolol | Beta-blocker | Oncorhynchus mykiss | Cardiotoxicity, reproductive issues |
| Clonazepam | Antiepileptic | Danio rerio | Neurological effects, behavioral changes |
| Lamotrigine | Antiepileptic | Pimephales promelas | Neurological effects, behavioral changes |
| Amitriptyline | Antidepressant | Oncorhynchus mykiss | Behavioral changes, reproductive issues |
2.3.1. Analgesics and antipyretic drugs
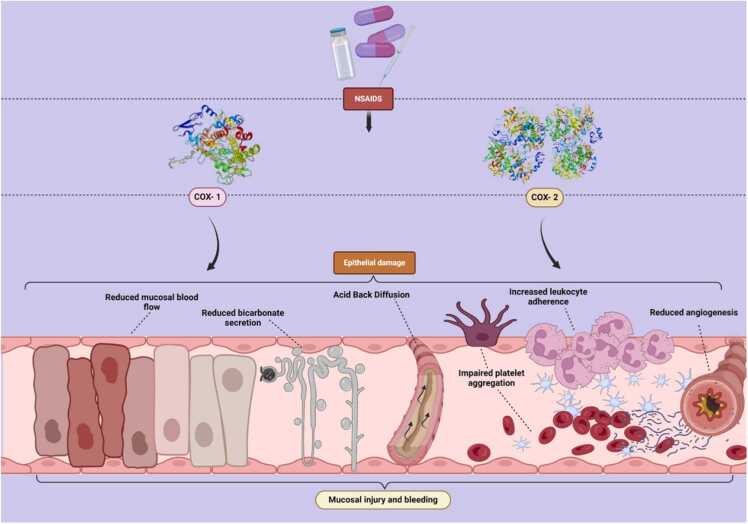
Nonsteroidal anti-inflammatory drugs (NSAIDs), including ibuprofen, diclofenac, naproxen, and aspirin, are extensively used for their analgesic, antipyretic, and anti-inflammatory properties. NSAIDs inhibit cyclooxygenase (COX) enzymes, which are crucial for the synthesis of prostaglandins. Prostaglandins play vital roles in regulating inflammation, pain, and fever, and in aquatic species, they are involved in critical physiological processes such as reproduction, development, and immune responses. COX-1 inhibition reduces mucosal blood flow and bicarbonate secretion, leading to decreased protective mucus production and direct epithelial damage. COX-2 inhibition increases leukocyte adherence, reduces angiogenesis, and impairs platelet aggregation, exacerbating epithelial damage through inflammation and impaired tissue repair. These combined effects cause significant mucosal injury and bleeding (Fig. 4). [87] In zebrafish, this inhibition leads to developmental abnormalities, including skeletal deformities, impaired fin development, and altered hatching rates. [10], [88] Exposure to diclofenac and ibuprofen at concentrations ranging from 0.5 to 1000 µg/L in rainbow trout, brown trout, and medaka results in gill damage, impaired ion regulation, cardiovascular abnormalities, and changes in reproductive patterns. NSAID exposure induces oxidative stress in species like zebrafish and common carp, leads to cytological alterations in primary organs, disruption of oocyte maturation and ovulation, and inhibition of cytochrome P450 enzymes (CYP2M), which are essential for detoxification and metabolism. Additionally it can interfere with endocrine functions by disrupting hormone synthesis and secretion, leading to impaired reproductive performance, such as testicular fibrosis, spermatic lobule shrinkage, and oocyte immaturity. Furthermore, the combined exposure paracetamol and ciprofloxacin, can severely induce teratogenic effects in zebrafish embryos, causing morphological alterations and reducing survival rates by up to 75 %. Realistic levels of paracetamol may activate the antioxidant defense system in freshwater crustaceans, leading to behavioral changes and hepatotoxicity in male fish Rhamdia quelen. [89] This disruption in developmental processes results in malformed embryos and reduced viability. NSAIDs can interfere with ion transport and regulation, particularly affecting sodium (Na+), potassium (K+), and calcium (Ca2+) channels. In rainbow trout and medaka, this leads to impairs gill function, difficulties in maintaining ion balance. This can cause osmoregulatory stress, reduced swimming performance, and overall poor health. Additionally, NSAIDs can cross the blood-brain barrier and interfere with neurotransmitter systems, particularly those involving serotonin and dopamine. [90], [91] This can lead to neurotoxic effects and behavioral changes in fish and common carp, such as changes in feeding, aggression, anxiety and social interactions.
Fig. 4.
Mechanism of NSAIDs-induced epithelial damage in aquatic species, illustrating the inhibition of COX-1 and COX-2, leading to mucosal injury, impaired blood flow, and increased leukocyte adhesion.
2.3.2. Antibiotics
The widespread use of antibiotics in both human and veterinary medicine has led to their presence in aquatic environments. Antibiotics can have teratogenic effects on fish embryos by disrupting normal bacterial flora and directly affecting cellular processes. Furthermore, the widespread use of antibiotics in aquaculture promotes antibiotic resistance in aquatic bacteria, contributing to environmental reservoirs of resistance genes that may transfer to human pathogens. Antibiotics such as penicillins, cephalosporins, macrolides, quinolones, tetracyclines, and aminoglycosides have been detected in aquatic environments. [92] Antibiotics like quinolones, aminoglycosides, and tetracyclines interfere with DNA replication and transcription by targeting bacterial DNA gyrase and topoisomerase IV. These antibodies generally bind to bacterial ribosomes, inhibiting protein synthesis for treatment therapy. This mechanism can extend to similar ribosomal structures in aquatic organisms, disrupting protein production. These disruptions can cause DNA strand breaks and mutations, leading to developmental abnormalities and impaired growth. Studies have shown that oxytetracycline, florfenicol, and sulfadimethoxine exposure in tilapia (Oreochromis niloticus) for 21 days at human therapeutic doses and maximum residual limit leads to DNA damage, contributing to liver impairment and skeletal deformities. [93], [94] Additionally, oxytetracycline and sulphonamides caused immune system impairments in medaka and rainbow trout through the disruption of gut microbial community potentially induce antibiotic-resistant bacteria. [92], [95] Similarly, oxytetracycline exposure in fish elevates hepatic enzymes alanine aminotransferase and aspartate aminotransferase, indicating hepatotoxic effects with simultaneous raising of cortisol and blood glucose levels, signaling a stress response. [92] Clarithromycin exposure in spring Chinook salmon (Oncorhynchus tshawytscha) improved survival rates and egg production in adults but led to mandible and fin teratogenesis in offspring because of ion homeostasis and hormonal alterations. [92] Doxycycline exposure in marine microalga (Tetraselmis suecica) for 96 hours resulted in growth inhibition and physiological and biochemical alterations due to the generation of ROS, which damages the cellular components. [96] Erythromycin exhibits genotoxic effects in fish, notably rainbow trout, at concentrations as low as 0.8 μg/L, leading to nuclear abnormalities in erythrocytes and oxidative stress, marked by lipid peroxidation. Chronic exposure affects gill health and reduces survival at high doses (1000 mg/L), though such levels are rare in natural habitats. Erythromycin also inhibits liver cytochrome P450 enzymes activity in fish, impacting drug metabolism and inducing enzymes like 7-ethoxyresorufin O-deethylase and anti-oxidant enzyme in carp tissue. [97]
2.3.3. Drugs for cardiovascular, endocrine, CNS, respiratory, and gastrointestinal conditions
Exposure to propranolol and metoprolol at 1–100 µg/L for 96 hpf in zebrafish larvae resulted in pericardial edema, altered heart structure, and reduced cardiac output, indicating significant oxidative damage to nucleic acids leading to DNA damage such as strand breaks, crosslinks, and mutations. Atenolol and gemfibrozil exposure led to cardiac malformations at 10 µg/L for 7 days and 50 µg/L for 14 days, respectively, causing reduced heart rates, impaired cardiac function, and skeletal deformities in zebrafish embryos. [86], [98]
Triclosan exposure (10 µg/L) led to developmental abnormalities in zebrafish, including pericardial edema and disrupted thyroid hormone signaling, affecting both heart and skeletal development through mimic or blocking action of thyroid hormone signalling. Furthermore, this exposure causes alterations in behavior and biochemical constituents in tilapia, even at a minimum concentration of 0.715 mg/L. [99] Additionally it induces changes in Phase I and Phase II metabolism enzymes and genes in swordtail fish, suggesting potential ecotoxicological risks to aquatic ecosystems. [100]
Fluoxetine exposure at 1 µg/L caused neurodevelopmental defects through increases serotonin levels, which can disrupt normal neuronal signalling, altered behavior, and skeletal malformations in zebrafish embryos. Fluoxetine exposure at environmentally relevant concentrations (40 ng/L) causes pericardial edema, hatching retardation, spine alterations, and craniofacial malformations in zebrafish. [101] Furthermore, short-term and long-term fluoxetine exposure in zebrafish and gold fish causes accelerated heart rates, postponed hatching times, and increased swimming speeds. [102] Theophylline exposure (100 µg/L) resulted in limb malformations and disrupted respiratory development in African clawed frog embryos through disrupting normal cellular metabolism by inhibiting phosphodiesterase enzymes, leading to an increase in cyclic AMP levels. Theophylline metabolites evaluated using the Frog Embryo Teratogenesis Assay - Xenopus suggest P-450 (CYP450) detoxification pathways may be responsible for its teratogenicity. [103] Carbamazepine exposure at environmental concentrations (0.01 µg/L) caused teratogenicity in sea urchins, resulting in developmental retardations through affects ion channels, particularly Na channels, disrupting neural and muscular function. [104] Carbamazepine exposure under multiple stress conditions resulted in a 10- to 25-fold higher toxicity in aquatic key organisms, especially New Zealand mud snail Potamopyrgus antipodarum. [105]
Ranitidine exposure (10 µg/L) led to developmental delays, reduced body length, and gastrointestinal tract malformations in zebrafish embryos [106] because it a histamine H2 receptor antagonist, disrupts gastrointestinal and hormonal functions by interfering with histamine signaling. Acute adverse effects of ranitidine are not expected in aquatic organisms like algae (Raphidocelis subcapitata), macrophyte (Lemna minor), cnidarian (Hydra attenuata), crustacean (Daphnia similis), and zebrafish at concentrations found in fresh surface waters. However, chronic exposure to ranitidine (100 mg/L) and its photoderivatives caused inhibition of growth population in rotifers and crustaceans, with genotoxic and mutagenic effects observed due to metabolic imbalances and oxidative stress. [107]
2.3.4. Chemotherapeutic agents
17β-estradiol, an estrogenic compound, binds to estrogen receptors in aquatic organisms, leading to the activation of estrogen response elements in the DNA, resulted in significant alterations in immune parameters, indicating immunomodulation in both fingerlings and juveniles, with teratogenic effects including disrupted immune function and potential developmental issues. [108] Cyclophosphamide and cisplatin cause alkylate DNA, forming crosslinks that inhibit DNA replication and transcription and causing changes in reproductive function, oxidative stress, genotoxicity, cytotoxicity, and neurotoxicity in aquatic species. Previous research evaluated the acute toxicity of six commonly used anti-cancer drugs and their metabolites (5-fluorouracil, ifosfamide, cyclophosphamide, imatinib, tamoxifen, methotrexate, and its metabolite 7-hydroxymethotrexate) on aquatic organisms such as Raphidocelis subcapitata, Vibrio fischeri, Lemna minor, and Daphnia magna. This study revealed Lemna minor as the most sensitive species, with 5-fluorouracil being highly toxic to algae (EC50 = 0.075 mg/L) because this drug inhibits thymidylate synthase, blocking DNA synthesis and repair. Methotrexate and tamoxifen very toxic to duckweed (EC50 = 0.08–0.23 mg/L) through inhibits dihydrofolate reductase, preventing nucleotide synthesis. [109] Exposure of 5-fluorouracil, capecitabine, cisplatin, etoposide, and imatinib to embryos of the African clawed frog at 0.01–50 mg/L increased developmental malformations, such as abdominal edema, axial flexure, and malformations of the head, eyes, gut, and heart, with significant effects at the highest concentrations tested (50 mg/L for 5-fluorouracil; 30 mg/L for etoposide; 20 mg/L for capecitabine and imatinib). [110]
2.3.5. Steroidal estrogens and progestogens
Exposure to steroidal estrogens and progestogens in aquatic environments disrupts the endocrine systems of fish, leading to reproductive and developmental issues. Levonorgestrel exposure in zebrafish reduces egg production and alters endocrine functions by lowering estradiol and testosterone levels in females, and 11-ketotestosterone in males. [111] Levonorgestrel exposure at environmentally relevant concentrations (0, 1, 10, 33, and 100 ng/L) for 63 days can cause androgenic activity and masculinization during zebrafish gonadal differentiation, potentially affecting fish populations. [112] Estrone exposure in fathead minnow at 15 ng/L for 3 weeks led to disrupted endocrine functions, altered reproductive behaviors with increased vitellogenin concentrations, decreased reproductive success, and significant impacts on gonad development. [113]
In Japanese medaka, chronic exposure (60 days) to ethinylestradiol caused feminization of male fish, reduced fertility, and skewed sex ratios, demonstrating strong endocrine-disrupting and teratogenic properties. [114] Exposure to sublethal concentrations (5 ng/L) of 17β-ethinylestradiol resulted in severe intersex conditions, altered gonadal development, and liver damage in least killifish (Heterandria formosa). [115] Paternal exposure to environmental 17α-ethinylestradiol (2.5, 5, and 10 ng/L) during spermatogenesis can cause skeletal distortions, lymphedema, and impaired estrogen receptor expression in zebrafish. [116] Progesterone exposure in fathead minnow for 21 days at 50–500 ng/L resulted in reduced egg production and disrupted endocrine systems, including decreased estradiol and testosterone levels in females, and reduced 11-ketotestosterone in males. [117], [118] Progestogen exposure in aquatic species like great pond snails (Lymnaea stagnalis) led to significant alterations in embryonic development time, heart rate, feeding, and gliding activities. [119]
2.3.6. Azoles (Aromatase inhibitors)
Azoles, which inhibit aromatase enzymes, are used to treat fungal infections and in certain cancer therapies. These compounds can cause significant disruptions in the reproductive systems of aquatic animals by inhibiting estrogen synthesis. Research has shown that exposure to azoles like dutasteride can negatively impact fish reproduction. [120]
2.3.7. Fibrates (Peroxisome proliferators)
Fibrates, used to manage cholesterol levels, have also been detected in aquatic environments. Their presence can lead to various developmental issues in fish embryos, although specific studies focusing on their teratogenic effects are limited. Fibrates' mode of action involves activating peroxisome proliferator-activated receptors, which play a role in lipid metabolism and can affect energy balance and growth in aquatic organisms. [121]
3. Aquaculture practices
Aquaculture, the cultivation of aquatic organisms, has become a critical sector for meeting global seafood demands. However, the intensive use of chemicals, feed additives, fertilizers, pesticides, disinfectants, and antibiotics in aquaculture practices poses significant risks to aquatic species.
3.1. Water and sediment treatment compounds
3.1.1. Liming compounds
Liming compounds such as calcium carbonate (agricultural lime), calcium hydroxide (hydrated lime), and calcium oxide (quicklime) are commonly applied during pond preparation to neutralize acidity and improve water quality. While their primary purpose is to balance pH levels, they are sometimes used to eliminate potential pests and predators. These compounds temporarily alter water quality parameters such as alkalinity, hardness, and pH, which can affect the development of aquatic organisms. [122]
3.1.2. EDTA and aluminium potassium sulfate
EDTA is used to reduce the bioavailability of heavy metals by chelating metal ions, thus preventing their uptake by aquatic organisms. This can protect organisms from metal toxicity but may also introduce temporary changes in water chemistry that affect embryonic development in fish and amphibians. [123] Aluminium potassium sulfate (alum) is applied to reduce turbidity, however, alum can introduce aluminum ions into the water, which can be toxic to aquatic life at high concentrations through binding to gill surfaces, disrupting ion transport and respiration, leading to physiological stress and potentially lethal. [124]
3.1.3. Zeolites
Zeolites are used to remove ammonia and other nitrogenous compounds from the water column. However, their efficacy in brackish water systems has been questioned, and improper use can lead to ammonia spikes that are harmful to aquatic larvae. [124]
3.2. Fertilizers
3.2.1. Organic fertilizers
Organic fertilizers, including cow, pig, and chicken manure, are employed to induce algal blooms and increase natural productivity in ponds. These fertilizers can raise concerns regarding food safety and environmental health due to the risk of bacterial proliferation and the introduction of antibiotic residues from livestock manure. The presence of antibiotics can lead to the development of antimicrobial-resistant bacterial strains, which pose significant risks to both aquatic species and human health. [125]
3.2.2. Inorganic fertilizers
Intensive and semi-intensive aquaculture systems often use inorganic nitrogenous and phosphate fertilizers during the early production stages. While these fertilizers enhance algal growth and natural food availability for young fish and shrimp, they also contribute to high organic matter discharges and eutrophication in receiving waters, leading to hypoxic conditions and developmental issues in aquatic species. [106]
3.2.3. Fungicides and insecticides
Twenty-nine different pesticides, including malachite green, copper sulfate, methylene blue, and trifluralin, are used in aquaculture to treat fungal and parasitic infections, kill unwanted organisms, and manage pests. Malachite green, a powerful bactericide, has been banned in many countries due to its carcinogenic properties [126]. Copper sulfate, although effective against planktonic organisms, poses risks of bioaccumulation and toxicity in cultured species and non-target organisms. [127] Insecticides such as endosulfan, trichlorfon, and dichlorvos are used to control ectoparasitic infections and crustaceans, with studies showing high toxicity to zooplankton and other non-target species. [128] Piscicides like rotenone and saponin compounds are used to kill fish in shrimp culture ponds before stocking. These substances cause significant declines in zooplankton populations, impacting the broader aquatic food web. [129]
3.2.4. Disinfectants
A wide range of disinfectants, including formaldehyde, potassium permanganate, chlorine, and iodine, are used in aquaculture to maintain hygiene, disinfect facilities, and treat disease outbreaks. These substances are moderately to highly toxic to planktonic and macroinvertebrate species. For instance, formaldehyde and chlorine-based disinfectants can cause significant harm to phytoplankton and crustaceans at treatment concentrations. Chlorine disinfectants also produce harmful organic chlorine compounds that are persistent environmental contaminants. [130], [131]
4. Environmental stress
4.1. Temperature
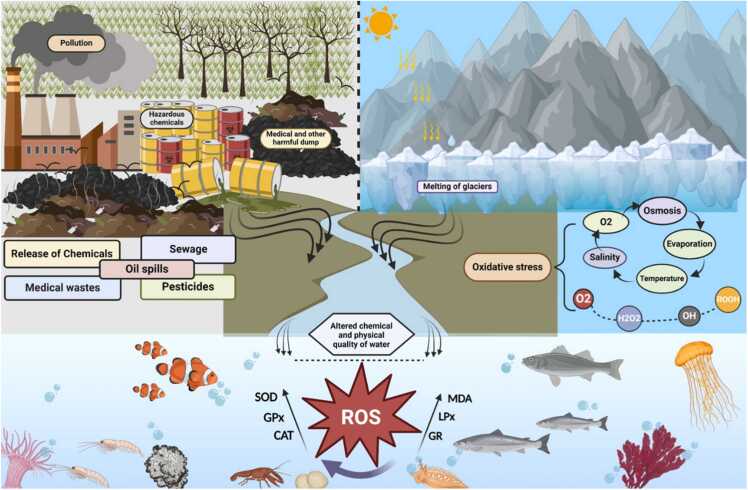
Fluctuating temperatures can cause proteins to denature and aggregate, disrupting critical developmental processes and leading to abnormalities like spinal deformities and craniofacial anomalies. While heat shock proteins (HSPs) typically refold denatured proteins, excessive damage overwhelms these mechanisms, causing cellular dysfunction. Temperature stress also increases ROS production, leading to oxidative stress and teratogenic effects (Fig. 5). [132] It alters the expression of crucial genes like Hox, resulting in developmental anomalies, and disrupts the retinoic acid signaling pathway, affecting limb, craniofacial, and organ development. [133], [134] Furthermore, temperature changes induce epigenetic modifications, impacting gene expression and development. [135] Stress-induced hormonal imbalances, involving cortisol and thyroid hormones, impair growth, neural development, and metabolic functions.
Fig. 5.
Pollutants and environmental stressors in aquatic ecosystems lead to oxidative stress, altering water quality and impacting aquatic species. Sources include industrial discharge, medical waste, pesticides, and melting glaciers, resulting in reactive oxygen species (ROS) formation and biological damage.
4.2. pH-Induced teratogenic effects
Acidosis (low pH) and alkalosis (high pH) disrupt the acid-base balance within cells and tissues, impairing enzyme activity, ion transport, and metabolic processes. This imbalance interferes with critical developmental pathways, leading to teratogenic effects. [136] Similar to temperature stress, pH fluctuations enhance ROS production, resulting in oxidative stress and subsequent damage to DNA, proteins, and lipids, which are associated with developmental defects. [132] pH changes significantly impact calcium ion availability and signaling, which are crucial for various developmental processes. Disruption of calcium signaling leads to abnormalities in skeletal formation and muscle development. Additionally, pH stress alters the expression of Hox genes, causing misexpression and resulting in segmental duplications, deletions, or transformations, similar to the effects seen with temperature fluctuations. [133] Changes in pH induce epigenetic modifications, including alterations in DNA methylation patterns, leading to the long-term impacts on gene expression and development. [135]
4.3. Salinity-induced teratogenic effects
Salinity-induced teratogenicity in aquatic species manifests through various developmental anomalies, influenced by the species' osmoregulatory abilities and genetic plasticity. For instance, the snail Radix balthica exhibits changes in developmental timing and hatchling morphology due to salinity fluctuations. [137] Similarly, salinity exposure impacts larval development in estuarine crabs, with different populations showing varying tolerance levels, likely due to environmental adaptation. The copepod Eurytemora affinis demonstrates genetic variation in salinity tolerance, influencing development time under different salinities. High salinity conditions also affect the compensatory growth potential in Litoria ewingii tadpoles. [138] In sturgeons, salinity levels are crucial for egg development and hatching success, with an optimal range identified for early stages. [139] The crown-of-thorns seastar shows delayed hatching and reduced fertilization success under low salinity conditions, threatening their populations. [136] Additionally, the freshwater snail Chilina dombeiana experiences decreased survival and physiological stress when exposed to brackish water. [140]
4.4. Oxygen level induced teratogenicity
Oxygen levels in the aquaculture environment are critical for the healthy development of aquatic organisms. Both hypoxia (low oxygen levels) and hyperoxia (high oxygen levels) can act as teratogenic agents, leading to developmental abnormalities.
4.4.1. Hypoxia-induced teratogenic effects
Hypoxia, disrupts cellular homeostasis by limiting oxygen needed for aerobic respiration, forcing cells to rely on anaerobic metabolism. This leads to lactate accumulation and decreased cellular pH, impairing cellular functions and developmental processes. Cells upregulate hypoxia-inducible factors (HIFs) to adapt, but prolonged hypoxia overwhelms these mechanisms, causing cellular dysfunction and developmental abnormalities such as impaired organogenesis and abnormal growth. Reoxygenation after hypoxia generates reactive oxygen species (ROS), causing oxidative stress and damaging DNA, proteins, and lipids, resulting in teratogenic effects like spinal deformities and craniofacial abnormalities. [141] Hypoxia also stabilizes and activates HIFs, regulating genes involved in angiogenesis, erythropoiesis, and metabolism. Chronic hypoxia dysregulates HIF signaling, disrupting blood vessel formation and organ development. Hypoxia can also induce changes in DNA methylation patterns, leading to altered gene expression. These epigenetic modifications can have lasting effects on development and phenotype. Research has shown that hypoxia during critical windows of development can lead to hypo- or hypermethylation of genes involved in developmental processes, resulting in teratogenic effects. Additionally, hypoxia can induce stress responses that elevate corticosteroid levels. Chronic stress can impair development and lead to teratogenic effects. Elevated cortisol levels have been linked to developmental abnormalities such as growth retardation, skeletal deformities, and impaired neural development. [142]
4.4.2. Hyperoxia-induced teratogenic effects
Hyperoxia, causes excessive ROS production, leading to oxidative stress and damage to DNA, proteins, and lipids, resulting in teratogenic effects. This oxidative stress is linked to developmental defects such as abnormal organ development and impaired growth. Hyperoxia also influences the Wnt signaling pathway, crucial for cell proliferation and differentiation, causing developmental abnormalities like defects in limb formation, craniofacial development, and organogenesis. [143] Additionally, hyperoxia affects histone acetylation and methylation, disrupting chromatin structure and gene expression, leading to the misregulation of genes essential for morphogenesis and differentiation. [144] These epigenetic changes interfere with normal development, contributing to the teratogenic effects of hyperoxia.
5. Global economic impact and environmental impact and management strategies
Teratogenicity in aquatic species, caused by various chemical pollutants and environmental stressors, poses significant threats to global biodiversity and ecological balance. The disruption of developmental processes in aquatic organisms can lead to severe ecological consequences, including reduced population sizes and biodiversity loss, which directly impact the productivity and sustainability of aquaculture industries. This decline in aquatic health affects global economic stability, as the aquaculture sector is crucial for food security and economic livelihoods worldwide. Moreover, the bioaccumulation of harmful substances in aquatic species can ascend the food chain, potentially impacting higher organisms, including humans. To mitigate the teratogenic effects of pollutants on aquatic species, comprehensive environmental management strategies are essential. These include enforcing stringent regulations and emission standards, adopting best management practices in industries and agriculture, and promoting sustainable aquaculture practices like Integrated Multi-Trophic Aquaculture (IMTA). The governance of aquatic pollution through frameworks like EU REACH, the U.S. Clean Water Act, Central and State Pollution Control Board reflects a commitment to protecting water resources from various pollution sources. Each entity employs specific regulations tailored to its jurisdiction's needs and promoting sustainable practices to safeguard aquatic ecosystems. Restoration efforts such as bioremediation and habitat restoration are crucial, along with public awareness and educational programs to foster community involvement. Ongoing research on pollutant toxicity and innovative pollution control technologies is vital. International cooperation and adherence to global treaties enhance the effectiveness of these strategies, ensuring the protection of aquatic ecosystems and public health.
6. Limitations, future perspective, and concluding remarks
This review highlights the significant teratogenic effects of chemical pollutants and environmental stressors on aquatic species, emphasizing the need for comprehensive environmental management strategies. However, future research should address several limitations such as conducting long-term studies to understand chronic impacts on development and reproduction, investigating the combined effects of multiple stressors to reveal synergistic or antagonistic interactions; examining species-specific responses to develop generalized models for predicting pollutant impacts on biodiversity; gaining a deeper mechanistic understanding of pollutant disruption at molecular and cellular levels using advanced techniques; exploring ecological and population-level consequences to understand effects on population dynamics, community structure, and ecosystem functioning; and translating scientific findings into effective policies and regulations to mitigate pollutant impacts.
Aquaculture practices, crucial for satisfying global seafood demands, introduce various chemicals and pollutants into aquatic environments, leading to teratogenic effects in aquatic organisms. This review comprehensively examines the teratogenic effects of chemical pollutants and environmental stressors on aquatic species, highlighting the critical need for sustainable management strategies. Rising global demand for aquaculture products has led to intensified practices, amplifying risks to aquatic life through pollutants like pesticides, heavy metals, and pharmaceuticals, alongside environmental stressors such as temperature fluctuations, hypoxia, and salinity changes, contribute to developmental abnormalities in aquatic species. Understanding the mechanisms through which these pollutants and stressors exert their effects is essential for developing effective mitigation strategies. Future research should prioritize long-term studies on cumulative stressor effects, species-specific responses, and ecological outcomes. Implementing informed policies and regulations based on scientific insights will be essential to safeguard aquatic biodiversity and promote ecosystem sustainability.
CRediT authorship contribution statement
S Madesh: Concepualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. Sanjai Gopi: Data curation, Formal analysis, Investigation, Writing – original draft. Avra Sau: Formal analysis, Investigation, Methodology, Writing – original draft. S Karthick Raja Namasivayam: Formal analysis, Methodology, Validation, Writing – review & editing. Jesu Arockiaraj: Concepualization, Methodology, Project administration, Resources, Validation, Visualization, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Consent for publication
All authors have given their consent to publish this paper.
Contributor Information
S. Karthick Raja Namasivayam, Email: biologiask@gmail.com.
Jesu Arockiaraj, Email: jesuaroa@srmist.edu.in.
Data availability
Data will be made available on request.
References
- 1.Kokturk M., Comaklı S., Ozkaraca M., Alak G., Atamanalp M. Teratogenic and neurotoxic effects of n-butanol on zebrafish development. J. Aquat. Anim. Health. 2021;33(2):94–106. doi: 10.1002/aah.10123. [DOI] [PubMed] [Google Scholar]
- 2.Grummer J.A., Beheregaray L.B., Bernatchez L., et al. Aquatic landscape genomics and environmental effects on genetic variation. Trends Ecol. Evol. 2019;34(7):641–654. doi: 10.1016/j.tree.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Bernhardt J.R., O’Connor M.I. Aquatic biodiversity enhances multiple nutritional benefits to humans. Proc. Natl. Acad. Sci. 2021;118(15) doi: 10.1073/pnas.1917487118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherry J.A., Pec G.J. Advances, applications, and prospects in aquatic botany. Appl. Plant Sci. 2022;10(4) doi: 10.1002/aps3.11488. [DOI] [Google Scholar]
- 5.Zhou J., Mogollón J.M., van Bodegom P.M., Barbarossa V., Beusen A.H.W., Scherer L. Effects of nitrogen emissions on fish species richness across the world’s freshwater ecoregions. Environ. Sci. Technol. 2023;57(22):8347–8354. doi: 10.1021/acs.est.2c09333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madesh S., Sudhakaran G., Meenatchi R., Guru A., Arockiaraj J. Interconnected environmental challenges: heavy metal–drug interactions and their impacts on ecosystems. Drug Chem. Toxicol. 2024:1–18. doi: 10.1080/01480545.2024.2342956. Published online April 24, Published online April 24, [DOI] [PubMed] [Google Scholar]
- 7.Franzellitti S., Canesi L., Auguste M., Wathsala R.H.G.R., Fabbri E. Microplastic exposure and effects in aquatic organisms: a physiological perspective. Environ. Toxicol. Pharm. 2019;68:37–51. doi: 10.1016/j.etap.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Lomartire S., Marques J.C., Gonçalves A.M.M. Biomarkers based tools to assess environmental and chemical stressors in aquatic systems. Ecol. Indic. 2021;122 doi: 10.1016/j.ecolind.2020.107207. [DOI] [Google Scholar]
- 9.Trotter B., Ramsperger A.F.R.M., Raab P., Haberstroh J., Laforsch C. Plastic waste interferes with chemical communication in aquatic ecosystems. Sci. Rep. 2019;9(1):5889. doi: 10.1038/s41598-019-41677-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez-Alvarez I., Islas-Flores H., Gomez-Oliván L.M., Garcia O.D. Vol. 96. Springer Science and Business Media Deutschland GmbH; 2020. N. Teratogenesis and Embryotoxicity Induced by Non-steroidal Anti-Inflammatory Drugs in Aquatic Organisms; pp. 115–129. (Handbook of Environmental Chemistry). [DOI] [Google Scholar]
- 11.Paschoalini A.L., Savassi L.A., Arantes F.P., Rizzo E., Bazzoli N. Heavy metals accumulation and endocrine disruption in Prochilodus argenteus from a polluted neotropical river. Ecotoxicol. Environ. Saf. 2019;169:539–550. doi: 10.1016/j.ecoenv.2018.11.047. [DOI] [PubMed] [Google Scholar]
- 12.Authman M.M. Use of Fish as Bio-indicator of the Effects of Heavy Metals Pollution. J. Aquac. Res Dev. 2015;06(04) doi: 10.4172/2155-9546.1000328. [DOI] [Google Scholar]
- 13.Mezzelani M., Gorbi S., Regoli F. Pharmaceuticals in the aquatic environments: Evidence of emerged threat and future challenges for marine organisms. Mar. Environ. Res. 2018;140:41–60. doi: 10.1016/j.marenvres.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Fabbri E. Pharmaceuticals in the environment: expected and unexpected effects on aquatic fauna. Ann. N. Y Acad. Sci. 2015;1340(1):20–28. doi: 10.1111/nyas.12605. [DOI] [PubMed] [Google Scholar]
- 15.Mazzitelli J.Y., Budzinski H., Cachot J., et al. Evaluation of psychiatric hospital wastewater toxicity: what is its impact on aquatic organisms? Environ. Sci. Pollut. Res. 2018;25(26):26090–26102. doi: 10.1007/s11356-018-2501-5. [DOI] [PubMed] [Google Scholar]
- 16.Tran C.M., Lee H., Lee B., Ra J.S., Kim K.T. Effects of the chorion on the developmental toxicity of organophosphate esters in zebrafish embryos. J. Hazard Mater. 2021;401 doi: 10.1016/j.jhazmat.2020.123389. [DOI] [PubMed] [Google Scholar]
- 17.Hasenbein S., Poynton H., Connon R.E. Contaminant exposure effects in a changing climate: how multiple stressors can multiply exposure effects in the amphipod Hyalella azteca. Ecotoxicology. 2018;27(7):845–859. doi: 10.1007/s10646-018-1912-x. [DOI] [PubMed] [Google Scholar]
- 18.Velasco J., Gutiérrez-Cánovas C., Botella-Cruz M., et al. Effects of salinity changes on aquatic organisms in a multiple stressor context. Philos. Trans. R. Soc. B Biol. Sci. 2019;374(1764):20180011. doi: 10.1098/rstb.2018.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng Y., Fang W., Krauss M., et al. Screening hundreds of emerging organic pollutants (EOPs) in surface water from the Yangtze River Delta (YRD): occurrence, distribution, ecological risk. Environ. Pollut. 2018;241:484–493. doi: 10.1016/j.envpol.2018.05.061. [DOI] [PubMed] [Google Scholar]
- 20.Bashir I., Lone F.A., Bhat R.A., Mir S.A., Dar Z.A., Dar S.A. In: Bioremediation and Biotechnology. Springer International Publishing; 2020. Concerns and Threats of Contamination on Aquatic Ecosystems; pp. 1–26. [DOI] [Google Scholar]
- 21.Vajargah M.F. A review on the effects of heavy metals on aquatic animals. J. Biomed. Res Environ. Sci. 2021;2(9):865–869. doi: 10.37871/jbres1324. [DOI] [Google Scholar]
- 22.Carere M., Antoccia A., Buschini A., et al. An integrated approach for chemical water quality assessment of an urban river stretch through Effect-Based Methods and emerging pollutants analysis with a focus on genotoxicity. J. Environ. Manag. 2021;300 doi: 10.1016/j.jenvman.2021.113549. [DOI] [PubMed] [Google Scholar]
- 23.Bradley P.M., Journey C.A., Button D.T., et al. Multi-region assessment of pharmaceutical exposures and predicted effects in USA wadeable urban-gradient streams. Cañedo-Argüelles Iglesias M, ed. PLoS One. 2020;15(1) doi: 10.1371/journal.pone.0228214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regoli F., Giuliani M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014;93:106–117. doi: 10.1016/j.marenvres.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Garces A., Pires I., Rodrigues P. Teratological effects of pesticides in vertebrates: a review. J. Environ. Sci. Heal Part B. 2020;55(1):75–89. doi: 10.1080/03601234.2019.1660562. [DOI] [PubMed] [Google Scholar]
- 26.Park S.E., Lim S.R., Choi H. kyoon, Bae J. Triazine herbicides inhibit relaxin signaling and disrupt nitric oxide homeostasis. Toxicol. Appl. Pharm. 2016;307:10–18. doi: 10.1016/j.taap.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Wirbisky S., Freeman J. Atrazine exposure and reproductive dysfunction through the hypothalamus-pituitary-gonadal (HPG) axis. Toxics. 2015;3(4):414–450. doi: 10.3390/toxics3040414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subramanian P., Arul G., Karthikeyeni S., et al. Influence of triazine herbicide exposure on guppies (Poecilia sphenops) aromatase activities, altered sex steroid concentration and vitellogenin induction. Indian J. Pharm. Sci. 2015;77(2):156. doi: 10.4103/0250-474X.156549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barcellos P., Araújo T., Gomes G., Bila D., Canela M.C. The fate of atrazine in tropical environments: photolysis, acute toxicity and endocrine disruptor potential. J. Braz. Chem. Soc. 2022 doi: 10.21577/0103-5053.20220030. (Published online) [DOI] [Google Scholar]
- 30.Saka M., Tada N., Kamata Y. Chronic toxicity of 1,3,5-triazine herbicides in the postembryonic development of the western clawed frog Silurana tropicalis. Ecotoxicol. Environ. Saf. 2018;147:373–381. doi: 10.1016/j.ecoenv.2017.08.063. [DOI] [PubMed] [Google Scholar]
- 31.Orton F., Tyler C.R. Do hormone-modulating chemicals impact on reproduction and development of wild amphibians? Biol. Rev. 2015;90(4):1100–1117. doi: 10.1111/brv.12147. [DOI] [PubMed] [Google Scholar]
- 32.Wiegand C., Krause E., Steinberg C., Pflugmacher S. Toxicokinetics of atrazine in embryos of the zebrafish (Danio rerio) Ecotoxicol. Environ. Saf. 2001;49(3):199–205. doi: 10.1006/eesa.2001.2073. [DOI] [PubMed] [Google Scholar]
- 33.Blahova J., Cocilovo C., Plhalova L., Svobodova Z., Faggio C. Embryotoxicity of atrazine and its degradation products to early life stages of zebrafish (Danio rerio) Environ. Toxicol. Pharm. 2020;77 doi: 10.1016/j.etap.2020.103370. [DOI] [PubMed] [Google Scholar]
- 34.Schmitt C., McManus M., Kumar N., Awoyemi O., Crago J. Comparative analyses of the neurobehavioral, molecular, and enzymatic effects of organophosphates on embryo-larval zebrafish (Danio rerio) Neurotoxicol. Teratol. 2019;73:67–75. doi: 10.1016/j.ntt.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Neylon J., Fuller J.N., van der Poel C., Church J.E., Dworkin S. Organophosphate insecticide toxicity in neural development, cognition, behaviour and degeneration: insights from zebrafish. J. Dev. Biol. 2022;10(4):49. doi: 10.3390/jdb10040049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva M.B. da, Fraga R.E., Nishiyama P.B., et al. Leukocyte profiles in odontophrynus carvalhoi (amphibia: odontophrynidae) tadpoles exposed to organophosphate chlorpyrifos pesticides. Water, Air, Soil Pollut. 2020;231(7):372. doi: 10.1007/s11270-020-04726-4. [DOI] [Google Scholar]
- 37.Boualit L., Cayuela H., Cattin L., Chèvre N. The amphibian short-term assay: evaluation of a new ecotoxicological method for amphibians using two organophosphate pesticides commonly found in nature—assessment of biochemical, morphological, and life-history traits. Environ. Toxicol. Chem. 2022;41(11):2688–2699. doi: 10.1002/etc.5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martyniuk C.J., Mehinto A.C., Denslow N.D. Organochlorine pesticides: Agrochemicals with potent endocrine-disrupting properties in fish. Mol. Cell Endocrinol. 2020;507 doi: 10.1016/j.mce.2020.110764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Senthilkumaran B. Pesticide- and sex steroid analogue-induced endocrine disruption differentially targets hypothalamo–hypophyseal–gonadal system during gametogenesis in teleosts – a review. Gen. Comp. Endocrinol. 2015;219:136–142. doi: 10.1016/j.ygcen.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 40.Wolmarans N.J., Du Preez L.H., Yohannes Y.B., et al. Linking organochlorine exposure to biomarker response patterns in Anurans: a case study of Müller’s clawed frog (Xenopus muelleri) from a tropical malaria vector control region. Ecotoxicology. 2018;27(9):1203–1216. doi: 10.1007/s10646-018-1972-y. [DOI] [PubMed] [Google Scholar]
- 41.Rathipriya A., Agarwal D., Suresh E., Rather M.A. In: Xenobiotics in Aquatic Animals. Springer Nature Singapore; 2023. Endocrine-Disrupting Compounds (EDCs) as Emerging Aquatic Contaminants: Emphasis on Reproduction and Development; pp. 415–427. [DOI] [Google Scholar]
- 42.Pham B., Miranda A., Allinson G., Nugegoda D. Evaluating the non-lethal effects of organophosphorous and carbamate insecticides on the yabby ( Cherax destructor) using cholinesterase (AChE, BChE), Glutathione S-Transferase and ATPase as biomarkers. Ecotoxicol. Environ. Saf. 2017;143:283–288. doi: 10.1016/j.ecoenv.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 43.Sule R.O., Condon L., Gomes A.V. In: Zhou X., editor. Vol. 2022. 2022. A common feature of pesticides: oxidative stress—the role of oxidative stress in pesticide-induced toxicity; pp. 1–31. (Oxid Med Cell Longev). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pham B., Miranda A., Allinson G., Nugegoda D. Assessing interactive mixture toxicity of carbamate and organophosphorus insecticides in the yabby (Cherax destructor) Ecotoxicology. 2018;27(9):1217–1224. doi: 10.1007/s10646-018-1973-x. [DOI] [PubMed] [Google Scholar]
- 45.Samarakoon H.M.T.R., Pathiratne A. Survival and cholinesterase activity of Asian common toad (Duttaphrynus melanostictus) tadpoles following short term exposure to a carbosulfan-based pesticide. Sri Lanka J. Aquat. Sci. 2017;22(1):29–37. doi: 10.4038/sljas.v22i1.7514. [DOI] [Google Scholar]
- 46.Wang Y., Chen C., Zhao X., Wang Q., Qian Y. Assessing joint toxicity of four organophosphate and carbamate insecticides in common carp (Cyprinus carpio) using acetylcholinesterase activity as an endpoint. Pest. Biochem Physiol. 2015;122:81–85. doi: 10.1016/j.pestbp.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 47.Jasrotia R., Langer S., Dhar M. Endocrine disrupting chemicals in aquatic ecosystem: an emerging threat to wildlife and human health. Proc. Zool. Soc. 2021;74(4):634–647. doi: 10.1007/s12595-021-00410-5. [DOI] [Google Scholar]
- 48.Li P., Zhang J., Xie H., et al. Heavy metal bioaccumulation and health hazard assessment for three fish species from nansi lake, China. Bull. Environ. Contam. Toxicol. 2015;94(4):431–436. doi: 10.1007/s00128-015-1475-y. [DOI] [PubMed] [Google Scholar]
- 49.Stara A., Pagano M., Capillo G., et al. Acute effects of neonicotinoid insecticides on Mytilus galloprovincialis: a case study with the active compound thiacloprid and the commercial formulation calypso 480 SC. Ecotoxicol. Environ. Saf. 2020;203 doi: 10.1016/j.ecoenv.2020.110980. [DOI] [PubMed] [Google Scholar]
- 50.Rodrigues A.C.M., Bordalo M.D., Golovko O., et al. Combined effects of insecticide exposure and predation risk on freshwater detritivores. Ecotoxicology. 2018;27(7):794–802. doi: 10.1007/s10646-017-1887-z. [DOI] [PubMed] [Google Scholar]
- 51.Madesh S., Sudhakaran G., Ramamurthy K., et al. Cadmium and ketoprofen accumulation influences aquatic ecosystem demonstrated using in-vivo zebrafish model. Drug Chem. Toxicol. 2024:1–16. doi: 10.1080/01480545.2024.2364240. Published online June 23, Published online June 23, [DOI] [PubMed] [Google Scholar]
- 52.Antunes dos Santos A., Ferrer B., Marques Gonçalves F., et al. Oxidative stress in methylmercury-induced cell toxicity. Toxics. 2018;6(3):47. doi: 10.3390/toxics6030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nielsen K.M., Soulen B.K., Overturf C.L., Drevnick P.E., Roberts A.P. Embryotoxicity of maternally transferred methylmercury to fathead minnows ( Pimephales promelas. Environ. Toxicol. Chem. 2016;35(6):1436–1441. doi: 10.1002/etc.3282. [DOI] [PubMed] [Google Scholar]
- 54.Malvandi H., Sarvary Korojdeh M., Azimi S. Assessment of mercury contamination in perch species in the southern Caspian Sea. Arch. Environ. Contam. Toxicol. 2020;79(1):147–155. doi: 10.1007/s00244-020-00730-1. [DOI] [PubMed] [Google Scholar]
- 55.Wang Q., Liu B., Yang H., Wang X., Lin Z. Toxicity of lead, cadmium and mercury on embryogenesis, survival, growth and metamorphosis of Meretrix meretrix larvae. Ecotoxicology. 2009;18(7):829–837. doi: 10.1007/s10646-009-0326-1. [DOI] [PubMed] [Google Scholar]
- 56.Kataba A., Botha T.L., Nakayama S.M.M., et al. Environmentally relevant lead (Pb) water concentration induce toxicity in zebrafish (Danio rerio) larvae. Comp. Biochem Physiol. Part C. Toxicol. Pharm. 2022;252 doi: 10.1016/j.cbpc.2021.109215. [DOI] [PubMed] [Google Scholar]
- 57.Lee J.W., Choi H., Hwang U.K., et al. Toxic effects of lead exposure on bioaccumulation, oxidative stress, neurotoxicity, and immune responses in fish: a review. Environ. Toxicol. Pharm. 2019;68:101–108. doi: 10.1016/j.etap.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 58.Trudeau V.L., Thomson P., Zhang W.S., Reynaud S., Navarro-Martin L., Langlois V.S. Agrochemicals disrupt multiple endocrine axes in amphibians. Mol. Cell Endocrinol. 2020;513 doi: 10.1016/j.mce.2020.110861. [DOI] [PubMed] [Google Scholar]
- 59.Yu S., Tang S., Mayer G.D., Cobb G.P., Maul J.D. Interactive effects of ultraviolet-B radiation and pesticide exposure on DNA photo-adduct accumulation and expression of DNA damage and repair genes in Xenopus laevis embryos. Aquat. Toxicol. 2015;159:256–266. doi: 10.1016/j.aquatox.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Zhu Q.L., Li W.Y., Zheng J.L. Life-cycle exposure to cadmium induced compensatory responses towards oxidative stress in the liver of female zebrafish. Chemosphere. 2018;210:949–957. doi: 10.1016/j.chemosphere.2018.07.095. [DOI] [PubMed] [Google Scholar]
- 61.Pan J., Huang X., Li Y., et al. Zinc protects against cadmium-induced toxicity by regulating oxidative stress, ions homeostasis and protein synthesis. Chemosphere. 2017;188:265–273. doi: 10.1016/j.chemosphere.2017.08.106. [DOI] [PubMed] [Google Scholar]
- 62.Liu J., Wang E., Jing W., Dahms H.U., Murugan K., Wang L. Mitigative effects of zinc on cadmium-induced reproductive toxicity in the male freshwater crab Sinopotamon henanense. Environ. Sci. Pollut. Res. 2020;27(14):16282–16292. doi: 10.1007/s11356-020-08074-y. [DOI] [PubMed] [Google Scholar]
- 63.Hu F., Yin L., Dong F., et al. Effects of long-term cadmium exposure on growth, antioxidant defense and DNA methylation in juvenile Nile tilapia (Oreochromis niloticus) Aquat. Toxicol. 2021;241 doi: 10.1016/j.aquatox.2021.106014. [DOI] [PubMed] [Google Scholar]
- 64.Xie D., Li Y., Liu Z., Chen Q. Inhibitory effect of cadmium exposure on digestive activity, antioxidant capacity and immune defense in the intestine of yellow catfish (Pelteobagrus fulvidraco) Comp. Biochem Physiol. Part C. Toxicol. Pharm. 2019;222:65–73. doi: 10.1016/j.cbpc.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 65.Jamwal A., Lemire D., Driessnack M., Naderi M., Niyogi S. Interactive effects of chronic dietary selenomethionine and cadmium exposure in rainbow trout (Oncorhynchus mykiss): a preliminary study. Chemosphere. 2018;197:550–559. doi: 10.1016/j.chemosphere.2018.01.087. [DOI] [PubMed] [Google Scholar]
- 66.Sun N., Wang H., Ju Z., Zhao H. Effects of chronic cadmium exposure on metamorphosis, skeletal development, and thyroid endocrine disruption in Chinese toad Bufo gargarizans tadpoles. Environ. Toxicol. Chem. 2018;37(1):213–223. doi: 10.1002/etc.3947. [DOI] [PubMed] [Google Scholar]
- 67.Wu C., Zhang Y., Chai L., Wang H. Oxidative stress, endocrine disruption, and malformation of Bufo gargarizans embryo exposed to sub-lethal cadmium concentrations. Environ. Toxicol. Pharm. 2017;49:97–104. doi: 10.1016/j.etap.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 68.Drag-Kozak E., Pawlica-Gosiewska D., Gawlik K., et al. Cadmium-induced oxidative stress in Prussian carp (Carassius gibelio Bloch) hepatopancreas: ameliorating effect of melatonin. Environ. Sci. Pollut. Res. 2019;26(12):12264–12279. doi: 10.1007/s11356-019-04595-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun H.J., Zhao W.J., Teng X.Q., et al. Antioxidant responses and pathological changes in the gill of zebrafish (Danio rerio) after chronic exposure to arsenite at its reference dose. Ecotoxicol. Environ. Saf. 2020;200 doi: 10.1016/j.ecoenv.2020.110743. [DOI] [PubMed] [Google Scholar]
- 70.Guidarelli A., Fiorani M., Carloni S., Cerioni L., Balduini W., Cantoni O. The study of the mechanism of arsenite toxicity in respiration-deficient cells reveals that NADPH oxidase-derived superoxide promotes the same downstream events mediated by mitochondrial superoxide in respiration-proficient cells. Toxicol. Appl. Pharm. 2016;307:35–44. doi: 10.1016/j.taap.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 71.Painefilu J.C., Pascual M.M., Bieczynski F., et al. Ex vivo and in vivo effects of arsenite on GST and ABCC2 activity and expression in the middle intestine of the rainbow trout Oncorhynchus mykiss. Comp. Biochem Physiol. Part C. Toxicol. Pharm. 2019;225 doi: 10.1016/j.cbpc.2019.108566. [DOI] [PubMed] [Google Scholar]
- 72.Jamwal A., Niyogi S. Dose and chemical species-specific effects of selenium against arsenite toxicity in cultured hepatocytes of rainbow trout (Oncorhynchus mykiss) Metallomics. 2017;9(6):744–756. doi: 10.1039/C7MT00006E. [DOI] [PubMed] [Google Scholar]
- 73.Xu H., Zhou X., Wen X., et al. Environmentally relevant concentrations of arsenite induce dose-dependent differential genotoxicity through poly(ADP-Ribose) polymerase inhibition and oxidative stress in mouse thymus cells. Toxicol. Sci. 2016;149(1):31–41. doi: 10.1093/toxsci/kfv211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mardirosian M.N., Lascano C.I., Ferrari A., Bongiovanni G.A., Venturino A. Acute toxicity of arsenic and oxidative stress responses in the embryonic development of the common South American toad Rhinella arenarum. Environ. Toxicol. Chem. 2015;34(5):1009–1014. doi: 10.1002/etc.2856. [DOI] [PubMed] [Google Scholar]
- 75.Mardirosian M.N., Ceschin D.G., Lascano C.I., Venturino A. Molecular effectors in the chronic exposure to arsenic as early and sensitive biomarkers in developing Rhinella arenarum toads. Aquat. Toxicol. 2017;186:19–27. doi: 10.1016/j.aquatox.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 76.Escudero-Lourdes C. Toxicity mechanisms of arsenic that are shared with neurodegenerative diseases and cognitive impairment: Role of oxidative stress and inflammatory responses. Neurotoxicology. 2016;53:223–235. doi: 10.1016/j.neuro.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 77.Chen T.H., Gross J.A., Karasov W.H. Chronic exposure to pentavalent arsenic of larval leopard frogs (Rana pipiens): bioaccumulation and reduced swimming performance. Ecotoxicology. 2009;18(5):587–593. doi: 10.1007/s10646-009-0316-3. [DOI] [PubMed] [Google Scholar]
- 78.Zhang J., Koch I., Gibson L.A., et al. Transcriptomic Responses During Early Development Following Arsenic Exposure in Western Clawed Frogs, Silurana tropicalis. Toxicol. Sci. 2015;148(2):603–617. doi: 10.1093/toxsci/kfv207. [DOI] [PubMed] [Google Scholar]
- 79.Xu J., Zhao M., Pei L., et al. Oxidative stress and DNA damage in a long-term hexavalent chromium-exposed population in North China: a cross-sectional study. BMJ Open. 2018;8(6) doi: 10.1136/bmjopen-2017-021470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shaw P., Mondal P., Bandyopadhyay A., Chattopadhyay A. Environmentally relevant concentration of chromium induces nuclear deformities in erythrocytes and alters the expression of stress-responsive and apoptotic genes in brain of adult zebrafish. Sci. Total Environ. 2020;703 doi: 10.1016/j.scitotenv.2019.135622. [DOI] [PubMed] [Google Scholar]
- 81.Mohamed A.A.R., El-Houseiny W., EL-Murr A.E., Ebraheim L.L.M., Ahmed A.I., El-Hakim Y.M.A. Effect of hexavalent chromium exposure on the liver and kidney tissues related to the expression of CYP450 and GST genes of Oreochromis niloticus fish: role of curcumin supplemented diet. Ecotoxicol. Environ. Saf. 2020;188 doi: 10.1016/j.ecoenv.2019.109890. [DOI] [PubMed] [Google Scholar]
- 82.Ni X., Wan L., Liang P., et al. The acute toxic effects of hexavalent chromium on the liver of marine medaka (Oryzias melastigma) Comp. Biochem Physiol. Part C. Toxicol. Pharm. 2020;231 doi: 10.1016/j.cbpc.2020.108734. [DOI] [PubMed] [Google Scholar]
- 83.Fernando V.A.K., Weerasena J., Lakraj G.P., et al. Lethal and sub-lethal effects on the Asian common toad Duttaphrynus melanostictus from exposure to hexavalent chromium. Aquat. Toxicol. 2016;177:98–105. doi: 10.1016/j.aquatox.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 84.Zhang X., Wang Y., Chen M., Zeng M. Hexavalent chromium-induced apoptosis in Hep3B cells is accompanied by calcium overload, mitochondrial damage, and AIF translocation. Ecotoxicol. Environ. Saf. 2021;208 doi: 10.1016/j.ecoenv.2020.111391. [DOI] [PubMed] [Google Scholar]
- 85.Ren X., Xia B., Chen Z., et al. Short-term and long-term exposure to hexavalent chromium alters 53BP1 via H3K18ac and H3K27ac. Chemosphere. 2019;229:284–294. doi: 10.1016/j.chemosphere.2019.04.113. [DOI] [PubMed] [Google Scholar]
- 86.Kathiravan M.K., Palaniappan S., Jayasankar N. Pharmaceuticals in Water. Book Publisher International (a part of SCIENCEDOMAIN International); 2021. Pharmaceuticals as Pollutants in the Aquatic Ecosystem – Cardiovascular, Anti-diabetic, Steroids and Related Drugs; pp. 32–38. [DOI] [Google Scholar]
- 87.Świacka K., Michnowska A., Maculewicz J., Caban M., Smolarz K. Toxic effects of NSAIDs in non-target species: a review from the perspective of the aquatic environment. Environ. Pollut. 2021;273 doi: 10.1016/j.envpol.2020.115891. [DOI] [PubMed] [Google Scholar]
- 88.Corcoran J., Winter M.J., Tyler C.R. Pharmaceuticals in the aquatic environment: A critical review of the evidence for health effects in fish. Crit. Rev. Toxicol. 2010;40(4):287–304. doi: 10.3109/10408440903373590. [DOI] [PubMed] [Google Scholar]
- 89.Nogueira A.F., Pinto G., Correia B., Nunes B. Embryonic development, locomotor behavior, biochemical, and epigenetic effects of the pharmaceutical drugs paracetamol and ciprofloxacin in larvae and embryos of Danio rerio when exposed to environmental realistic levels of both drugs. Environ. Toxicol. 2019;34(11):1177–1190. doi: 10.1002/tox.22819. [DOI] [PubMed] [Google Scholar]
- 90.Hou L., Fu Y., Zhao C., Fan L., Hu H., Yin S. Ciprofloxacin and enrofloxacin can cause reproductive toxicity via endocrine signaling pathways. SSRN Electron J. 2022 doi: 10.2139/ssrn.4064359. (Published online) (Published online) [DOI] [PubMed] [Google Scholar]
- 91.Xia L., Zheng L., Zhou J.L. Effects of ibuprofen, diclofenac and paracetamol on hatch and motor behavior in developing zebrafish ( Danio rerio) Chemosphere. 2017;182:416–425. doi: 10.1016/j.chemosphere.2017.05.054. [DOI] [PubMed] [Google Scholar]
- 92.Bojarski B., Kot B., Witeska M. Antibacterials in Aquatic Environment and Their Toxicity to Fish. Pharmaceuticals. 2020;13(8):189. doi: 10.3390/ph13080189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qiu W., Liu X., Yang F., et al. Single and joint toxic effects of four antibiotics on some metabolic pathways of zebrafish (Danio rerio) larvae. Sci. Total Environ. 2020;716 doi: 10.1016/j.scitotenv.2020.137062. [DOI] [PubMed] [Google Scholar]
- 94.Botelho R.G., Christofoletti C.A., Correia J.E., Ansoar Y., Olinda R.A., Tornisielo V.L. Genotoxic responses of juvenile tilapia (Oreochromis niloticus) exposed to florfenicol and oxytetracycline. Chemosphere. 2015;132:206–212. doi: 10.1016/j.chemosphere.2015.02.053. [DOI] [PubMed] [Google Scholar]
- 95.Rodrigues S., Antunes S.C., Correia A.T., Nunes B. Rainbow trout (Oncorhynchus mykiss) pro-oxidant and genotoxic responses following acute and chronic exposure to the antibiotic oxytetracycline. Ecotoxicology. 2017;26(1):104–117. doi: 10.1007/s10646-016-1746-3. [DOI] [PubMed] [Google Scholar]
- 96.Payne C.J., Turnbull J.F., MacKenzie S., Crumlish M. Investigating the effect of an oxytetracycline treatment on the gut microbiome and antimicrobial resistance gene dynamics in nile tilapia (Oreochromis niloticus) Antibiotics. 2021;10(10):1213. doi: 10.3390/antibiotics10101213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang C., Song G., Lim W. A review of the toxicity in fish exposed to antibiotics. Comp. Biochem Physiol. Part C. Toxicol. Pharm. 2020;237 doi: 10.1016/j.cbpc.2020.108840. [DOI] [PubMed] [Google Scholar]
- 98.Kovalakova P., Cizmas L., McDonald T.J., Marsalek B., Feng M., Sharma V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere. 2020;251 doi: 10.1016/j.chemosphere.2020.126351. [DOI] [PubMed] [Google Scholar]
- 99.Deepika S., Padmavathy P., Srinivasan A., Sugumar G., Jawahar P. Effect of triclosan (TCS) on the protein content and associated histological changes on tilapia, Oreochromis mossambicus (Peters, 1852) Environ. Sci. Pollut. Res. 2021;28(42):59899–59907. doi: 10.1007/s11356-021-14990-4. [DOI] [PubMed] [Google Scholar]
- 100.Liang X., Nie X., Ying G., An T., Li K. Assessment of toxic effects of triclosan on the swordtail fish (Xiphophorus helleri) by a multi-biomarker approach. Chemosphere. 2013;90(3):1281–1288. doi: 10.1016/j.chemosphere.2012.09.087. [DOI] [PubMed] [Google Scholar]
- 101.Orozco-Hernandez J.M., Gomez-Olivan L.M., Elizalde-Velazquez G.A., et al. Effects of oxidative stress induced by environmental relevant concentrations of fluoxetine on the embryonic development on Danio rerio. Sci. Total Environ. 2022;807 doi: 10.1016/j.scitotenv.2021.151048. [DOI] [PubMed] [Google Scholar]
- 102.Pan C., Yang M., Xu H., Xu B., Jiang L., Wu M. Tissue bioconcentration and effects of fluoxetine in zebrafish (Danio rerio) and red crucian cap (Carassius auratus) after short-term and long-term exposure. Chemosphere. 2018;205:8–14. doi: 10.1016/j.chemosphere.2018.04.082. [DOI] [PubMed] [Google Scholar]
- 103.Fort D.J., Stover E.L., Propst T., Hull M.A., Bantle J.A. Evaluation of the developmental toxicity of theophylline, dimethyluric acid, and methylxanthine metabolites using xenopus. Drug Chem. Toxicol. 1996;19(4):267–278. doi: 10.3109/01480549608998237. [DOI] [PubMed] [Google Scholar]
- 104.Aguirre-Martinez G.V., Owuor M.A., Garrido-Perez C., Salamanca M.J., Del Valls T.A., Martin-Diaz M.L. Are standard tests sensitive enough to evaluate effects of human pharmaceuticals in aquatic biota? Facing changes in research approaches when performing risk assessment of drugs. Chemosphere. 2015;120:75–85. doi: 10.1016/j.chemosphere.2014.05.087. [DOI] [PubMed] [Google Scholar]
- 105.Heye K., Graumnitz S., Rybicki M., et al. Laboratory-to-field extrapolation: Increase in carbamazepine toxicity in a higher tier, multiple-stress experiment. Ecotoxicol. Environ. Saf. 2019;183 doi: 10.1016/j.ecoenv.2019.109481. [DOI] [PubMed] [Google Scholar]
- 106.Ferrera C.M., Watanabe A., Miyajima T., et al. Phosphorus as a driver of nitrogen limitation and sustained eutrophic conditions in Bolinao and Anda, Philippines, a mariculture-impacted tropical coastal area. Mar. Pollut. Bull. 2016;105(1):237–248. doi: 10.1016/j.marpolbul.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 107.Godoy A.A., Domingues I., de Carvalho L.B., et al. Assessment of the ecotoxicity of the pharmaceuticals bisoprolol, sotalol, and ranitidine using standard and behavioral endpoints. Environ. Sci. Pollut. Res. 2020;27(5):5469–5481. doi: 10.1007/s11356-019-07322-0. [DOI] [PubMed] [Google Scholar]
- 108.Thilagam H., Gopalakrishnan S. Environmental deterioration due to existing and emerging persistent organic pollutants: an overview. In. 2022:59–89. doi: 10.1007/978-3-030-72441-2_3. [DOI] [Google Scholar]
- 109.Bialk-Bielinska A., Mulkiewicz E., Stokowski M., Stolte S., Stepnowski P. Acute aquatic toxicity assessment of six anti-cancer drugs and one metabolite using biotest battery – Biological effects and stability under test conditions. Chemosphere. 2017;189:689–698. doi: 10.1016/j.chemosphere.2017.08.174. [DOI] [PubMed] [Google Scholar]
- 110.Isidori M., Piscitelli C., Russo C., Smutná M., Bláha L. Teratogenic effects of five anticancer drugs on Xenopus laevis embryos. Ecotoxicol. Environ. Saf. 2016;133:90–96. doi: 10.1016/j.ecoenv.2016.06.044. [DOI] [PubMed] [Google Scholar]
- 111.Teigeler M., Schaudien D., Böhmer W., Länge R., Schäfers C. Effects of the gestagen levonorgestrel in a life cycle test with Zebrafish ( Danio rerio) Environ. Toxicol. Chem. 2022;41(3):580–591. doi: 10.1002/etc.5008. [DOI] [PubMed] [Google Scholar]
- 112.Hua J., Han J., Guo Y., Zhou B. The progestin levonorgestrel affects sex differentiation in zebrafish at environmentally relevant concentrations. Aquat. Toxicol. 2015;166:1–9. doi: 10.1016/j.aquatox.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 113.Dammann A.A., Shappell N.W., Bartell S.E., Schoenfuss H.L. Comparing biological effects and potencies of estrone and 17β-estradiol in mature fathead minnows, Pimephales promelas. Aquat. Toxicol. 2011;105(3-4):559–568. doi: 10.1016/j.aquatox.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 114.Langston W.J., Burt G.R., Chesman B.S., Vane C.H. Partitioning, bioavailability and effects of oestrogens and xeno-oestrogens in the aquatic environment. J. Mar. Biol. Assoc. U. Kingd. 2005;85(1):1–31. doi: 10.1017/S0025315405010787h. [DOI] [Google Scholar]
- 115.Jackson L.M., Felgenhauer B.E., Klerks P.L. Feminization, altered gonadal development, and liver damage in least killifish (Heterandria formosa) exposed to sublethal concentrations of 17α-ethinylestradiol. Ecotoxicol. Environ. Saf. 2019;170:331–337. doi: 10.1016/j.ecoenv.2018.11.094. [DOI] [PubMed] [Google Scholar]
- 116.Valcarce D.G., Vuelta E., Robles V., Herráez M.P. Paternal exposure to environmental 17-alpha-ethinylestradiol concentrations modifies testicular transcription, affecting the sperm transcript content and the offspring performance in zebrafish. Aquat. Toxicol. 2017;193:18–29. doi: 10.1016/j.aquatox.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 117.Liang Y.Q., Huang G.Y., Lin Z., et al. Reproductive effects of synthetic progestin norgestrel in zebrafish (Danio rerio) Chemosphere. 2018;190:17–24. doi: 10.1016/j.chemosphere.2017.09.127. [DOI] [PubMed] [Google Scholar]
- 118.Frankel T., Yonkos L., Ampy F., Frankel J. Exposure to levonorgestrel increases nest acquisition success and decreases sperm motility in the male fathead minnow ( Pimephales promelas. Environ. Toxicol. Chem. 2018;37(4):1131–1137. doi: 10.1002/etc.4054. [DOI] [PubMed] [Google Scholar]
- 119.Svigruha R., Fodor I., Padisak J., Pirger Z. Progestogen-induced alterations and their ecological relevance in different embryonic and adult behaviours of an invertebrate model species, the great pond snail (Lymnaea stagnalis) Environ. Sci. Pollut. Res. 2021;28(42):59391–59402. doi: 10.1007/s11356-020-12094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Margiotta-Casaluci L., Hannah R.E., Sumpter J.P. Mode of action of human pharmaceuticals in fish: the effects of the 5-alpha-reductase inhibitor, dutasteride, on reproduction as a case study. Aquat. Toxicol. 2013;128-129:113–123. doi: 10.1016/j.aquatox.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 121.Kwan W.S., Roy V.A.L., Yu K.N. Review on toxic effects of Di(2-ethylhexyl) phthalate on zebrafish embryos. Toxics. 2021;9(8):193. doi: 10.3390/toxics9080193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Boyd C.E., Massaut L. Risks associated with the use of chemicals in pond aquaculture. Aquac. Eng. 1999;20(2):113–132. doi: 10.1016/S0144-8609(99)00010-2. [DOI] [Google Scholar]
- 123.McDougall D.R., Chan A., McGillivray D.J., de Jonge M.D., Miskelly G.M., Jeffs A.G. Examining the role of ethylenediaminetetraacetic acid (EDTA) in larval shellfish production in seawater contaminated with heavy metals. Aquat. Toxicol. 2019;217 doi: 10.1016/j.aquatox.2019.105330. [DOI] [PubMed] [Google Scholar]
- 124.Santore R.C., Ryan A.C., Kroglund F., et al. Development and application of a biotic ligand model for predicting the chronic toxicity of dissolved and precipitated aluminum to aquatic organisms. Environ. Toxicol. Chem. 2018;37(1):70–79. doi: 10.1002/etc.4020. [DOI] [PubMed] [Google Scholar]
- 125.Van den Meersche T., Rasschaert G., Vanden Nest T., et al. Longitudinal screening of antibiotic residues, antibiotic resistance genes and zoonotic bacteria in soils fertilized with pig manure. Environ. Sci. Pollut. Res. 2020;27(22):28016–28029. doi: 10.1007/s11356-020-09119-y. [DOI] [PubMed] [Google Scholar]
- 126.Souza A.C.P., Melo K.M., de Azevedo L.F.C., de Almada Vilhena A.O., Nagamachi C.Y., Pieczarka J.C. Lethal and sublethal exposure of Hemichromis bimaculatus (Gill, 1862) to malachite green and possible implications for ornamental fish. Environ. Sci. Pollut. Res. 2020;27(26):33215–33225. doi: 10.1007/s11356-020-09615-1. [DOI] [PubMed] [Google Scholar]
- 127.Zhang J., Zhu M., Wang Q., Yang H. The combined use of copper sulfate and trichlorfon exerts stronger toxicity on the liver of zebrafish. Int J. Mol. Sci. 2023;24(13):11203. doi: 10.3390/ijms241311203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Islam M.A., Amin S.M.N., Brown C.L., Juraimi A.S., Uddin M.K., Arshad A. Determination of median lethal concentration (LC50) for endosulfan, heptachlor and dieldrin pesticides to african catfish, clarias gariepinus and their impact on its behavioral patterns and histopathological responses. Toxics. 2021;9(12):340. doi: 10.3390/toxics9120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sanden M., Olsvik P.A., Søfteland L., et al. Dietary pesticide chlorpyrifos-methyl affects arachidonic acid metabolism including phospholipid remodeling in Atlantic salmon (Salmo salar L.) Aquaculture. 2018;484:1–12. doi: 10.1016/j.aquaculture.2017.10.033. [DOI] [Google Scholar]
- 130.Leal J.F., Neves M.G.P.M.S., Santos E.B.H., Esteves V.I. Use of formalin in intensive aquaculture: properties, application and effects on fish and water quality. Rev. Aquac. 2018;10(2):281–295. doi: 10.1111/raq.12160. [DOI] [Google Scholar]
- 131.Hanigan D., Truong L., Simonich M., Tanguay R., Westerhoff P. Zebrafish embryo toxicity of 15 chlorinated, brominated, and iodinated disinfection by-products. J. Environ. Sci. 2017;58:302–310. doi: 10.1016/j.jes.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 132.Lushchak V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011;101(1):13–30. doi: 10.1016/j.aquatox.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 133.Amores A., Force A., Yan Y.L., et al. Zebrafish hox clusters and vertebrate genome evolution. Science (80-) 1998;282(5394):1711–1714. doi: 10.1126/science.282.5394.1711. [DOI] [PubMed] [Google Scholar]
- 134.Niederreither K., Dolle P. Retinoic acid in development: towards an integrated view. Nat. Rev. Genet. 2008;9(7):541–553. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- 135.Varriale A., Bernardi G. DNA methylation and body temperature in fishes. Gene. 2006;385:111–121. doi: 10.1016/j.gene.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 136.Allen J., Schrage K., Foo S., Watson S.A., Byrne M. The effects of salinity and pH on fertilization, early development, and hatching in the crown-of-thorns seastar. Diversity. 2017;9(1):13. doi: 10.3390/d9010013. [DOI] [Google Scholar]
- 137.Tills O., Spicer J., Rundle S. Salinity-induced heterokairy in an upper-estuarine population of the snail Radix balthica (Mollusca: Pulmonata) Aquat. Biol. 2010;9:95–105. doi: 10.3354/ab00231. [DOI] [Google Scholar]
- 138.Kearney B.D., Pell R.J., Byrne P.G., Reina R.D. Anuran larval developmental plasticity and survival in response to variable salinity of ecologically relevant timing and magnitude. J. Exp. Zool. Part A Ecol. Genet Physiol. 2014;321(10):541–549. doi: 10.1002/jez.1887. [DOI] [PubMed] [Google Scholar]
- 139.Gaisina A.A., Aliyev M.K., Salmanli Z.S. Impact of water salinity on the early stages of sturgeon development. Azerbaijan J. Physiol. 2022;2:33–40. doi: 10.59883/ajp.36. [DOI] [Google Scholar]
- 140.Barrios-Figueroa R., Urbina M.A. In: Cooke S., editor. Vol. 11. 2023. Behavioural and physiological responses to salinization and air exposure during the ontogeny of a freshwater South American snail. (Conserv Physiol). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mansoor S., Ali A., Kour N., et al. Heavy metal induced oxidative stress mitigation and ROS scavenging in plants. Plants. 2023;12(16):3003. doi: 10.3390/plants12163003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dunwoodie S.L. The role of hypoxia in development of the mammalian embryo. Dev. Cell. 2009;17(6):755–773. doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 143.Schuijers J., Clevers H. Adult mammalian stem cells: the role of Wnt, Lgr5 and R-spondins. EMBO J. 2012;31(12):2685–2696. doi: 10.1038/emboj.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Strahl B.D., Allis C.D. The language of covalent histone modifications. Nature. 2000;403(6765):41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.