Abstract
Purpose: To investigate how home optical coherence tomography (OCT) influences the clinical decision-making of retina specialists for the management of neovascular age-related macular degeneration (nAMD). Methods: In this retrospective imaging review, 15 retina specialists each evaluated 10 home OCT data segments from 29 eyes being treated for nAMD. Based on OCT data, indications were identified for when eyes should be treated, which antivascular endothelial growth factor should be used, and the specific retinal fluid and time thresholds for notification. Results: Withholding treatment was recommended in 64 (42.7%) of 150 data segments (95% CI, 34.7-50.6), whereas 100% of eyes received treatment on the last day of each data segment. Treatment was recommended in 86 cases (57.3%), with treatment occurring 7 or more days before the actual treatment was advised in 52 (60.5%) of 86 data segments. This earlier treatment would have prevented the accumulation of intraretinal fluid (IRF), subretinal fluid (SRF), and total retinal fluid for 69.1 nL, 162.2 nL, and 231.2 nL days. Retina specialists chose a different type of treatment agent in 35 (40%) of 86 cases. The following notification values were set: IRF, mean 9.8 ± 14.9 nL (median, 5; IQR, 5); SRF, mean 10.2 ± 16.1 nL (median, 5.5; IQR, 5); total retinal fluid, mean 15.2 ± 24.0 nL (median, 10; IQR, 5). The time-based notification interval was set at a mean of 34.7 ± 21.9 days (median, 30; IQR, 2). Conclusions: Home OCT-based decision-making by retina specialists differed substantially from actual clinical care. Home OCT has the potential to facilitate personalized care in nAMD.
Keywords: optical coherence tomography, neovascular age-related macular degeneration, home OCT, imaging review, retina specialists, telemedicine, overtreatment, undertreatment, remote monitoring, artificial intelligence
Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness in the United States. 1 Over the past 2 decades, the use of antivascular endothelial growth factor (anti-VEGF) agents coupled with optical coherence tomography (OCT) imaging has revolutionized the management of neovascular AMD (nAMD). What was once a blinding condition is now treatable and, as well-documented in large clinical trials, patients with nAMD now typically maintain or improve their vision with timely anti-VEGF treatments.2 –5
Unlike in the clinical trial setting, there is no standardized anti-VEGF administration schedule for the treatment of nAMD in typical real-world practice. 6 A variety of administration schedules have been used, such as monthly dosing intervals (fixed), treat-and-extend with subsequent maintenance, and as-needed treatment regimens, among others. Treat-and-extend protocols remain the most popular in the US as clinicians try to balance efficacy, treatment burden, and costs. Therapies for nAMD currently cost the US healthcare system approximately $4 billion per year. 7
Despite the high cost, studies have found that the visual outcomes for patients in the real world are significantly worse than those reported in clinical trials.8,9 This is often attributed to undertreatment because a significantly smaller number of injections are administered per patient in the real-world setting than in clinical trials. Although overtreatment also occurs, cases in which a treatment could have been avoided if physicians had access to more detailed information about disease dynamics are harder to quantify. A patient’s disease state may allow for a reduction in treatment frequency that is missed as a result of fixed-interval dosing after implementation of an initial treat-and-extend protocol. Analyses of patients on as-needed regimens have shown that the ideal number of treatments can vary over the entire observation period, from monthly treatments to a single treatment. 10
Ideally, patients would receive the minimum number of anti-VEGF treatments necessary to control or eliminate disease activity. Avoiding unnecessary treatments minimizes the low but real risk of injections and reduces costs to the healthcare system. However, this would require a personalized treatment regimen based on each individual patient’s disease pattern, treatment response, medication durability, and appropriate regimen readjustments over time. At present, we are limited by the use of in-clinic eye examinations, visual acuity (VA) measurements, and OCT snapshots of the macular anatomy taken at each visit, with clinicians making treatment decisions and inferring interval changes weeks to months apart.
Recently, studies of a patient-operated spectral-domain OCT system, the Notal Vision Home OCT System (NVHO, Notal Vision Inc), showed excellent feasibility and performance of daily self-imaging at home.11,12 With support from a virtual monitoring center, patients self-acquire OCT images. Those images are subsequently uploaded to a centralized database, and the scans are analyzed by a validated artificial intelligence–based software that automatically detects and quantifies the volume of intraretinal fluid (IRF) and subretinal fluid (SRF). The scans are also available for viewing and manipulation via a browser-based system. 13 Fluid quantification allows for detailed graphic and mathematic analyses of temporal retinal fluid volume trajectories to theoretically inform clinical decision-making.
The purpose of the current study was to retrospectively investigate how the use of home OCT data influences clinical decision-making by retina specialists for the management of nAMD. We compared home OCT data–informed treatment decisions with actual care decisions that were made based solely on routine in-clinic OCT scans. The variations in parameters and thresholds set by different retina specialists using remote monitoring via the in-home OCT system were also compared.
Methods
This was a retrospective review of data collected during 2 prospective observational clinical studies, the Home OCT Performance Study 11 and the Diabetic Retinopathy Clinical Research (DRCR) Retina Network Protocol AK study. The Advarra Institutional Review Board approved the study, which was conducted in accordance with the tenets of the Declaration of Helsinki.
The details of the Home OCT Performance Study have been reported, 11 and the design of the DRCR Retina Network Protocol AK study is similar. These studies recruited patients from 5 sites in Massachusetts, Missouri, Illinois, and Texas. Patients with a diagnosis of nAMD with a Snellen VA of 20/320 or better and undergoing active treatment (Home OCT Performance Study) or initiating treatment after a new diagnosis (Protocol AK) were included.
The NVHO device and a detailed user guide were delivered to each participant’s home. After setting up the device and viewing a tutorial video, participants were asked to perform daily self-imaging in each study eye for a predetermined period of time; that is, 3 months for the Home OCT Performance Study and 6 months for the DRCR Retina Network study. The captured scans were automatically uploaded by the device to a Notal Health cloud via a built-in cellular modem and then analyzed by a previously validated deep learning–based algorithm, the Notal OCT Analyzer.14,15
The results were available for remote review by a retina specialist through a password-protected, end-to-end encrypted web viewer. Fourteen of 40 eligible patients in Protocol AK started the scanning, with travel and inadequate cell reception being the main reasons they eventually declined. All 29 patients from both studies who started the program completed the full follow-up. The mean time reported for patients to scan was 42 seconds in the Performance Study and 47 seconds in Protocol AK. Patients showed high scanning adherence (5.7 scans/week in the Performance Study; 6.3 scans/week in Protocol AK). The home OCT data were not used to inform clinical decision-making on the care of enrolled patients. All patients received standard-of-care treatment per the investigator’s discretion during the study period.
Fifteen fellowship-trained retina specialists with experience ranging between 1 year and 38 years of practice after their fellowship used the Notal Home OCT Web Viewer and reviewed the home OCT data. The choice of retina specialist was made to account for diversity of experience. After selecting the study eye, retina specialists could review individual home OCT B-scans, volume scans, and Notal OCT Analyzer analytics, including retinal fluid volume trajectory over time and the retinal fluid volume map. Scans from any 2 different dates could be compared.
To prepare home OCT data for review, the previously acquired data were edited into segments consisting of continuous time periods during which the study eye received no treatment until the last day of the data segment. In other words, the data segments were between 2 treatment dates or between the program start date and the first treatment date (Figure 1). The data segments included OCT volumes, fluid thickness maps, and IRF and SRF volume trajectories over this period. Data segments of less than 30 days or with fewer than 10 scans were excluded. All available segments from the data pool that met the criterion were included. The data segments were selected to best simulate physician decision-making with home OCT data, while working within the retrospective nature of the dataset. Segments were chosen such that no actual treatments were present in the displayed segment because a treatment would result in fluid volume change. Extending data segments to the date of treatment allowed for the selection of the longest possible segments without interference of actual care treatment decisions.
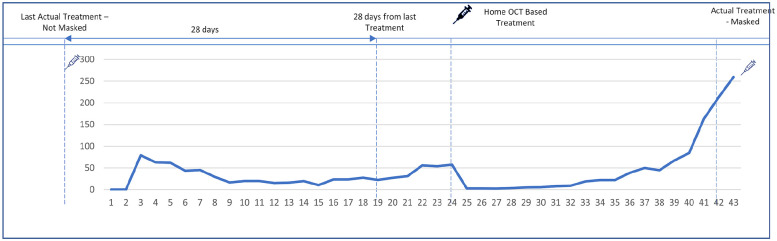
Figure 1.
Home optical coherence tomography (OCT) web viewer interface for data review. An example of a home OCT data segment is shown. Day 0 is the day the patient received the last treatment. Day 43 is the day the patient received the next treatment. The retina specialists were asked to determine whether they would offer a treatment during this time period and if so, which day and what treatment. They were only allowed to offer a treatment at least 28 days from the previous one.
In addition to the home OCT data, retina specialists were also provided with the date of the study eye’s last treatment as well as the type of anti-VEGF agent used; however, they were masked to the fact that the patient received treatment on the last day of the data segment. Each retina specialist was presented with 10 home OCT data segments and asked to decide whether and at what timepoint they would have offered anti-VEGF treatment. One common data segment was presented to all 15 retina specialists, while the other 9 segments were selected randomly from all other eligible segments. If the retina specialist decided to offer treatment, he or she was asked to select the date and medication type for treatment. Given the restrictions of on-label dosing, the treatment date had to be at least 28 days from the previous treatment.
The decisions made by the retina specialists after reviewing the home OCT data were compared with those made by the actual treating physicians. The mean and SD of the difference in treatment days between the home OCT–based decision and the regular treatment decision was calculated, including the number and proportion of cases in which the difference in treatment dates was 7 days or more. In addition, in cases in which the home OCT–based decision was to treat earlier than the actual treatment, the patient’s additional fluid burden (compared with the actual treatment schedule) was estimated as the area under the curve of the retinal fluid volume trajectory before the actual treatment. The area under the curve for the fluid volume trajectory is measured in units of nL days.
The retina specialists were asked to select eye-specific retinal fluid volume thresholds that would prompt provider notification. Fluid volumes were provided in units of nanoliters rather than from microns, which are commonly used with in-office OCT. For this part of the study, the retina specialists were presented with home OCT data from both eyes of a patient over a period of 30 days in the same previously mentioned web viewer. Available metrics included OCT volumes, fluid thickness maps, and fluid volume trajectories. Additional clinical information was provided, including recent treatment dates. After reviewing this information, the retina specialists were asked to select the level of IRF, SRF, and total retinal fluid volumes beyond which a notification would be triggered. In addition to retinal fluid volume notifications, after a certain number of days had elapsed since a patient’s last data review, retina specialists received a time-based notification reminding them to review the data.
Each retina specialist reviewed data from 3 patients; 1 common patient was included for all retina specialists to review, and 2 other patients were randomly selected from all available patients. The mean ± SD, median, IQR, and total range for all IRF, SRF, and total retinal fluid volume thresholds set by individual retina specialists were analyzed for the common patient. The same outcomes were calculated for all patients reviewed by the same retina specialist.
Results
The study included 37 eyes of 29 patients with a history of nAMD study. The mean patient age was 74.4 ± 7.6 years. Of the patients, 48.3% were women and 100% identified as non-Hispanic White.
A total of 60 segments of home OCT data were eligible for review by the retina specialists. On average, the segments lasted 45 ± 10 days (range, 30-62).
Comparison of Home OCT Data–Informed and Actual Care Treatment Decisions
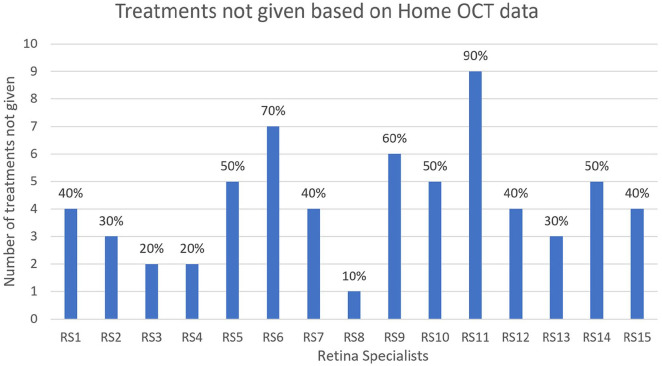
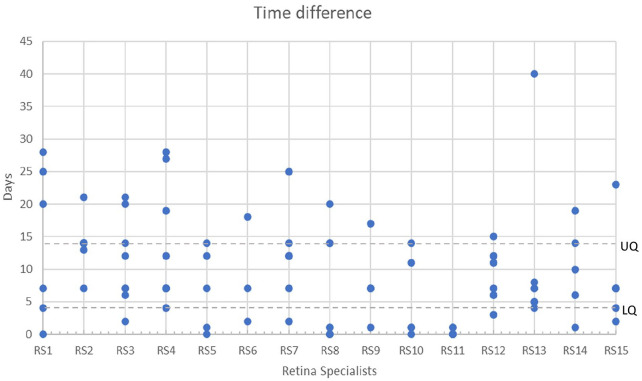
Fifteen retina specialists performed reviews of 10 data segments each from the pool of 60 available segments, resulting in a total of 150 reviews. The treatments for these reviews comprised aflibercept in 42% (63/150), ranibizumab in 30% (45/150), and bevacizumab in 28% (42/150). Different decisions were made for the timing of treatment in 149 data segments (99.3%) compared with decisions made in the patients’ actual care. More specifically, retina specialists chose not to treat 64 patients (42.7%) (95% CI, 34.7-50.6) at all during the data segment period, whereas in actual practice all eyes were treated on the last day of the data segment (Figure 2). For the remaining data segments reviewed, retina specialists advised treatment sooner than the actual treatment in 85 (98.8%) of 86 cases based on the at-home OCT data scans. For these segments, treatment was offered a mean of 10 ± 8.04 days (range, 0-40) before the actual treatment visit. In 52 (60.5%) of 86 cases, the advised treatment date preceded the actual treatment date by 7 days or more. Figure 3 shows the time difference between home OCT–guided treatment and the actual treatment in the cases in which the retina specialists decided to treat the patient earlier.
Figure 2.
The number and percentage of times when a retina specialist decided not to treat the patient based on the home optical coherence tomography (OCT) data but the patient did receive treatment in actual care based on in-clinic OCT data alone.
Figure 3.
The time difference between home OCT–guided treatment and the actual treatment in cases in which retina specialists decided to treat the patient earlier, as shown by each individual retina specialist.
Abbreviations: LQ, lower quartile; OCT, optical coherence tomography; UQ, upper quartile.
The retinal fluid burden was associated with the difference in treatment timing. The mean of the area under the curve between the home OCT–determined treatment date and the actual treatment date for total retinal fluid, IRF, and SRF was 231.2 ± 355.1 nL days, 69.1 ± 157.7 nL days, and 162.6 ± 344.3 nL days, respectively. A different anti-VEGF agent from the actual medication used was recommended in 35 (40%) of 86 cases based on home OCT review.
For the single data segment reviewed by all 15 retina specialists, 9 (60%) of 15 elected not to treat the patient during the data segment period. The 6 remaining retina specialists recommended treatment at a mean of 13.3 ± 14.2 days before the actual treatment.
Retinal Fluid Notification Thresholds
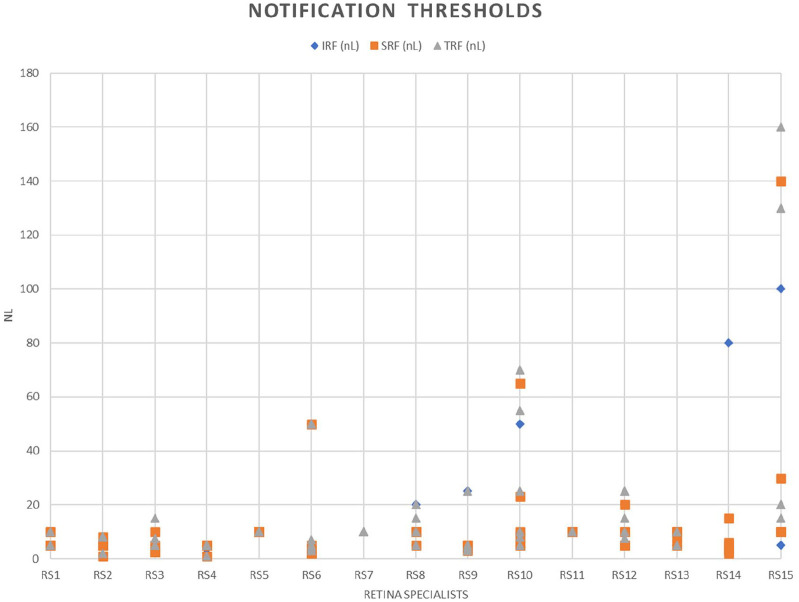
On average, after home OCT data from both eyes of a patient were reviewed, the retina specialists set the total retinal fluid notification level at a mean of 15.2 ± 24.0 nL (median, 10 nL; IQR, 5; range, 1-160). The IRF notification level was set at a mean of 9.8 ± 14.9 nL (median, 5; IQR, 5; range, 1-100). The SRF notification level was set at a mean of 10.2 ± 16.1 nL (median, 5.5; IQR, 5; range, 1-100). The retina specialists set time-based notifications at a mean of 34.7 ± 21.9 days after the last review (median, 30; IQR, 2; range, 1-180). Figure 4 shows the fluid notification levels set by each individual retina specialist.
Figure 4.
The distribution of retinal fluid notification thresholds for volume of intraretinal fluid (IRF), subretinal fluid (SRF), and total retinal fluid (TRF) as determined by 15 retina specialists across 6 different eyes.
For the 1 patient whose data were reviewed by all 15 retina specialists, the total retinal fluid notification level was set at a mean of 8.2 ± 4.3 nL (median, 8; IQR, 5; range, 1-20). The IRF notification level was set at a mean of 5.6 ± 3.1 nL (median, 5; IQR, 5.5; range, 1-10). The SRF notification level was set at a mean of 6.5 ± 3.3 nL (median, 5; IQR, 5.5; range, 1-10). The retina specialists set time-based notifications at a mean of 34.8 ± 15.4 days after the last review (median, 30; IQR, 2; range, 28-90).
To determine notification thresholds, physicians reviewed an average of 42% of individual OCT volume scans for treatment visit decisions and 39% of individual OCT volume scans.
Conclusions
Current treatment strategies for nAMD are fundamentally limited by the sparseness and accessibility of data, requiring retina specialists to optimize their treatment plans. In practice today, as-needed and treat-and-extend are the 2 most common dosing regimens. 16 In a PRONTO-style, as-needed regimen, patients are evaluated in the office setting using OCT at a fixed interval, typically monthly, and treatment is provided only when retinal fluid is detected on OCT. 17 In a treat-and-extend regimen, physicians treat patients at each visit but adjust treatment intervals based on the presence or absence of retinal fluid. Intervals are typically extended once fluid has resolved and shortened if fluid recurs or worsens.
A meta-analysis comparing these 2 treatment strategies 16 found 1.8 fewer treatments were administered under an as-needed regimen; however, numerically better visual outcomes were achieved with treat-and-extend dosing. Because the visual outcome difference was not statistically significant, neither strategy was deemed superior.
Both strategies suffer from limited temporal information on disease dynamics. Reports of real-world visual outcomes are significantly worse than those seen in controlled clinical trial environments.8,9 Home OCT monitoring has the potential to overcome these limitations. Patient-acquired, near-daily home OCT monitoring can better inform clinicians of retinal fluid trajectories and allow for a more individualized treatment plan. This theoretically avoids undertreatment by optimizing visual outcomes while avoiding overtreatment, thereby minimizing patient risk and cost to the healthcare system. Moreover, adjustments to treatment intervals would be made continually, allowing optimal control, not just initially but throughout the dynamic course of the disease.
This study found that clinical decisions made using daily home OCT data differed significantly from those made based on in-clinic OCT data. Retina specialists recommended delaying treatment, relative to the actual real-world treatment date, in more than 40% of cases. For the 1 common patient that was reviewed by all 15 retina specialists, 60% of the specialists chose to delay treatment. This is probably driven by the use of a treat-and-extend regimen in which treatment decisions are made based on the timing of previous recurrences rather than on home OCT, which can provide insights into disease dynamics immediately before the clinic visit. In addition, clinicians tend to cautiously extend intervals to avoid undertreatment, creating a tendency toward earlier treatment; however, some physicians elected earlier treatment dates. This is likely because of implementation of an as-needed regimen or the fluctuations in retinal fluid that are not evident on the date of routine in-clinic OCT imaging.
The diversity of experience of the retina specialist in the study makes the results more generalizable to the real world. Either way, our study found that there is a substantial mismatch between treatment timeline and fluctuations in retinal fluid. Both the patients’ vision and our healthcare system stand to benefit if treatments can be delivered in a more precise and personalized fashion.
Use of a home OCT-guided treatment paradigm may provide direct and indirect cost savings. Although a randomized controlled trial is required to understand the exact impact on cost, based on the current study, we attempted to estimate the overall change in patient management costs while using home OCT monitoring. We assumed the future course of the disease and the treatment frequency are not influenced by an earlier treatment or treatment withheld for estimation purposes. The cost of therapies for 150 reviewed segments was allocated using average market price for off-label drugs and Medicare-allowable rates for on-label drugs published from July to September 2023.
The costs for a single treatment with bevacizumab, ranibizumab, aflibercept, and faricimab were $68, $1183, $1751, and $2225, respectively. The total drug cost for the 150 treatments administered was estimated to be $168 084, while the total drug cost using home OCT data was estimated to be $120 675, a reduction of 28%. In 2022, the expenditure for anti-VEGF treatments on direct Medicare beneficiaries was approximately $4.6 billion. If such saving rates are generalizable, the US healthcare system could save more than $1 billion annually. 7 For this estimate to be accurate, such analysis assumes treatment distributions in the study dataset are representative and remain consistent.
Beyond the pharmaceutical costs, additional savings can be obtained based on a reduced need for procedures and diagnostic testing. Using mean values from the US Centers for Medicare & Medicaid Services fee schedule 18 for the year 2023 for codes 67028 and 92314, respectively, we estimated the injection procedure to cost $116.90 and the in-office OCT examination to cost $42.06. This equals a total cost of $158.96. Assuming an average of 6 treatment visits for nAMD per year, this amounts to additional costs of $953.76 related to in-office procedures per year per patient. As shown in this study, a 42.7% reduction would mean additional savings of $407.26 per year per patient. These estimates of costs and savings are conservative because they do not account for instances in which an additional evaluation and management fee may be applicable. Any program providing home OCT–based monitoring will also bring additional costs. The net benefit to the system will be the projected savings subtracted by the costs incurred to provide such a monitoring service.
Our study found significant variabilities in practice pattern among the 15 retina specialists. For the common home OCT segment reviewed by all retina specialists, 9 opted to not treat while 6 recommended treatment an average 13 days before the actual treatment date. This amount of variability could reflect differences in the specialists’ years in practice, tolerance to fluid on OCT, and practice pattern. These variations further reflect that the use of fluid volume–imaging biomarkers has not yet been established as standard of care; however, retina specialists did have access to full OCT volumes.
In addition, practical considerations about patient preference regarding treatment frequency are not provided in this retrospective study and can also affect such decisions. On the other hand, there was relative uniformity in the fluid volume notification thresholds. On average, a slightly higher threshold was used for SRF than for IRF. The availability of high-density home OCT volume scans with a resolution similar to in-office OCT may explain the observed consistency in threshold selection, showing that although the decision-making process for treatments can be complex and nuanced, participating retina specialists in general acted to optimize visual outcomes.
Our study has several limitations. First is its retrospective nature. Even though we detected substantial differences between home OCT–based decision-making and actual practice, the study was not designed to detect differences in visual outcomes or treatment-related costs. These would best be evaluated with a future prospective randomized controlled trial comparing a home OCT–based treatment protocol with a standardized treat-and-extend regimen. A prospective study would also allow an analysis of outcomes in patients in whom home OCT data suggested delaying care, which was not evaluable in the current study. In essence, the current study was biased toward earlier treatment compared with actual care because it was not possible to delay care beyond the date of actual treatment. In this manner, the study was more likely to find undertreatment (treating too late) than overtreatment (treating too early). Additional studies could further highlight the overall mismatch and help reduce overtreatment (thus reducing healthcare costs) and undertreatment (improving clinical outcomes).
The current study does not account for practical challenges of coordinating practice staff, caregivers, and patients to respond to home OCT data insights. In addition, the relatively small number of physicians, patients, and eyes in this study limits the generalizability of the findings to all nAMD patients in the US. The study looked at physician decision-making; however, it did not explore the reasons for their specific decisions, including those for opting for treatment, treatment type, and setting threshold levels.
In addition, the retina specialists did not have a full patient history including treatment responses, which can influence the choice of treatments. Also, the decisions were made based solely on anatomic data obtained from home OCT. Other parameters, such as changes in patients’ VA over time, can influence decision-making as well; however, these were not accounted for in this study, again limiting the generalizability of the findings. While choosing the treatment type after reviewing home OCT data, the physicians did not have limitations posed by payers in regard to the choice of drugs; thus, the difference in the types of treatment selected may not be exactly comparable with real-life treatment choices.
Last, our study is limited by its short duration. To evaluate how visual outcome can benefit from home OCT, a study of a longer duration is required.
We believe that frequent macular scans with home OCT may lead to less retinal, especially intraretinal, fluid reaccumulation without treatment over time, and as a result, potentially better long-term visual outcomes. Despite its limitations, this study provides an unprecedented understanding of how near-daily home OCT data influences physician decision-making in the management of nAMD.
Footnotes
Authors’ Note: Drs. Heier and Liu contributed equally to this work and are joint first authors.
Ethical approval: This study received an exemption by the Advarra Institutional Review Board and was conducted in accordance with the tenets of the Declaration of Helsinki. The collection and evaluation of all protected patient health information were performed in a US Health Insurance Portability and Accountability Act–compliant manner.
Statement of Informed Consent: No human subjects were recruited for this study.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: G: Grant, C: Consultant, H: Honorarium, T: Travel Support, S: Shareholder: A: Advisory or Safety Board Member, L: Leadership. JSH: 4D Molecular Therapuetics (G,C,S), Annexon (G,C), Apellis (G,C), AsclepiX Therapeutics (G,C), Ashvattha (G), Bayer (G), Cognition Therapeutics (G), Curacle (G,C), Genentech/Roche (G,C), Gyroscope Therapeutics (G,C), Iveric Bio (G,C), Janssen R&D (G,C), Kodiak (G), NGM (G,C), Novartis (G,C), OcuTerra (G,C), Perceive Bio (G), Regeneron Pharmaceuticals (G,C), RegenexBio (G,C), Abpro (C), Adverum (C,S), AffaMed Therapeutics (C), Applied Genetics Technologies Corporation (C), Akouos (C), Aviceda (C,S), Bausch + Lomb (C), Biovisics (C), Clearside (C), DTx Pharma (C,S), Exegenesis (C), Glaukos (C), Gyroscope Therapeutics (C), Immunogen (C), jCyte (C,S), Kriya (C), Nanoscope (C), Notal Vision (C), Ocular Therapeutix (C,L), Ocuphire (C,S), OliX (C), ONL Therapeutics (C), Outlook Therapeutics (C), Palatin Technologies (C), Perceive Biotherapeutics (C), Ray Therapeutics (C), RetinAI (C), RevOpsis Therapeutics (C,S), Stealth BioTherapeutics (C), Théa Pharmaceuticals (C), Vanotech (C), Aldeyra Therapeutics (S), Allegro (S), Vinci Pharmaceuticals (S), Vitranu (S); YL: Genentech (H), Horizon Therapeutics (H), Apellis Pharmaceuticals (H), Regeneron Healthcare Solutions (H), Notal Vision (T), Center for Eye Research and Education (T); NMH: Notal Vision (G,A,S);MHA: Notal (M), Alimera Science (C), Abbvie (C); KJB: Notal Vision (M), Regeneron (C,H), Genentech (C,H), Bausch & Lomb (C,H), Biogen (C); MAB: Notal Vision (M,C,H,T); MAC: Notal Vision (M,C); MJE: Notal Vision (G, T); JGF: Notal Vision (M), Roche (C, A, H), Regeneron (C, A, H); PH: Notal Vision (M), Adverum (G,C), Apellis (G,C,A), Eyepoint (G,C,H,A), Genentech (G,C,H,A), Regeneron (G), RegenxBio (G), Samsara (G), Alcon (C,A), DORC (C,A), ASRS (L); NL: Notal Vision (M), Roche (C), Regeneron (C,A), RegenxBio, (C), Ionis (C), Apellis (C,H), Annexon (C), Genentech (H,A), Iveric Bio (H), EyePoint Pharmaceuticals (A), Opthea (A); TM: Notal Vision (M); YSM: Notal Vision (C), Alimera (C), Allergan (C), Genentech (C), DORC (C), Thea (C), Zeiss (C), Iveric Bio (C), Apellis, Regeneron (C); AR: AGTC (G), Apellis Pharmaceuticals (G,C,), DRCR Retina Network (G), Roche/Genentech (G,C), Abbvie/Allergan (C), Alcon (C), Regeneron (C), Iveric Bio (C), Ocular Therapeutix (C); EWS: Notal Vision (M,C), Carl Zeiss (C); JCW: Genetech (C,A), Carl Zeiss (C); ARS: Notal Vision (M,C), RegenxBio (C), Regeneron (C), Apellis (S). The sponsor of the study is a provider of home OCT monitoring services.
Funding: The study was sponsored by Notal Vision Inc, Manassas, VA, USA. The sponsor organization participated in the design of the study, the conduct of the study, data collection, data management, data analysis, and review and approval of the manuscript.
ORCID iDs: Nancy M. Holekamp  https://orcid.org/0000-0001-7850-8515
https://orcid.org/0000-0001-7850-8515
Mohsin H. Ali  https://orcid.org/0000-0002-1077-2341
https://orcid.org/0000-0002-1077-2341
Kevin J. Blinder  https://orcid.org/0000-0003-3648-3772
https://orcid.org/0000-0003-3648-3772
Miguel A. Busquets  https://orcid.org/0000-0001-9522-8486
https://orcid.org/0000-0001-9522-8486
Michael J. Elman  https://orcid.org/0000-0001-7726-9508
https://orcid.org/0000-0001-7726-9508
Jordana G. Fein  https://orcid.org/0000-0001-7198-3823
https://orcid.org/0000-0001-7198-3823
Aleksandra Rachitskaya  https://orcid.org/0000-0001-7725-1631
https://orcid.org/0000-0001-7725-1631
References
- 1. Fleckenstein M, Keenan TDL, Guymer RH, et al. Age-related macular degeneration. Nat Rev Dis Primers. 2021;7(1):31. [DOI] [PubMed] [Google Scholar]
- 2. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-1431. [DOI] [PubMed] [Google Scholar]
- 3. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537-2548. [DOI] [PubMed] [Google Scholar]
- 4. Busbee BG, Ho C, Brown DM, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120(5):1046-1056. [DOI] [PubMed] [Google Scholar]
- 5. Heier JS, Khanani AM, Quezada Ruiz C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399(10326):729-740. [DOI] [PubMed] [Google Scholar]
- 6. García-Quintanilla L, Luaces-Rodríguez A, Gil-Martínez M, et al. Pharmacokinetics of intravitreal anti-VEGF drugs in age-related macular degeneration. Pharmaceutics. 2019;11(8):E365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel S, Sternberg P. Is there a cost benefit to the ranibizumab port delivery system? JAMA Ophthalmol. 2022;140(7):723-724. [DOI] [PubMed] [Google Scholar]
- 8. Khanani AM, Skelly A, Bezlyak V, Griner R, Torres LR, Sagkriotis A. SIERRA-AMD: a retrospective, real-world evidence study of patients with neovascular age-related macular degeneration in the United States. Ophthalmol Retina. 2020;4(2):122-133. [DOI] [PubMed] [Google Scholar]
- 9. Ho AC, Kleinman DM, Lum FC, et al. Baseline visual acuity at wet AMD diagnosis predicts long-term vision outcomes: an analysis of the IRIS registry. Ophthalmic Surg Lasers Imaging Retina. 2020;51(11):633-639. [DOI] [PubMed] [Google Scholar]
- 10. Martin DF. Evolution of intravitreal therapy for retinal diseases-from CMV to CNV: the LXXIV Edward Jackson memorial lecture. Am J Ophthalmol. 2018;191:xli-lviii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu Y, Holekamp NM, Heier JS. Prospective, longitudinal study: daily self-imaging with home OCT for neovascular age-related macular degeneration. Ophthalmol Retina. 2022;6(7):575-585. [DOI] [PubMed] [Google Scholar]
- 12. Keenan TDL, Goldstein M, Goldenberg D, Zur D, Shulman S, Loewenstein A. Daily self-imaging with patient-operated home OCT in neovascular age-related macular degeneration. Ophthalmol Sci. 2021;1(2):100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim JE, Tomkins-Netzer O, Elman MJ, et al. Evaluation of a self-imaging SD-OCT system designed for remote home monitoring. BMC Ophthalmol. 2022;22(1):261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chakravarthy U, Goldenberg D, Young G, et al. Automated identification of lesion activity in neovascular age-related macular degeneration. Ophthalmology. 2016;123(8):1731-1736. [DOI] [PubMed] [Google Scholar]
- 15. Keenan TDL, Chakravarthy U, Loewenstein A, Chew EY, Schmidt-Erfurth U. Automated quantitative assessment of retinal fluid volumes as important biomarkers in neovascular age-related macular degeneration. Am J Ophthalmol. 2021;224:267-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fang HS, Bai CH, Cheng CK. Strict pro re nata versus treat-and-extend regimens in neovascular age-related macular degeneration: a systematic review and meta-analysis. Retina. 2023;43(3):420-432. [DOI] [PubMed] [Google Scholar]
- 17. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143(4):566-583. [DOI] [PubMed] [Google Scholar]
- 18. CMS.gov. Search the physician fee schedule. 2024. Accessed February 10, 2024. https://www.cms.gov/medicare/physician-fee-schedule/search