Abstract
Background
The prevalence of bronchiectasis is significantly higher among adult Aboriginal Australians (the Indigenous peoples of Australia) compared to non-Aboriginal Australians. Currently, there is no well-established tool to assess bronchiectasis severity specific to Indigenous peoples. Nor has the applicability and validity of the two well-established bronchiectasis severity assessment tools - The “Bronchiectasis Severity Index” (BSI) and “FACED” scale been vigorously tested in an Indigenous population. This retrospective study evaluated the validity of the BSI and FACED amongst an adult Aboriginal Australian cohort with bronchiectasis in the Top End Northern Territory (NT) of Australia.
Methods
Patients with CT confirmed bronchiectasis identified between 2011 and 2020, residing in the Top End of the NT were eligible to be enrolled. The primary endpoint of 4-year mortality was assessed via hospital records, and sensitivity and specificity of the BSI and FACED assessed against this using area under the curve (AUC) receiver operating characteristics analysis. For patients with missing data, a relative BSI / FACED score was used which divided the score recorded for that patient by the total potential score based on their available clinical data.
Results
A total of 456 adult Aboriginal Australian patients >18 years of age were included (55.5% female, median age 49 years). According to the BSI score 43.4% of patients were assessed to have mild, 30.5% moderate and 26.1% severe bronchiectasis (median score 4 (IQR 2, 8)). According to the FACED 80.9% were assessed to have mild, 17.8% moderate and 1.3% severe (median score of 1 (IQR 0, 2)). Four-year mortality was 11.2% (median age of death 55.6 years). Sensitivity and specificity of the BSI combining moderate and severe were 86.3 and 47.2% respectively, and for severe alone 51% and 77%. Sensitivity and specificity of the FACED combining moderate and severe were 21.6% and 81.2%, respectively, and for severe alone 2% and 98.8%. The AUC for the continuous total BSI was 0.703, and the FACED 0.515. Utilising a relative score, based only on data available for patients with missing data (ie lung function or BMI) resulted in slightly improved AUCs for both the BSI (0.717) and FACED (0.571).
Conclusion
Both BSI and FACED bronchiectasis assessment tools may not be ideal in an Indigenous/Aboriginal people’s context. However, it may be reasonable to utilise the relative BSI score in this population until Indigenous people’s specific bronchiectasis severity assessment tools are developed.
Keywords: assessment, hospital admissions, scale, severity, spirometry, tool
Plain Language Summary
Adult Indigenous people globally have a higher prevalence of chronic respiratory disorders, and bronchiectasis is no exception. To assess the bronchiectasis severity and to predict future mortality, there are well-established assessment tools. However, the existing bronchiectasis assessment tools are developed predominately from data gathered from non-Indigenous population cohorts. To date, it is unclear if these existing bronchiectasis assessment tools are appropriate or applicable for Indigenous people. Therefore, this study assessed how existing bronchiectasis tools, namely the “Bronchiectasis Severity Index” (BSI) and “FACED” [Forced expiratory volume in 1 s, Age, Chronic colonization, Extension, and Dyspnea] fit for an adult Indigenous/ Aboriginal Australian cohort diagnosed to have bronchiectasis. The results of the study showed that both BSI and FACED assessment tools may not be ideal in the Australian Indigenous/Aboriginal population, due to population demographics and other social determinants, including geographical isolation. Hence, further research is warranted in developing Indigenous/Aboriginal specific bronchiectasis assessment tools.
Introduction
The prevalence of bronchiectasis among adult Aboriginal Australians is estimated to be up to 19.4/1000 people1 and is associated with significant hospital admission rates and mortality.2–8 In order to predict disease severity, exacerbations and mortality among patients with bronchiectasis, there are two well-established and validated tools that are utilised widely in day-to-day clinical practice worldwide, namely; the bronchiectasis severity index (BSI) and the FACED scale [Forced expiratory volume in 1 s (FEV1), Age, Chronic colonization, Extension, and Dyspnea].9,10 Moreover, both of these tools have been endorsed to be used in the Australian setting via “The Bronchiectasis Toolbox”.11 The clinical variables utilised in the BSI and FACED tools include: Age; Body mass index (BMI); forced expiratory volume in 1 second (FEV1); respiratory tract colonization by pseudomonas aeruginosa and other microorganisms; radiological extension/severity; previous exacerbation/hospitalisations; and dyspnoea/Medical Research Council (MRC) Breathlessness Score.
Nevertheless, the applicability and validity of the parameters utilised in the BSI and FACED tools within an Indigenous population with bronchiectasis is questionable for various reasons and their validity has never been tested (from here on “Indigenous” is used to refer to global First nations people/populations, while “Aboriginal Australian/ population/patients/people” is used to specifically to refer to Australia’s First Nations people). First and foremost, a greater proportion of Aboriginal Australians, as well as Indigenous people worldwide, reside in remote and rural communities, hence, access to chest computed tomography (CT) is very limited12 – therefore assessment of the radiological extent of disease is challenging, especially at the primary care level where the majority of patients present. More broadly, substantial chest radiology (CT) data is lacking among the Aboriginal Australian populations.13 Therefore, health practitioners are very much reliant on basic modalities like chest X-Ray in the diagnosis and management of patients with bronchiectasis. Secondly, spirometry reference norms are not well established for Indigenous people. Among adult Aboriginal Australians, the forced vital capacity (FVC) and FEV1 are reported to be up to 20% lower than for their Caucasian counterparts14 in both apparently healthy patients, and those with known chronic obstructive pulmonary disease (COPD).15 Moreover, FEV1 values are noted to be as low as 36% predicted among Aboriginal Australian patients with bronchiectasis with a restrictive impairment being the most commonly observed pattern.16 Furthermore, acceptability, repeatability and the validity of spirometry tests may impose an issue as well.17 Therefore, it is reasonable to assume that using spirometry criteria, such as the FEV1 threshold recommended in the FACED and BSI tools may present a significant barrier to the accurate assessment of bronchiectasis severity in Indigenous patients. Thirdly, data pertaining to sputum microbiology is nearly non-existent for adult Indigenous patients with bronchiectasis. Hence, how important colonization with pseudomonas aeruginosa or other micro-organisms is in an Indigenous population with bronchiectasis is not known. A recent study showcased that colonisation with fungal species was associated with increased rates of hospitalisation and mortality among Aboriginal Australians, in addition to the known increased risk associated with pseudomonas.8 Fourth, measurement of dyspnoea by the MRC dyspnoea scale has not yet been shown as an accurate or valid assessment of symptoms and daily burden of respiratory disease among Indigenous populations. In the remote context, physical disability among Aboriginal Australians is more than often measured on the ability to undertake activities in the community setting such as participating in ceremonial activities or hunting – factors not utilised in the MRC dyspnoea scale – and thus the cultural appropriateness and validity is questionable. Finally, adopting the age cut off criteria used in both BSI and FACED scores presents a significant problem in Indigenous populations. For the majority of Indigenous patients, bronchiectasis is a lifelong disease diagnosed either in childhood and accompanied by poor transition of care into adulthood, or at a much younger age in adulthood.18,19 Aboriginal Australian patients with bronchiectasis also die significantly younger (median age at death 60 years) in comparison to non-Indigenous patients.1,4 Hence, up to half of Aboriginal Australian patients may be deceased before their age is even considered a relevant factor in the existing severity tools. Thus, adopting the recommended age criteria would spuriously lower the overall bronchiectasis severity scores or may not be applicable at all. These factors combined suggest an urgent need to assess the applicability and validity of the BSI and FACED tools, which have not been tested vigorously in the past among Indigenous patients with bronchiectasis, more specifically among adult Aboriginal Australians. Therefore, in this study, we evaluated the sensitivity and specificity of the BSI and FACED amongst an adult Aboriginal Australian cohort diagnosed to have bronchiectasis over a ten-year period (2011–2020) from the Top End Health Service (TEHS) region of the Northern Territory (NT) of Australia.
Methods
Setting and Study Participants
Approximately 3.3% of Australians self-identify as Aboriginal and/or Torres Strait Islanders. The NT is an Australian federal territory occupying the central-northern region of Australia. The TEHS region within the NT covers approximately 35% or 475,338 km2 of the total area of the NT and contains an estimated adult population (>18 years) of 129,000 people, representing almost 80% of the total NT adult population. In the TEHS region, 22% of the adult population are Aboriginal Australians, the highest proportion compared to all other Australian states and Territory, of whom approximately 77% to 80% reside in remote or very remote communities as defined by the Australian Statistical Geographic Standard Level 4 or Level 5.20,21 This study was conducted at the respiratory and sleep division based at the Royal Darwin Hospital, a university affiliated tertiary care teaching hospital in the TEHS region of NT and Darwin Respiratory and Sleep Health, Darwin Private Hospital. All adult Aboriginal patients aged 18 years and over with chest CT confirmed bronchiectasis from our previous study (between 2011 and 2020) in the TEHS, NT regions were included.1 Further details of the study setting and study participants included in this study are detailed in our recent reports from our centre.1,8
Ethics
This study is a part of a larger research project examining various clinical profiles of adult Aboriginal Australians with bronchiectasis and was approved by the human research ethics governance/committee of the TEHS, NT and Menzies School of Health Research (Reference: HREC; 2019–3547). This study complies with the Declaration of Helsinki. Individual patient consent was not required, as it was a retrospective study and no active interventions were undertaken and the need for individual consent was waived by the Human Research Ethics governance/committee.
Bronchiectasis Severity Assessment Data
The BSI is calculated from the following parameters: Age (maximum 6 points), BMI (2 points), FEV1 (3 points), significant hospital admission before the study (5 points), exacerbations before the study (2 points), MRC dyspnoea score (3 points), pseudomonas colonisation (3 points), colonisation with other organisms (1 point) and radiological severity (1 point).9 Thus, the maximum score for the BSI is 26 points, with breakdown to mild (score 0–4), moderate (score 5–8) and severe (score ≥9). Due to the reasons outlined above, MRC dyspnoea data is not routinely collected in this study population and therefore was not available for our cohort, thus the maximum BSI score was 23. Furthermore, BMI data and lung function data (FEV1) were not available for all patients, as these data were collected only for patients undergoing lung function testing, which not all patients did – thus for many patients, the maximum potential score was 18. As such, we used both the absolute BSI score (ie score out of 26 possible points) to define the severity level, and also calculated a relative score as a percentage of the total score possible from the available data (ie if a patient scored 10 but did not have FEV1, BMI or MRC dyspnoea available, their potential maximum score was only 18, thus 10/18 = 55.6%). Within the relative score, mild bronchiectasis was defined as <16%, moderate 16–31% and severe >31% in line with the relative thresholds used in the traditional BSI.
The FACED is calculated from FEV1 (2 points), age (2 points), pseudomonas colonisation (1 point), radiological extent (1 point) and dyspnoea (1 point).10 Thus, the maximum score of the FACED is 7, with a breakdown into mild (score 0–2), moderate (score 3–4) and severe (score ≥5) Again, we did not have dyspnoea data available; thus, the maximum score in our cohort was 6, and as lung function data was not available for many patients, their maximum score was 4. Similar to the BSI, we therefore calculated a relative score, with mild bronchiectasis defined as 0–41%, moderate as 42–70% and severe as ≥71% in line with the relative thresholds used in the traditional FACED.
In the current cohort, as participants were diagnosed with bronchiectasis at different timepoints, due to the high prevalence of bronchiectasis among children and the poor transition of care into adulthood, all patients were assumed to have bronchiectasis at the beginning of the study period (1 January 2011) or if they entered into the study at a later point upon turning 18 years of age, they were also assumed to have bronchiectasis already present. As the BSI uses a 2-year prior exacerbation history, we defined a census date for our cohort as 1 January 2013, or when the patient reached 20 years of age if they were enrolled at the point when they turned 18. Patients who were deceased prior to this census date were excluded from the analysis. Hospital admissions were restricted to respiratory-related presentations with an International Classification of Diseases (ICD) code “J” and related sub-class. Significant prior hospitalisation was defined as any respiratory-related hospitalisation of at least 24 hours in length within the 2 years prior to the census date. Community/primary care level exacerbation data were not collected, and therefore to calculate the number of exacerbations in the past year we summed hospital presentations under ICD code J47 specifically, in the 1 year prior to the census date. Pseudomonas colonisation, or colonisation with other organisms were defined as any recording of pseudomonas or other organisms within the 10-year period of data collection. This differs somewhat from the definitions used in the BSI or FACED, however due to this study being based on retrospective audit data, we were unable to assess specific temporal patterns of micro-organism colonisation. Patients were excluded if they were deceased prior to the imposed census date (n = 3). Our primary outcomes were mortality and respiratory related hospitalisation in 4 years post the defined census date as was used in the original BSI derivation study.9 Our secondary outcomes were mortality and respiratory related hospitalisation at one, two, three and 5 years post the defined census date, with these time periods chosen to also replicate the varied follow-up times used in the validation of the BSI where follow-up duration ranged from 12 months to a mean 49 months,9 and to match validation studies of the FACED, which followed patients for up to 5 years.10 Mortality and hospitalisation data, used as the reference to define sensitivity and specificity of the BSI and FACED, and all clinical data (lung function tests, sputum results, demographics etc) were extracted for patients from the Royal Darwin Hospital electronic medical records.
Statistical Analyses
All data was presented as median (interquartile range (IQR)) or number (%). The individual effects of the BSI and FACED categories on four-year mortality and hospitalisations were assessed by a univariate Poisson model with robust error variances reporting relative risk ratios (RRs) and 95% confidence intervals (CIs). The validity of absolute and relative BSI and FACED score categories in predicting mortality and hospitalisations were assessed as 1) moderate against mild, 2) severe against mild, 3) combined moderate and severe against mild, and 4) severe against combined moderate and mild using the aforementioned Poisson model in addition to area under the curves (AUCs) (95% CIs) of receiver operating characteristics (ROCs). Due to the large proportion of patients missing BMI and lung function data, a secondary assessment was carried out for patients with complete and incomplete data separately and presented in the Supplementary materials 1–3. Alpha was set to 0.05 throughout, and all analyses were conducted in STATA IC 15 (CollegeStation, Texas).
Results
Clinical and Severity Scale Assessment
Of the 456 patients included for analysis, the majority were female (55.5%), resided in remote areas (92.5%) and had comorbid COPD (82.7%) or hypertension (62.9%) (Table 1). Most (n = 287 (62.9%)) were missing BMI and lung function data, which heavily influenced the absolute BSI and FACED scoring. Overall, according to the absolute BSI score, 43.4% of patients were categorised as having mild bronchiectasis, 30.5% as moderate and 26.1% as severe with a median score of 4 (IQR 2, 8) (Table 2). When utilising the relative scoring these proportions reversed, with 27.9% categorised as mild, 28.5% as moderate and 43.6% as severe with a median score of 27.8% (IQR 11.1, 44.4%). According to the absolute FACED score 80.9% of patients were categorised as mild, 17.8% as moderate and 1.3% as severe with a median score of 1 (IQR 0, 2). Using the relative FACED score, 70.8% were categorised as mild, 25.4% as moderate and 3.7% as severe with a median score of 25% (IQR 0, 50%). Utilising the relative scoring appeared to more evenly categorise patients with the BSI, however in the FACED there remained significant proportion classified as ‘mild’ severity in both absolute and relative scores.
Table 1.
Included Cohort’s Demographic and Clinical Characteristics
| Clinical Parameters | Total Cohort (n=456) |
|---|---|
| Age (years) | 48.84 (41.29, 58.09) |
| Sex (female) | 253 (55.5%) |
| Remote residence* | 422 (92.5%) |
| BMI (kg/m2) | 23.1 (19.38, 27) |
| Underweight (BMI >18.5 kg/m2) | 35 (20.7%) |
| Normal weight (BMI 18.5–24.9 kg/m2) | 72 (42.6%) |
| Overweight (BMI 25–29.9 kg/m2) | 34 (20.1%) |
| Obese (BMI ≥ 30 kg/m2) | 28 (16.6%) |
| FEV1 (%) | 38 (28, 52) |
| FVC (%) | 50 (40, 64) |
| FEV1/FVC | 0.65 (0.49, 0.75) |
| Hospitalisation past 2 years | 161 (35.3%) |
| Pseudomonas spp. | 141 (30.9%) |
| Non-Aspergillus fungi | 27 (5.9%) |
| Moraxella spp. | 117 (25.7%) |
| Staphylococcus spp. | 63 (13.8%) |
| Streptococcus spp. | 149 (32.7%) |
| Aspergillus spp. | 37 (8.1%) |
| Bilateral radiological extent | 337 (73.9%) |
| Lobes affected | 2 (1, 3) |
| COPD | 377 (82.7%) |
| Hypertension | 287 (62.9%) |
| Diabetes | 221 (48.5%) |
| Coronary artery disease | 158 (34.7%) |
| Asthma | 117 (25.7%) |
| Four-year mortality | 51 (11.2%) |
| Age of death (years) | 55.59 (47.52, 63.2) |
| Four-year hospitalisation | 311 (68.2%) |
| BSI | 5 (2, 9) |
| FACED | 1 (0, 2) |
Notes: Data presented as median (IQR) or number (%). *Remote residence defined as residing in Australian statistical geographic standard areas 4 or 5 [21].
Abbreviations: BMI, body mass index; FEV1; Forced expiratory volume in one second; FVC, Forced vital capacity; COPD, Chronic obstructive pulmonary disease; BSI, Bronchiectasis severity index; FACED, (F: forced expiratory volume in 1 s [FEV1]; A: age; C: chronic colonization by Pseudomonas aeruginosa [PA], E: radiological extension [number of pulmonary lobes affected], and D: dyspnea).
Table 2.
BSI Score Breakdowns for Total Cohort, Including Proportion of Patients with 4-Year Mortality or 4-Year Hospitalisation and Risk Ratios
| Total Cohort (n, %) | Mortality (n, %) | Relative Risk (RR, CI) | Hospitalisation (n, %) | Relative Risk (RR, CI) | |
|---|---|---|---|---|---|
| Age | |||||
| - <50 years | 249 (54.6%) | 19 (7.6%) | Reference | 160 (64.3%) | Reference |
| - 50–69.9 years | 176 (38.6%) | 23 (13.1%) | 1.71 (0.96, 3.05) | 125 (71%) | 1.11 (0.97, 1.26) |
| - 70–79.9 years | 27 (5.9%) | 6 (22.2%) | 2.91 (1.27, 6.67)* | 22 (81.5%) | 1.27 (1.04, 1.55)* |
| - ≥80 years | 4 (0.9%) | 3 (75%) | 9.83 (4.82, 20.05)* | 4 (100%) | 1.56 (1.42, 1.71)* |
| BMI | |||||
| - BMI ≥18.5 kg/m2 | 134 (79.3%) | 5 (3.7%) | Reference | 90 (67.2%) | Reference |
| - BMI <18.5 kg/m2 | 35 (20.7%) | 3 (8.6%) | 2.3 (0.57, 9.19) | 27 (77.1%) | 1.15 (0.93, 1.43) |
| FEV1 | |||||
| - FEV1 ≥ 80% | 3 (1.8%) | 0 (0%) | – | 2 (66.7%) | Reference |
| - FEV1 50–79.9% | 49 (29%) | 3 (6.1%) | Reference^ | 24 (49%) | 0.73 (0.31, 1.72) |
| - FEV1 30–49.9% | 68 (40.2%) | 2 (2.9%) | 0.48 (0.08, 2.78) | 52 (76.5%) | 1.15 (0.51, 2.59) |
| - FEV1 < 30% | 49 (29%) | 3 (6.1%) | 1 (0.21, 4.74) | 39 (79.6%) | 1.19 (0.53, 2.7) |
| Significant hospitalisation in past 2 years | |||||
| - No | 295 (64.7%) | 16 (5.4%) | Reference | 150 (50.8%) | Reference |
| - Yes | 161 (35.3%) | 35 (21.7%) | 4.01 (2.29, 7.02)* | 161 (100%) | 1.97 (1.76, 2.2)* |
| Exacerbations in past year | |||||
| - < 3 | 448 (98.3%) | 49 (10.9%) | Reference | 303 (67.6%) | Reference |
| - ≥ 3 | 8 (1.8%) | 2 (25%) | 2.29 (0.67, 7.82) | 8 (100%) | 1.48 (1.39, 1.58)* |
| Pseudomonas colonisation | |||||
| - No | 315 (69.1%) | 25 (7.9%) | Reference | 191 (60.6%) | Reference |
| - Yes | 141 (30.9%) | 26 (18.4%) | 2.32 (1.39, 3.88)* | 120 (85.1%) | 1.4 (1.25, 1.57)* |
| Other culture colonisation | |||||
| - No | 79 (17.3%) | 4 (5.1%) | Reference | 36 (45.6%) | Reference |
| - Yes | 377 (82.7%) | 47 (12.5%) | 2.46 (0.91, 6.64) | 275 (72.9%) | 1.6 (1.25, 2.05)* |
| Radiological extent | |||||
| - <3 lobes and cystic type not reported | 272 (59.7%) | 31 (11.4%) | Reference | 190 (69.9%) | Reference |
| - ≥3 lobes or cystic type reported | 184 (40.4%) | 20 (10.9%) | 0.95 (0.56, 1.62) | 121 (65.8%) | 0.94 (0.83, 1.07) |
| Severity (absolute) | |||||
| - Mild | 198 (43.4%) | 7 (3.5%) | Reference | 84 (42.4%) | Reference |
| - Moderate | 139 (30.5%) | 18 (12.9%) | 3.66 (1.57, 8.54)* | 112 (80.6%) | 1.9 (1.58, 2.28)* |
| - Severe | 119 (26.1%) | 26 (21.8%) | 6.18 (2.77, 13.81)* | 115 (96.6%) | 2.28 (1.93, 2.69)* |
| Severity (relative) | |||||
| - Mild | 127 (27.9%) | 2 (1.6%) | Reference | 52 (40.9%) | Reference |
| - Moderate | 130 (28.5%) | 9 (6.9%) | 4.4 (0.97, 19.98) | 71 (54.6%) | 1.33 (1.03, 1.73)* |
| - Severe | 199 (43.6%) | 40 (20.1%) | 12.76 (3.13, 51.97)* | 188 (94.5%) | 2.31 (1.87, 2.85)* |
Notes: Data presented as number (%) or RR (95% CI). *Indicates statistical significance at p<0.05. ^No hospitalisations were recorded among patients with an FEV1 ≥ 80%, therefore the reference category was changed to FEV1 50–79.9% in this instance.
Abbreviations: BMI, Body mass index; FEV1, Forced expiratory volume in one second; RR, Relative risk; CI, Confidence interval.
BSI Assessment
The majority of patients received no score in the BSI based on age as they were <50 years (54.6%) (Table 2). The BSI age categorisation was associated with increased risk of both mortality and hospitalisations, however there was no significant difference in risk between the 50–69.9 year and 70–79.9-year age groups, nor between the 70–79.9 and ≥80-year age groups. Data and risk ratios for patients with complete or incomplete data separately are shown in Supplement 1. Among patients with BMI available there was no significant effect of being underweight on either mortality (RR 2.3 (95% CI 0.57, 9.19), p = 0.240) or hospitalisations (RR 1.15 (95% CI 0.93, 1.43), p = 0.210). Two thirds of the cohort had an FEV1 <50% predicted, however more severe FEV1 categorisation was not associated with either mortality or hospitalisations. One-third of patients had a prior significant hospitalisation, which was associated with a significantly increased risk of mortality (RR 4.01 (95% CI 2.29, 7.02), p < 0.001). Furthermore, all patients who had a previous significant hospitalisation were hospitalised again within 4 years (RR 1.97 (95% CI 1.76, 2.2)), p < 0.001). A higher frequency of previous exacerbations (>3 per year) did not show any significant association with mortality (RR 2.29 (95% CI 0.67, 7.82), p = 0.188), though all patients with frequent exacerbations (>3 per year) were hospitalised within 4 years (n = 8) (RR 1.48 (95% CI 1.39, 1.58), p < 0.001). Colonisation with pseudomonas spp. was significantly associated with both mortality and hospitalisations, while colonisation with other pathogenic organisms was only associated with hospitalisations. Radiological extent did not show any significant association with either mortality or hospitalisations.
BSI Severity to Mortality and Hospitalisations
There was a significant effect of both absolute and relative BSI severity on four-year mortality and hospitalisations (Table 2). Moderate and severe categorisation showed increased risk of both mortality and hospitalisation compared to mild categorisation by the absolute BSI score. There was no significant difference in risk of mortality between the Moderate and Severe categories (p = 0.062), however there was an increased risk of hospitalisation (p < 0.001). Using the relative BSI score, there was no significant increase in risk of mortality between Moderate and Mild categorisation, though there was a significant increase in risk between Severe and Mild (p < 0.001). For hospitalisations, however, there was a significant increase in risk between mild and moderate (p < 0.001) and between moderate and severe (p < 0.001).
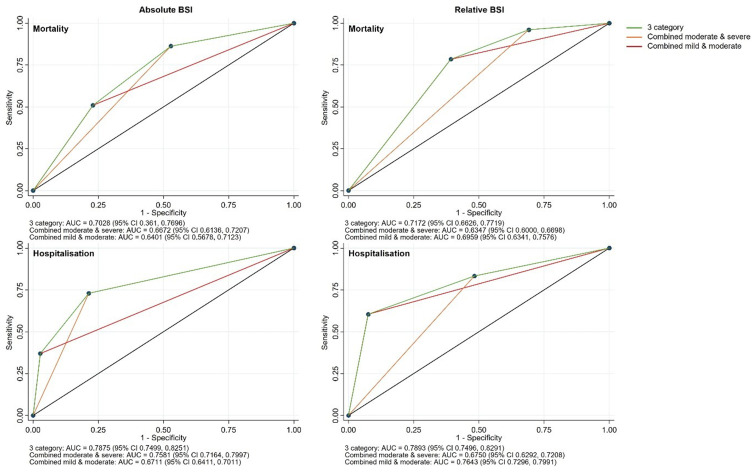
The AUC of the absolute score for mortality was 0.703 (95% CI 0.636, 0.770) with sensitivity and specificity for combined moderate and severe at 86.3% and 47.2%, respectively, and for severe alone at 51% and 77%, respectively (Figure 1). The AUC of the relative score for mortality was 0.717 (95% CI 0.663, 0.772) with sensitivity and specificity for combined moderate and severe at 96.1% and 30.9%, respectively, and for severe alone at 78.4% and 60.7%, respectively. The AUC of the absolute score for hospitalisations was 0.788 (95% CI 0.750, 0.825) with sensitivity and specificity for combined moderate and severe at 73% and 78.6%, respectively, and for severe alone at 37% and 97.2%, respectively. The AUC of the relative score for hospitalisations was 0.789 (95% CI 0.750, 0.829) with sensitivity and specificity for combined moderate and severe at 83.3% and 51.7%, respectively, and for severe alone at 60.5% and 92.4%, respectively.
Figure 1.
AUCs of absolute and relative BSI categorisation against four-year mortality and hospitalisations.
Secondary Outcome Analyses for BSI Assessment
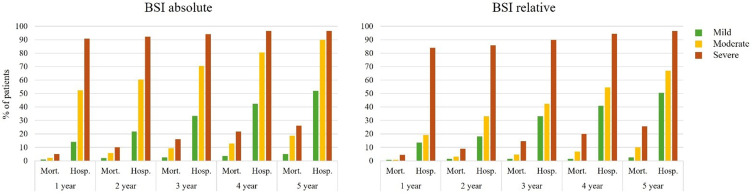
Relative risk for mortality was significantly increased among patients categorised as severe via the absolute BSI compared to both mild alone, and moderate and mild combined at 1–5 years of follow-up (Figure 2 and Table 3). Among patients categorised as moderate alone or moderate and severe combined compared to mild, the risk for mortality was significantly increased at years 3–5 and 2–5, respectively. There was a significantly increased risk of hospitalisations at years 1–5 for all categorisations.
Figure 2.
Mortality and hospitalisations at 1–5 years post-census date by BSI severity categorisation.
Table 3.
Risk Ratios for Mortality and Hospitalisation at 1–5 Years of Follow Up Post Census Date by Absolute and Relative BSI Category
| BSI Category | BSI Severity | 1 Year (RR, CI) | 2 Year (RR, CI) | 3 Year (RR, CI) | 4 Year (RR, CI) | 5 Year (RR, CI) |
|---|---|---|---|---|---|---|
| Absolute BSI; Mortality | Moderate vs Mild | 2.14 (0.36, 12.64) | 2.85 (0.87, 9.29) | 3.7 (1.35, 10.16)* | 3.66 (1.57, 8.54)* | 3.7 (1.84, 7.44)* |
| Severe vs Mild | 4.99 (1.02, 24.38)* | 4.99 (1.65, 15.14)* | 6.32 (2.42, 16.51)* | 6.18 (2.77, 13.81)* | 5.16 (2.62, 10.14)* | |
| Moderate & Severe vs Mild | 3.45 (0.75, 15.83) | 3.84 (1.33, 11.06)* | 4.91 (1.95, 12.39)* | 4.82 (2.22, 10.49)* | 4.37 (2.29, 8.35)* | |
| Severe vs Moderate & Mild | 3.4 (1.06, 10.94)* | 2.83 (1.31, 6.14)* | 2.99 (1.62, 5.5)* | 2.95 (1.77, 4.9)* | 2.44 (1.58, 3.76)* | |
| Relative BSI; Mortality | Moderate vs Mild | 0.98 (0.06, 15.5) | 1.95 (0.36, 10.5) | 2.93 (0.6, 14.28) | 4.4 (0.97, 19.98) | 4.23 (1.23, 14.52)* |
| Severe vs Mild | 5.74 (0.73, 44.89) | 5.74 (1.35, 24.37)* | 9.25 (2.24, 38.17)* | 12.76 (3.13, 51.97)* | 10.85 (3.46, 34.06)* | |
| Moderate & Severe vs Mild | 3.86 (0.5, 29.92) | 4.25 (1.01, 17.82)* | 6.76 (1.65, 27.72)* | 9.46 (2.33, 38.37)* | 8.24 (2.63, 25.77)* | |
| Severe vs Moderate & Mild | 5.81 (1.27, 26.64)* | 3.87 (1.57, 9.59)* | 4.68 (2.19, 10.02)* | 4.7 (2.47, 8.92)* | 4.12 (2.42, 7)* | |
| Absolute BSI; Hospitalisation | Moderate vs Mild | 3.71 (2.54, 5.42)* | 2.78 (2.07, 3.75)* | 2.12 (1.69, 2.65)* | 1.9 (1.58, 2.28)* | 1.73 (1.5, 2)* |
| Severe vs Mild | 6.42 (4.53, 9.09)* | 4.26 (3.25, 5.57)* | 2.82 (2.31, 3.46)* | 2.28 (1.93, 2.69)* | 1.86 (1.62, 2.13)* | |
| Moderate & Severe vs Mild | 4.96 (3.49, 7.06)* | 3.46 (2.63, 4.55)* | 2.44 (1.99, 3)* | 2.07 (1.75, 2.45)* | 1.79 (1.56, 2.05)* | |
| Severe vs Moderate & Mild | 3.03 (2.55, 3.6)* | 2.45 (2.12, 2.84)* | 1.93 (1.72, 2.18)* | 1.66 (1.51, 1.83)* | 1.43 (1.32, 1.55)* | |
| Relative BSI; Hospitalisation | Moderate vs Mild | 1.44 (0.82, 2.53) | 1.83 (1.17, 2.85)* | 1.28 (0.93, 1.76) | 1.33 (1.03, 1.73)* | 1.33 (1.08, 1.64)* |
| Severe vs Mild | 6.27 (4.01, 9.8)* | 4.74 (3.26, 6.9)* | 2.72 (2.11, 3.5)* | 2.31 (1.87, 2.85)* | 1.91 (1.61, 2.28)* | |
| Moderate & Severe vs Mild | 4.36 (2.77, 6.85)* | 3.59 (2.46, 5.24)* | 2.15 (1.66, 2.78)* | 1.92 (1.55, 2.39)* | 1.68 (1.41, 2.01)* | |
| Severe vs Moderate & Mild | 5.14 (3.87, 6.82)* | 3.35 (2.7, 4.15)* | 2.38 (2.02, 2.81)* | 1.97 (1.73, 2.25)* | 1.64 (1.48, 1.83)* |
Notes: *Denotes statistically significant difference at p<0.05. Data displayed as RR (95% CI).
Abbreviations: BSI, Bronchiectasis severity index; RR, Relative risk; CI, Confidence interval.
Relative risk for mortality was significantly increased among patients categorised as severe via the relative BSI compared to mild alone at 2–5 years follow-up and compared to moderate and mild combined at 1–5 years of follow-up (Figure 2 and Table 3). Among patients categorised as moderate alone, there was an increased risk of mortality only at 5 years of follow-up, while among those categorised as moderate and severe combined, there was an increased risk at 2–5 years follow-up.
FACED Assessment
The majority of patients received no score in the FACED based on age as they were <70 years (93.2%) (Table 4). However, FACED age categorisation did show a significant association with both mortality and hospitalisations (both p < 0.001). More severe FEV1 categorisation was not associated with mortality (p = 0.674), however was associated with increased risk of hospitalisation (p = 0.003). Colonisation with pseudomonas spp. was significantly associated with both mortality and hospitalisations, while radiological extent did not show any significant association with either mortality or hospitalisations. The majority of patients were categorised as having mild severity by the absolute FACED score (80.9%), with only six patients (1.3%) categorised as being severe. Using the relative FACED score slightly alleviated this bias, however 70.8% remained classified as having mild bronchiectasis.
Table 4.
FACED Score Breakdowns for Total Cohort, Including Proportion of Patients with 4-Year Mortality or 4-Year Hospitalisation and Risk Ratios
| Total Cohort (n, %) | Mortality (n, %) | Relative Risk (RR, CI) | Hospitalisation (n, %) | Relative Risk (RR, CI) | |
|---|---|---|---|---|---|
| FEV1 | |||||
| - FEV1 ≥ 50% | 52 (30.8%) | 3 (5.8%) | Reference | 26 (50%) | Reference |
| - FEV1 < 50% | 117 (69.2%) | 5 (4.3%) | 0.74 (0.18, 3) | 91 (77.8%) | 1.56 (1.16, 2.08)* |
| Age | |||||
| - < 70 years | 425 (93.2%) | 42 (9.9%) | Reference | 285 (67.1%) | Reference |
| - ≥ 70 years | 31 (6.8%) | 9 (29%) | 2.94 (1.58, 5.47)* | 26 (83.9%) | 1.25 (1.06, 1.48)* |
| Pseudomonas colonisation | |||||
| - No | 315 (69.1%) | 25 (7.9%) | Reference | 191 (60.6%) | Reference |
| - Yes | 141 (30.9%) | 26 (18.4%) | 2.32 (1.39, 3.88)* | 120 (85.1%) | 1.4 (1.25, 1.57)* |
| Radiological extent | |||||
| - < 2 lobes | 290 (63.6%) | 31 (10.7%) | Reference | 199 (68.6%) | Reference |
| - ≥ 3 lobes | 166 (36.4%) | 20 (12%) | 1.13 (0.66, 1.91) | 112 (67.5%) | 0.98 (0.86, 1.12) |
| Severity (absolute) | |||||
| - Mild | 369 (80.9%) | 40 (10.8%) | Reference | 240 (65%) | Reference |
| - Moderate | 81 (17.8%) | 10 (12.3%) | 1.14 (0.59, 2.18) | 66 (81.5%) | 1.25 (1.1, 1.42)* |
| - Severe | 6 (1.3%) | 1 (16.7%) | 1.54 (0.25, 9.44) | 5 (83.3%) | 1.28 (0.89, 1.85) |
| Severity (relative) | |||||
| - Mild | 323 (70.8%) | 31 (9.6%) | Reference | 198 (61.3%) | Reference |
| - Moderate | 116 (25.4%) | 13 (11.2%) | 1.17 (0.63, 2.15) | 97 (83.6%) | 1.36 (1.21, 1.54)* |
| - Severe | 17 (3.7%) | 7 (41.2%) | 4.29 (2.22, 8.3)* | 16 (94.1%) | 1.54 (1.33, 1.78)* |
Notes: Data displayed as number (%) and RR (95% CI). *Denotes statistically significant association at p<0.05.
Abbreviations: FACED, Forced expiratory volume in 1 s (FEV1), Age, Chronic colonization, Extension, and Dyspnea; FEV1, Forced expiratory volume in one second; RR, Relative risk; CI, Confidence interval.
FACED Severity to Mortality and Hospitalisations
There was no significant effect of absolute FACED score on mortality (Table 4). Moderate categorisation via the absolute FACED showed increased risk of hospitalisation compared to mild categorisation (RR 1.25, (95% CI 1.1, 1.42), p = 0.001); however, there was no significant association with severe categorisation. Using the relative FACED score, there was a significant increase in risk of mortality between severe and mild categorisation (p < 0.001), though not moderate and mild (p = 0.620). For hospitalisations, however, there was a significant increase in risk between moderate and mild (p < 0.001) and between severe and mild (p < 0.001) but not between severe and moderate (p = 0.107).
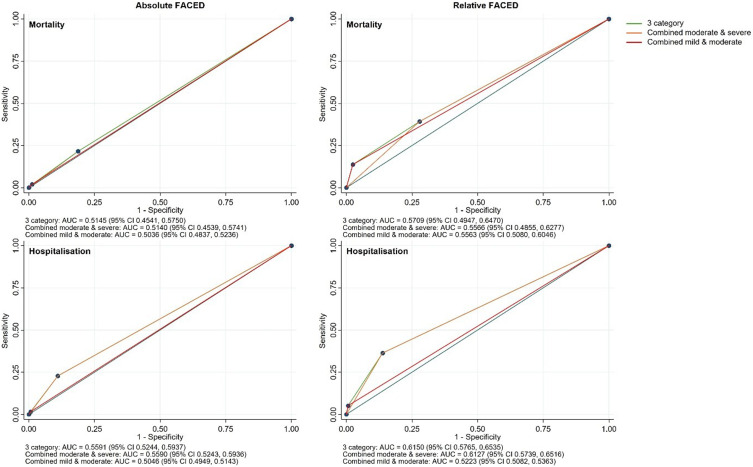
The AUC of the absolute FACED for mortality was 0.515 (95% CI 0.454, 0.575) with sensitivity and specificity for combined moderate and severe at 21.6% and 81.2%, respectively, and for severe alone at 2% and 98.8%, respectively (Figure 3). The AUC of the relative FACED for mortality was 0.571 (95% CI 0.495, 0.647) with sensitivity and specificity for combined moderate and severe at 39.2% and 72.1%, respectively, and for severe alone at 13.7% and 97.5%, respectively. The AUC of the absolute FACED for hospitalisations was 0.559 (95% CI 0.524, 0.594) with sensitivity and specificity for combined moderate and severe at 22.8% and 89%, respectively, and for severe alone at 1.6% and 99.3%, respectively. The AUC of the relative FACED for hospitalisations was 0.615 (95% CI 0.577, 0.654) with sensitivity and specificity for combined moderate and severe at 36.3% and 86.2%, respectively, and for severe alone at 5.1% and 99.3%, respectively.
Figure 3.
AUCs of absolute and relative FACED categorisation against four-year mortality and hospitalisations.
Secondary Outcome Analyses for FACED Assessment
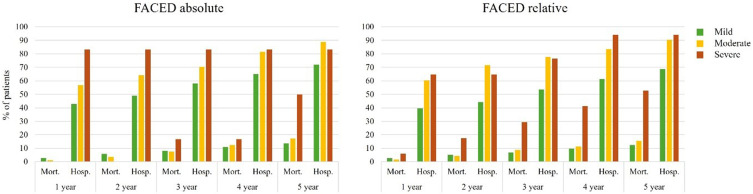
Using the absolute FACED, there was a heightened risk of mortality among those categorised as severe compared to mild alone, and moderate and mild combined only at 5-years of follow-up (Table 5 and Figure 4). In the same comparison categories, there was an increased risk of hospitalisations only at 1 and 2 years of follow-up. Among patients categorised as moderate alone, or moderate and severe combined there was no increased risk of mortality at any time point compared to those in the mild category. In both comparison categories, however, there was an increased risk of hospitalisation compared to mild patients at every year of follow-up.
Table 5.
Risk Ratios for Mortality at 1–5 Years of Follow Up Post Census Date by Absolute and Relative FACED Category
| FACED Category | FACED Severity | 1 Year (RR, CI) | 2 Year (RR, CI) | 3 Year (RR, CI) | 4 Year (RR, CI) | 5 Year (RR, CI) |
|---|---|---|---|---|---|---|
| Absolute; Mortality | Moderate vs Mild | 0.46 (0.06, 3.52) | 0.65 (0.2, 2.13) | 0.91 (0.39, 2.12) | 1.14 (0.59, 2.18) | 1.28 (0.74, 2.19) |
| Severe vs Mild | – | – | 2.05 (0.33, 12.7) | 1.54 (0.25, 9.44) | 3.69 (1.59, 8.56)* | |
| Moderate & Severe vs Mild | 0.42 (0.05, 3.28) | 0.61 (0.18, 1.99) | 0.99 (0.45, 2.18) | 1.17 (0.62, 2.18) | 1.44 (0.88, 2.37) | |
| Severe vs Moderate & Mild | – | – | 2.08 (0.34, 12.84) | 1.5 (0.25, 9.17) | 3.52 (1.53, 8.08)* | |
| Relative; Mortality | Moderate vs Mild | 0.7 (0.15, 3.24) | 0.87 (0.33, 2.32) | 1.27 (0.62, 2.59) | 1.17 (0.63, 2.15) | 1.25 (0.75, 2.1) |
| Severe vs Mild | 2.38 (0.31, 17.96) | 3.56 (1.15, 11.07)* | 4.32 (1.86, 10.01)* | 4.29 (2.22, 8.3)* | 4.28 (2.51, 7.3)* | |
| Moderate & Severe vs Mild | 0.91 (0.25, 3.39) | 1.21 (0.53, 2.77) | 1.66 (0.89, 3.09) | 1.57 (0.93, 2.65) | 1.64 (1.05, 2.56)* | |
| Severe vs Moderate & Mild | 2.58 (0.35, 19.08) | 3.69 (1.22, 11.19)* | 4.03 (1.8, 9.06)* | 4.11 (2.18, 7.75)* | 4.01 (2.41, 6.67)* | |
| Absolute; Hospitalisation | Moderate vs Mild | 1.33 (1.06, 1.66)* | 1.32 (1.08, 1.6)* | 1.21 (1.03, 1.43)* | 1.25 (1.1, 1.42)* | 1.23 (1.12, 1.36)* |
| Severe vs Mild | 1.95 (1.33, 2.84)* | 1.71 (1.18, 2.48)* | 1.44 (0.99, 2.08) | 1.28 (0.89, 1.85) | 1.16 (0.8, 1.66) | |
| Moderate & Severe vs Mild | 1.37 (1.11, 1.69)* | 1.34 (1.12, 1.62)* | 1.23 (1.05, 1.44)* | 1.25 (1.11, 1.42)* | 1.23 (1.11, 1.36)* | |
| Severe vs Moderate & Mild | 1.84 (1.27, 2.67)* | 1.62 (1.12, 2.34)* | 1.38 (0.96, 2) | 1.23 (0.85, 1.76) | 1.11 (0.77, 1.59) | |
| Relative; Hospitalisation | Moderate vs Mild | 1.52 (1.25, 1.86)* | 1.62 (1.37, 1.91)* | 1.45 (1.26, 1.67)* | 1.36 (1.21, 1.54)* | 1.32 (1.2, 1.45)* |
| Severe vs Mild | 1.63 (1.12, 2.38)* | 1.46 (1.01, 2.12)* | 1.43 (1.08, 1.89)* | 1.54 (1.33, 1.78)* | 1.37 (1.19, 1.58)* | |
| Moderate & Severe vs Mild | 1.54 (1.27, 1.86)* | 1.6 (1.35, 1.88)* | 1.45 (1.26, 1.66)* | 1.39 (1.24, 1.55)* | 1.32 (1.21, 1.45)* | |
| Severe vs Moderate & Mild | 1.43 (0.99, 2.07) | 1.26 (0.87, 1.81) | 1.28 (0.97, 1.68) | 1.4 (1.22, 1.6)* | 1.26 (1.11, 1.44)* |
Notes: *Denotes statistically significant difference at p<0.05. Data displayed as RR (95% CI).
Abbreviations: FACED, Forced expiratory volume in 1 s (FEV1), Age, Chronic colonization, Extension, and Dyspnea; RR, Relative risk; CI, Confidence interval.
Figure 4.
Mortality and hospitalisations at 1–5 years post-census date by FACED severity categorisation.
Using the relative FACED scores, there was a heightened risk of mortality among those categorised as severe compared to mild alone, and moderate and mild combined at 2-5-years of follow-up (Table 5). Among those categorised as severe compared to mild alone, there was a significantly increased risk of hospitalisation at each year of follow-up, while when compared to moderate and mild combined, there was only an increased risk at years 4 and 5 of follow-up. Among patients categorised as moderate alone, there was no increased risk of mortality at any time point compared to mild patients, however among those categorised as moderate and severe combined there was an increased risk of mortality at 5-years of follow-up compared to mild patients. In both comparison categories, however, there was an increased risk of hospitalisation at every year of follow-up.
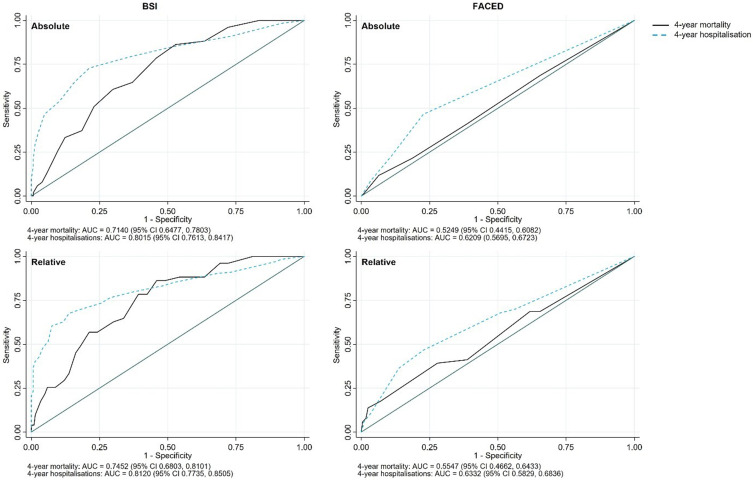
Comparison Between BSI and FACED Tools
Using the total scores for the BSI and FACED showed significant differences between the scoring systems for the predictive capacity of both mortality and hospitalisations, and within scoring systems for the predictive capacity between mortality and hospitalisations (Figure 5). For both BSI and FACED, the AUCs were larger for hospitalisations than for mortality (absolute BSI hospitalisations 0.802 (95% CI 0.761, 0.842) vs mortality 0.714 (95% CI 0.648, 0.78) and absolute FACED hospitalisations 0.621 (95% CI 0.57, 0.672) vs mortality 0.525 (95% CI 0.442, 0.608)). Furthermore, using the relative scores instead of absolute scores resulted in slightly greater AUCs, with a greater effect seen for mortality than for hospitalisations.
Figure 5.
Receiver operating characteristics for total absolute and relative BSI and FACED scores on 4-year mortality and hospitalisations.
Discussion
To the best of our knowledge, this is the first study to undertake an in-depth assessment of the commonly utilised bronchiectasis severity assessment tools within an Indigenous patient cohort, more specifically among the Aboriginal Australians in the Top End, NT of Australia. This study demonstrated 43% of patients to be categorised as having mild bronchiectasis by the BSI, and 81% according to the FACED scoring tools. However, a significant proportion (63%) of patients within our cohort were missing data including lung function data, BMI and MRC dyspnoea score. Thus, utilising a relative scoring system, instead 28% and 71% of patients were categorised as having mild bronchiectasis by the BSI and FACED, respectively. The risk of mortality at four-year follow-up significantly increased in line with increased severity according to both the absolute and relative scores for the BSI; however, according to the FACED, there was only increased risk when using the relative scoring system. Using the absolute BSI severity assessment, the AUC for four-year mortality was 0.703. In the FACED, however, the AUC was significantly lower at 0.515. Utilising the relative scoring systems resulted in a minor improvement for both the BSI (AUC = 0.717) and the FACED scores (AUC 0.571).
Both the BSI and FACED tools are utilised in predicting future mortality. In our study, the AUC for four-year mortality utilising the total absolute BSI score in the current cohort (0.714 (95% CI 0.648, 0.78)) was significantly lower than what has previously been shown in multiple European cohorts (mean AUCs ranging from 0.81 to 0.849 and a pooled European estimate of 0.76 (95% CI 0.74, 0.78).22 Utilising the total relative BSI score, however, resulted in an AUC of 0.745 (95% CI 0.68, 0.81) which better approximated the pooled European estimates. In addition, severe categorisation via the relative BSI showed significantly greater sensitivity and specificity (79% and 61%) for four-year mortality compared to severe categorisation via the absolute BSI (51% and 77%). This suggests that in this cohort, a relative scoring system, taking into account only the available data, can be used as a substitute for the absolute score. In a population where strict data collection is not always possible, and existing tools have not always been culturally validated, this is a boon and provides an alternate avenue to score bronchiectasis severity for rural and remote primary settings. Furthermore, the AUCs for four-year hospitalisations (absolute score AUC 0.802 (95% CI 0.761, 0.842) and relative score AUC 0.812 (95% CI 0.774, 0.851)) were similar to what has previously been reported (AUCs mean 0.80–0.88).9,22 This is despite the differences in definitions used to assess hospitalisations between the current cohort, and those in the existing literature. The current study defined hospitalisations as any hospitalisation with an ICD-10 J code. This is due to the significant degree of comorbidities and multimorbidity present in this cohort (83% having comorbid COPD, 26% asthma, 35% coronary artery disease (CAD)) – and therefore the ability to define an exacerbation or hospitalisation due purely to bronchiectasis is limited. It is noteworthy that although the current cohort displayed significantly lower lung function, were significantly younger, had a significantly greater proportion of prior hospitalisations and a greater prevalence of pseudomonas8 that the BSI still showed adequate accuracy and validity to predict four-year mortality and hospitalisations.
While the BSI showed relatively close accuracy to that displayed in the original validation cohorts, the FACED score was extremely poor in predicting both mortality and hospitalisations in our study cohort, contrary to previous reports.23 The FACED AUC for 5-year mortality in the original study was 0.83 in the validation cohort,10 although it declined to 0.80 in a follow-up study,24 and down to 0.76 (95% CI 0.74, 0.78) in a pooled European cohort.22 In contrast, the current study's FACED AUC was 0.50 for mortality and 0.51 for hospitalisations. There are several potential reasons for this 1) the age threshold for severity in the FACED is 70 years – in the current cohort the median age was 49 years (IQR 41, 58), and median age of death for those with four-year mortality was 56 years (IQR 48, 63) – therefore, most patients would be deceased before receiving any increased score due to age under the FACED system 2) Dyspnoea data was not available in the current cohort – given dyspnoea accounts for 14% of the total potential score, having this unavailable has a significant effect on the potential score 3) the FEV1 cut-off for the FACED is 50%, and in the current cohort the median FEV1 was 37% (IQR 28, 52) – therefore almost 75% of patients received a greater score due to this, limiting its capacity to accurately differentiate patients.
A significant limitation of utilising either of these scoring systems in the current population is the lack of required data such as lung function testing and BMI. Due to the remote residence of the majority of participants (93%), spirometry is not always available in primary care, and therefore lung function testing is not consistently carried out, unless the patients present to an outreach specialist respiratory team5 or during a presentation to a secondary or a tertiary care referral hospital. In addition to this, there are currently no reference norms for this population, and though the need for ethnicity-specific references in lung function is controversial,25,26 data from our centre has shown that referred patients from this population do not fit the available references norms for older Aboriginal Australians.14 In our study, lung function did appear to predict four-year hospitalisations; however, it was not associated with four-year mortality. This may be due to the low number of patients with lung function data, or it may be due to the high number of patients with reduced lung function values, and therefore the thresholds not being sensitive enough to delineate between those at risk or not for mortality.
In the current cohort, previous hospitalisations showed a significant association with both future hospitalisation and future mortality. Yet, previous exacerbation history did not show such an association. This may partially be due to the definition of exacerbation used in the current study (limited to a hospital presentation under ICD code J47) – however, this raises a fundamental question with regard to the definition of exacerbations, and the availability of this information. Healthcare systems operate differently between countries, and sometimes within countries between jurisdictions – therefore, it is plausible that when a patient has an exacerbation and is managed at the primary health clinic, this information is not then transferred to the patient’s hospital records, or, if a patient is managed at the local hospital, this information may not be transferred back to primary care. Furthermore, in the case of mobile and transient populations or patients, there is the issue of medical information (mis)communication between jurisdictions. As such, in primary care, patient recall of exacerbation history may be necessary – yet the validity and efficacy of this method in both a general assessment, and for use within the severity scoring systems has not yet been tested. In this manner, self-assessment scales such as the MARKO questionnaire27 or the CAT28 used for COPD assessment may be useful, although they have yet to be tested amongst an Indigenous population.
In line with previous studies, colonisation with pseudomonas was associated with both mortality and hospitalisations.9,29,30 However, within the BSI, patients may score an increased severity based on colonisation of “other” pathogenic organisms. This thought raises the question of what is considered a pathogenic organism in this context. Previous literature has suggested that Streptococcus spp., Staphylococcus spp. or Moraxella spp. may be significant such organisms,31 however, a previous study from our centre in this cohort found no significant association between these organisms and mortality or hospitalisations.8 On the other hand, we identified a significant association between non-aspergillus fungal species and mortality.8 Indeed, previous literature has also identified significant variations in predominant organisms across geographies.32 Therefore, definitions of “other” pathogenic organisms must be geographic and context specific in order to enable greater accuracy of severity classification systems.
Notably, in the current cohort, radiological extent did not show any significant association with either mortality or hospitalisations. From one perspective, this is a positive outcome from the current study, as access to CT scans is significantly impaired in rural and remote areas where the majority of the Indigenous populations reside and as such regular CT checkups to assess disease progression are not feasible. The current results suggest that CT scans may not be required to assess the severity of the disease. However, many of the CTs utilised in the current cohort were not conducted specifically to assess the type and extent of lung disease, but rather to assess other issues, with bronchiectasis only opportunistically identified and reported upon. Without specific reporting on high-resolution images, vital details may be missed, the accuracy of reports diminished and thus the influence of radiological extent on adverse outcomes is not wholly accurate.
A significant omission from the current severity scoring scales is assessment of the comorbidity burden of patients. Previous work has highlighted that bronchiectasis with comorbid COPD has earlier and more severe sequalae than bronchiectasis alone, as does the total degree of multimorbidity.33 Yet, comorbidities are not considered in either the BSI or FACED scales,9,10 prompting the development of the bronchiectasis comorbidity index (BCI), and the bronchiectasis aetiology and comorbidity index (BACI).33 In the current cohort, there was significant presence of comorbidities with 83% demonstrating comorbid COPD, 63% hypertension, 48% diabetes and 35% CAD and thus it is reasonable to presume that these would have a strong influence on both mortality and hospitalisations in Indigenous populations.
This study clearly demonstrates the limitations in using the existing bronchiectasis severity assessment tools in Indigenous patients. This may be related to remoteness, geographic isolation and differing population demographics, lower lung function parameters and presence of multimorbidity’s.34–58 It is imperative that health care practitioners caring for Indigenous people with bronchiectasis are made aware of these limitations. However, until Indigenous patients’ specific bronchiectasis severity tools are developed in the near future, it may be reasonable, as demonstrated in this study, to utilise the relative BSI score. Furthermore, further research is warranted taking into account symptomatology and precision diagnostic modalities, including biomarkers59–63 in the future amongst Indigenous populations globally.
Limitation
The authors acknowledge that there are several limitations in this retrospective study that may have its inherent limitations. First, the data represented in this study are limited to Aboriginal Australian people residing in the single TEHS region of the NT of Australia and absent confirmatory data, it is unclear whether they will be able to be generalised to the wider Aboriginal Australian populations or for Indigenous people globally. Second, our study relied on existing clinical and laboratory parameters, more specifically the availability of BMI and MRC dyspnoea score. Third, lung function data was not available in all patients. However, this also represents the realistic clinical scenarios in a remote residing Indigenous populations. Nevertheless, this is the first study to evaluate the utility and validity of assessing bronchiectasis through the established assessment tools in a relatively large Aboriginal Australian cohort and establishing pathways to develop Indigenous people’s specific bronchiectasis assessment tools in the future.
Conclusion
This study has demonstrated that while taking into account remoteness and access to specialist health care both BSI and FACED bronchiectasis assessment tools may not be ideal in an Indigenous/Aboriginal patient with bronchiectasis while acknowledging the study limitations. Nonetheless, until Indigenous people specific bronchiectasis assessment tools are developed, it may be reasonable to utilise the relative BSI score in this population, and further research is clearly warranted in developing Indigenous people’s specific bronchiectasis severity assessment tool.
Acknowledgments
We thank the Thoracic Society of Australia and New Zealand (TSANZ) for their support through Robert Pierce Grant-In-Aid for Indigenous Lung Health. We thank Associate Professor Linda Ford - Indigenous Australian woman, a Mak Mak Marranunggu descendent from the Delissaville, Wagait Larrakia Aboriginal Land Trust and the Gurudju Aboriginal Land Trust in the Northern Territory for the support and facilitating Mrs Adriana Ticoalu from the Northern Institute, Faculty of Arts & Society, Charles Darwin University, Darwin, NT, Australia to assist with population data collection for this study. We also extend our sincere apparition to Dr Shiidheshwar Ravichandran and Dr Davaadorj Erdenebayar for their help during this study. We would like to thank our respiratory clinical nurse consultants, Mrs Raelene Messenger and Mrs Siji Issac from the respiratory chronic disease unit, at the Royal Darwin Hospital, including, rural and remote community Aboriginal health workers, all the Royal Darwin Hospital medical services, in particular radiology and pathology services, including patients travel division for coordinating care for Aboriginal people living in the remote and rural communities. Finally, we thank all the Darwin Respiratory and Sleep Health staff – Ms Ara J Perez, Mr Jessie Crespo, Mr Mark L Ramirez, Ms Bianca Al Dossary, Ms Caira Reyes, Ms Kyrah Abregana, Mrs Zaranaben Patel, Ms Van Thanh Huynh and Ms Angelou Castro in coordinating, what rather could be a complex process to make it simple and efficient against all odds in facilitating patients care/research in this Top End, Northern Territory region.
Funding Statement
This work was supported by the Thoracic Society of Australia and New Zealand (TSANZ) Robert Pierce Grant-In-Aid for Indigenous Lung Health. The TSANZ did not have any role in the study design, data collection, analysis, or interpretation.
Abbreviations
AUC, Area under the curve; BMI, Body mass index; BSI, bronchiectasis severity index; CAD, Coronary artery disease; CI, Confidence interval; COPD, Chronic obstructive pulmonary disease; CT, Computed tomography; FACED, Forced expiratory volume in 1 s (FEV1), Age, Chronic colonization, Extension, and Dyspnea; FEV1, Forced expiratory volume in 1 second; ICD, International Classification of Diseases; IQR, Interquartile range; MRC, Medical Research Council; NT, Northern Territory; RR Relative risk.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
This study was approved by the Human Research Ethics governance/committee of the Top End Health Service (TEHS), Northern Territory (NT) and Menzies School of Health Research (Reference: HREC; 2019-3547). Individual patient consent was not required, as it was a retrospective study and no active interventions were undertaken and the need for individual consent was waived by the Human Research Ethics governance/committee of the TEHS, NT and Menzies School of Health Research.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
All authors declare no conflicts of interest for this study.
References
- 1.Gibbs C, Howarth T, Ticoalu A. et al. Bronchiectasis among indigenous adults in the top end of the northern territory, 2011-2020: a retrospective cohort study. Med J Aust. 2024;220:188–195. doi: 10.5694/mja23.00615 [DOI] [PubMed] [Google Scholar]
- 2.Howarth TP, Jersmann HPA, Majoni SW, et al. The ‘ABC’ of respiratory disorders among adult Indigenous people: asthma, bronchiectasis and COPD among Aboriginal Australians - a systematic review. BMJ Open Respir Res. 2023;10(1):e001738. doi: 10.1136/bmjresp-2023-001738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howarth T, Heraganahally SS, Heraganahally SS. Bronchiectasis among adult first nations indigenous people – a scoping review. Curr Respir Med Rev. 2023;19:36–51. doi: 10.2174/1573398X19666221212164215 [DOI] [Google Scholar]
- 4.Mehra S, Chang AB, Lam CK, et al. Bronchiectasis among Australian Aboriginal And Non-Aboriginal patients in the regional and remote population of the Northern Territory of Australia. Rural Remote Health. 2021;21(2):6390. doi: 10.22605/RRH6390 [DOI] [PubMed] [Google Scholar]
- 5.Kruavit A, Fox M, Pearson R, Heraganahally S. Chronic respiratory disease in the regional and remote population of the northern territory top end: a perspective from the specialist respiratory outreach service. Aust J Rural Health. 2017;25:275–284. doi: 10.1111/ajr.12349 [DOI] [PubMed] [Google Scholar]
- 6.Heraganahally SS, Wasgewatta SL, McNamara K, et al. Chronic obstructive pulmonary disease with and without bronchiectasis in Aboriginal Australians – a comparative study. Int Med J. 2020;50(12):1505–1513. doi: 10.1111/imj.14718 [DOI] [PubMed] [Google Scholar]
- 7.Heraganahally SS, Ghimire RH, Howarth T, Kankanamalage OM, Palmer D, Falhammar H. Comparison and outcomes of emergency department presentations with respiratory disorders among Australian indigenous and non-indigenous patients. BMC Emerg Med. 2022;22:11. doi: 10.1186/s12873-022-00570-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howarth T, Gibbs C, Heraganahally SS, Abeyaratne A. Hospital admission rates and related outcomes among adult Aboriginal Australians with bronchiectasis - a ten-year retrospective cohort study. BMC Pulm Med. 2024;24(1):118. doi: 10.1186/s12890-024-02909-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med. 2014;189(5):576–585. doi: 10.1164/rccm.201309-1575OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martínez-García MÁ, de Gracia J, Vendrell Relat M, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J. 2014;43(5):1357–1367. doi: 10.1183/09031936.00026313 [DOI] [PubMed] [Google Scholar]
- 11.Nicolson CH, Holland AE, Lee AL. The bronchiectasis toolbox-a comprehensive website for the management of people with bronchiectasis. Med Sci. 2017;5(2):13. doi: 10.3390/medsci5020013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howarth T, Gahreman D, Ben Saad H, Ng L, Heraganahally SS. Correlation of spirometry indices to chest radiology in the diagnosis of chronic airway disease among regional and rural Indigenous Australians. Int Med J. 2023;53(11):1994–2006. doi: 10.1111/imj.16023 [DOI] [PubMed] [Google Scholar]
- 13.Heraganahally SS, Howarth TP, Sorger L. Chest computed tomography findings among adult Indigenous Australians in the Northern Territory of Australia. J Med Imaging Radiat Oncol. 2022;66(3):337–344. doi: 10.1111/1754-9485.13295 [DOI] [PubMed] [Google Scholar]
- 14.Heraganahally SS, Howarth T, White E, Sorger L, Biancardi E, Ben Saad H. Lung function parameters among Australian Aboriginal “apparently healthy” adults: an Australian caucasian and global lung function initiative (GLI-2012) various ethnic norms comparative study. Expert Rev Respir Med. 2020;23:1–11. doi: 10.1080/17476348.2021.1847649 [DOI] [PubMed] [Google Scholar]
- 15.Sze DFL, Howarth TP, Lake CD, Ben Saad H, Heraganahally SS. Differences in the spirometry parameters between indigenous and non-indigenous patients with COPD: a matched control study. Int J Chron Obstruct Pulmon Dis. 2022;Volume 17(17):869–881. doi: 10.2147/COPD.S361839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heraganahally SS, Howarth T, Mo L, Sorger L, Ben Saad H. Critical analysis of spirometric patterns in correlation to chest computed tomography among adult Indigenous Australians with chronic airway diseases. Expert Rev Respir Med. 2021;15(9):1229–1238. doi: 10.1080/17476348.2021.1928496 [DOI] [PubMed] [Google Scholar]
- 17.Schubert J, Kruavit A, Mehra S, Wasgewatta S, Chang AB, Heraganahally S. Prevalence and nature of lung function abnormalities among Indigenous Australians referred to specialist respiratory outreach clinics in the Northern Territory. Int Med J. 2019;49(49):217–224://10.1111/imj.14112. doi: 10.1111/imj.14112 [DOI] [PubMed] [Google Scholar]
- 18.Schutz KL, Fancourt N, Chang AB, et al. Transition of pediatric patients with bronchiectasis to adult medical care in the Northern Territory: a retrospective chart audit. Front Pediatr. 2023;11:1184303. doi: 10.3389/fped.2023.1184303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCallum GB, Oguoma VM, Versteegh LA, et al. Comparison of profiles of first nations and non-first nations children with bronchiectasis over two 5-year periods in the Northern Territory, Australia. Chest. 2021;160(4):1200–1210. doi: 10.1016/j.chest.2021.04.057 [DOI] [PubMed] [Google Scholar]
- 20.Australian Bureau of Statistics. Northern Territory: aboriginal and Torres Strait Islander population summary. 2022. Available from: https://www.abs.gov.au/articles/northern-territory-aboriginal-and-torres-strait-islander-population-summary. Accessed 26, November 2024.
- 21.Australian Bureau of Statistics. Australian Statistical Geography Standard (ASGS): Volume 5—Remoteness Structure ABS cat. no. 1270.0.55.005. Canberra: Australian Bureau of Statistics; 2013a. [Google Scholar]
- 22.McDonnell MJ, Aliberti S, Goeminne PC, et al. Multidimensional severity assessment in bronchiectasis: an analysis of seven European cohorts. Thorax. 2016;71(12):1110–1118. doi: 10.1136/thoraxjnl-2016-208481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He M, Zhu M, Wang C, et al. Prognostic performance of the FACED score and bronchiectasis severity index in bronchiectasis: a systematic review and meta-analysis. Biosci Rep. 2020;40(10):BSR20194514. doi: 10.1042/BSR20194514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellis HC, Cowman S, Fernandes M, Wilson R, Loebinger MR. Predicting mortality in bronchiectasis using bronchiectasis severity index and FACED scores: a 19-year cohort study. Eur Respir J. 2016;47(2):482–489. doi: 10.1183/13993003.01312-2015 [DOI] [PubMed] [Google Scholar]
- 25.Ekström M, Mannino D. Race-specific reference values and lung function impairment, breathlessness and prognosis: analysis of NHANES 2007–2012. Respir Res. 2022;23:271. doi: 10.1186/s12931-022-02194-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhakta NR, Bime C, Kaminsky DA, et al. Race and ethnicity in pulmonary function test interpretation: an official American thoracic society statement. Am J Respir Crit Care Med. 2023;207(8):978–995. doi: 10.1164/rccm.202302-0310ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vrbica Ž, Labor M, Košćec Đuknić A, et al. Development and the initial validation of a new self-administered questionnaire for an early detection of health status changes in smokers at risk for chronic obstructive pulmonary disease (MARKO questionnaire). Croat Med J. 2016;57(5):425–433. doi: 10.3325/cmj.2016.57.425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 29.Martinez-García MA, Oscullo G, Posadas T, et al.; Spanish Registry of Bronchiectasis Group of SEPAR (RIBRON). Pseudomonas aeruginosa and lung function decline in patients with bronchiectasis. Clin Microbiol Infect. 2021;27(3):428–434. doi: 10.1016/j.cmi.2020.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Araújo D, Shteinberg M, Aliberti S, et al. The independent contribution of Pseudomonas aeruginosa infection to long-term clinical outcomes in bronchiectasis. Eur Respir J. 2018;51(2):1701953. doi: 10.1183/13993003.01953-2017 [DOI] [PubMed] [Google Scholar]
- 31.Amati F, Simonetta E, Gramegna A, et al. The biology of pulmonary exacerbations in bronchiectasis. Eur Respir Rev. 2019;28(154):190055. doi: 10.1183/16000617.0055-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chalmers JD, Polverino E, Crichton ML, et al. EMBARC Registry Investigators. Bronchiectasis in Europe: data on disease characteristics from the European Bronchiectasis registry (EMBARC). Lancet Respir Med. 2023;11(7):637–649. doi: 10.1016/S2213-2600(23)00093-0 [DOI] [PubMed] [Google Scholar]
- 33.McDonnell MJ, Aliberti S, Goeminne PC, et al. Comorbidities and the risk of mortality in patients with bronchiectasis: an international multicentre cohort study. Lancet Respir Med. 2016;4(12):969–979. doi: 10.1016/S2213-2600(16)30320-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heraganahally SS, Gibbs C, Ravichandran SJ, Erdenebayar D, Abeyaratne A, Howarth T. Factors influencing survival and mortality among adult aboriginal Australians with bronchiectasis—Ten-year retrospective study. Front Med. 2024;11:1366037. doi: 10.3389/fmed.2024.1366037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pal A, Howarth TP, Rissel C, et al. COPD disease knowledge, self-awareness and reasons for hospital presentations among a predominately Indigenous Australian cohort – a study to explore preventable hospitalization. BMJ Open Respir Res. 2022;9:e001295. doi: 10.1136/bmjresp-2022-001295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heraganahally SS, Mortimer N, Howarth T, et al. Utility and outcomes among indigenous and non-indigenous patients requiring domiciliary oxygen therapy in the regional and rural Australian population. Aust J Rural Health. 2021;29(6):918–926. doi: 10.1111/ajr.12782 [DOI] [PubMed] [Google Scholar]
- 37.Heraganahally SS, Wasgewatta SL, McNamara K, et al. Chronic obstructive pulmonary disease in aboriginal patients of the northern territory of Australia: a landscape perspective. Int J Chron Obstruct Pulmon Dis. 2019;14:2205–2217. doi: 10.2147/COPD.S213947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heraganahally SS, Howarth TP, Lloyd A, White E, Veale A, Ben Saad H. The prevalence of bronchodilator responsiveness “asthma” among adult indigenous Australians referred for lung function testing in the top end northern territory of Australia. J Asthma Allergy. 2022;Volume 15(15):1305–1319. doi: 10.2147/JAA.S376213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heraganahally SS, Sams S, Howarth TP, Kangaharan N, Majoni SW. Comparison of clinical manifestation among Australian Indigenous and non‐ indigenous patients presenting with pleural effusion. Int Med J. 2022;52(7):1232–1241. [DOI] [PubMed] [Google Scholar]
- 40.Seyedshahabedin MM, Howarth TP, Mo L, Biancardi E, Heraganahally SS. Flexible bronchoscopy indications and outcomes between Indigenous and non-Indigenous patients in the Northern Territory of Australia. Int Med J. 2022. doi: 10.1111/imj.15865 [DOI] [PubMed] [Google Scholar]
- 41.Heraganahally SS, Kruavit A, Oguoma VM, et al. Sleep apnoea among Australian Aboriginal and Non- Aboriginal patients in the Northern Territory of Australia– a comparative study. Sleep. 2020;43(3):zsz248. doi: 10.1093/sleep/zsz248 [DOI] [PubMed] [Google Scholar]
- 42.Heraganahally SS, Howarth TP, Perez AJ, et al. Acceptability, adaptability and adherence to CPAP therapy among Aboriginal Australians with OSA - “The A5 study”. Sleep Med. 2023;102:147–156. doi: 10.1016/j.sleep.2022.12.024 [DOI] [PubMed] [Google Scholar]
- 43.Heraganahally SS, Rajaratnam B, Silva SAAS, et al. Obstructive sleep apnoea and cardiac disease among Aboriginal patients in the Northern Territory of Australia. Heart Lung Circ. 2021;30:S1443–9506(21)00044–5. doi: 10.1016/j.hlc.2021.01.007 [DOI] [PubMed] [Google Scholar]
- 44.Heraganahally SS, Howarth T, Sorger L, Ben Saad H. Sex differences in pulmonary function parameters among Indigenous Australians with and without chronic airway disease. PLoSONE. 2022;17(2):e0263744. doi: 10.1371/journal.pone.0263744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heraganahally S, Howarth TP, White E, Ben Saad H. Implications of using the GLI-2012, GOLD and Australian COPD-X recommendations in assessing the severity of airflow limitation on spirometry among an Indigenous population with COPD: an Indigenous Australians perspective study. BMJ Open Respir Res. 2021;8:e001135. doi: 10.1136/bmjresp-2021-001135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howarth TP, Saad HB, Perez AJ, Atos CB, White E, Heraganahally SS. Comparison of diffusing capacity of carbon monoxide (DLCO) and total lung capacity (TLC) between Indigenous Australians and Australian Caucasian adults. PLoS One. 2021;16(4):e0248900. doi: 10.1371/journal.pone.0248900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nockles V, Hill E, Howarth TP, et al. Effects of environmental smoke exposure on respiratory conditions—a report of an aboriginal man fire hunting for mud turtles in the top end, Northern Territory of Australia. Am J Trop Med Hyg. 2024. doi: 10.4269/ajtmh.24-0366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howarth T, Ben Saad H, Heraganahally SS. The impact of lung function parameters on sleep among Aboriginal Australians – a polysomnography and spirometry relationship study. Nat Sci Sleep. 2023;Volume 15(15):449–464. doi: 10.2147/NSS.S409883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heraganahally SS, Ponneri TR, Howarth TP, Saad HB. The effects of inhaled airway directed pharmacotherapy on decline in lung function parameters among indigenous Australian adults with and without underlying airway disease. Int J Chron Obstruct Pulmon Dis. 2021;16:2707–2720. doi: 10.2147/COPD.S328137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doss AX, Howarth TP, Ng L, Doss SA, Heraganahally SS. Significance and prognostication of mediastinal lymph node enlargement on chest computed tomography among adult Indigenous Australians. J Med Imaging Radiat Oncol. 2023;67:726–733. doi: 10.1111/1754-9485.13569 [DOI] [PubMed] [Google Scholar]
- 51.Mishra K, Fazal R, Howarth T, Mutai J, Doss AX, Heraganahally SS. Cystic lung disease in adult Indigenous Australians in the Northern Territory of Australia. J Med Imaging Radiat Oncol. 2023;68:67–73. doi: 10.1111/1754-9485.13593 [DOI] [PubMed] [Google Scholar]
- 52.Heraganahally SS, Monsi E, Gadil E, Maze D, Lynch S. Catastrophic effects of using cannabis via bucket bong in top end Northern Territory of Australia. Am J Trop Med Hyg. 2023;1–6. doi: 10.4269/ajtmh.23-0393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ng LY, Howarth TP, Doss AX, et al. Significance of lung nodules detected on chest CT among adult Aboriginal Australians - A retrospective descriptive study. J Med Radiat Sci. 2024:1–10. doi: 10.1002/jmrs.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heraganahally SS, Howarth T, Gibbs C, Heraganahally S, Sorger L. Chest computed tomography findings among adult Aboriginal Australians with bronchiectasis in the top end Northern Territory of Australia. J Med Imaging Radiat Oncol. 2024;68:545–552. doi: 10.1111/1754-9485.13671 [DOI] [PubMed] [Google Scholar]
- 55.Heraganahally S, Howarth TP, Issac S, et al. Exploring the appropriateness of prescribing practice of inhaled pharmacotherapy among Aboriginal Australians in the top end Northern Territory of Australia: a retrospective cohort study. BMJ Open Respir Res. 2023;10:e001508. doi: 10.1136/bmjresp-2022-001508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benn E, Wirth H, Short T, Howarth T, Heraganahally SS. The top end sleepiness scale (TESS): a new tool to assess subjective daytime sleepiness among indigenous Australian Adults. Nat Sci Sleep. 2021;13:315–328. doi: 10.2147/NSS.S298409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heraganahally SS, Howarth TP, Wirth H, Short T, Benn E. Validity of the new “top end sleepiness scale” (TESS) against the STOP-bang tool in predicting obstructive sleep apnoea among indigenous Australian adults. Int Med J. 2021; 53(3):339–47. [DOI] [PubMed] [Google Scholar]
- 58.Heraganahally SS, Howarth T, Chen W. A clinical approach to chronic respiratory disorders in ‘Aboriginal and Torres Strait Islander in primary care. Aust J Gen Pract. 2024;2024:1 [Google Scholar]
- 59.Shih VH, Jison M, Bark E, Venerus M, Meyers O, Chalmers JD. The bronchiectasis exacerbation diary: a novel patient-reported outcome for non-cystic fibrosis bronchiectasis. ERJ Open Res. 2023;9(3):00712–2022. doi: 10.1183/23120541.00712-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tiddens HAWM, Meerburg JJ, van der Eerden MM, Ciet P. The radiological diagnosis of bronchiectasis: what’s in a name? Eur Respir Rev. 2020;29(156):190120. doi: 10.1183/16000617.0120-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Green O, Liautaud S, Knee A, Modahl L. Measuring accuracy of international classification of diseases codes in identification of patients with non-cystic fibrosis bronchiectasis. ERJ Open Res. 2024;10(2):00715–2023. doi: 10.1183/23120541.00715-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raboso B, Pou C, Abril R, et al. Bronchiectasis. Open Respir Arch. 2024;6(3):100339. doi: 10.1016/j.opresp.2024.100339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinez-García MÁ, Olveira C, Girón R, et al. Peripheral neutrophil-to-lymphocyte ratio in bronchiectasis: a marker of disease severity. Biomolecules. 2022;12(10):1399. doi: 10.3390/biom12101399 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.