Abstract
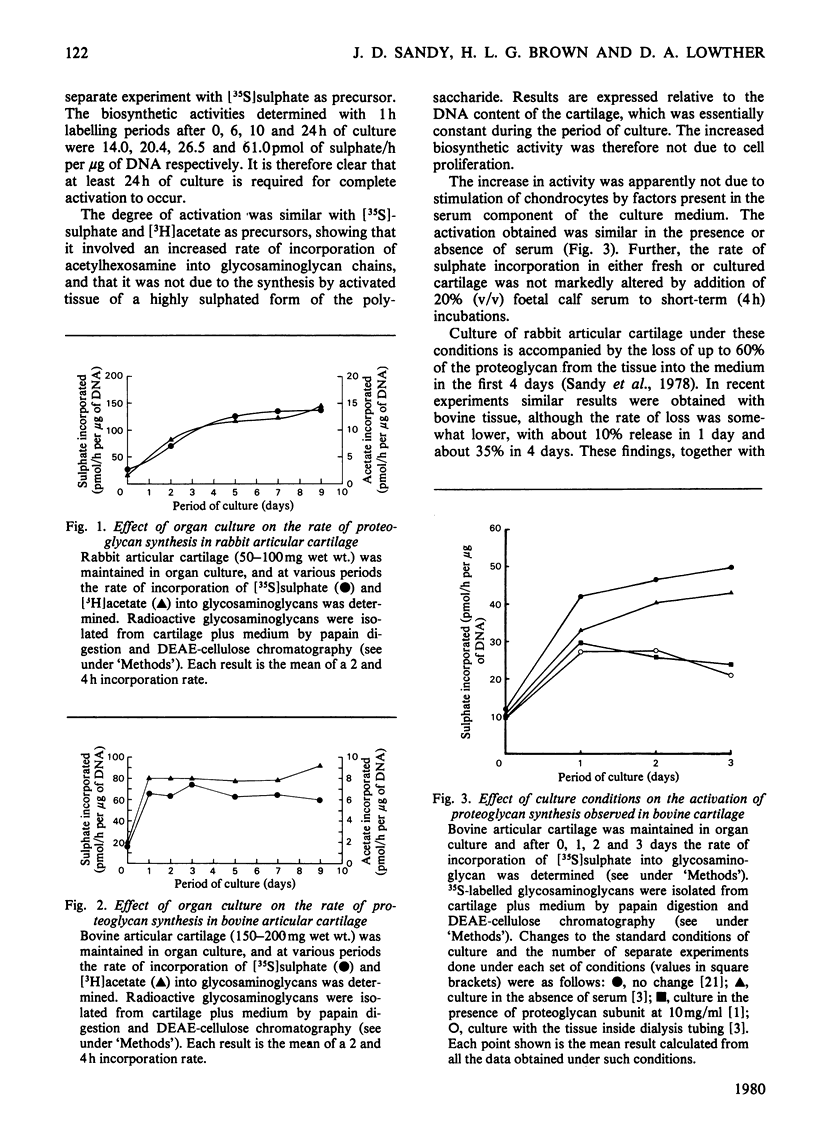
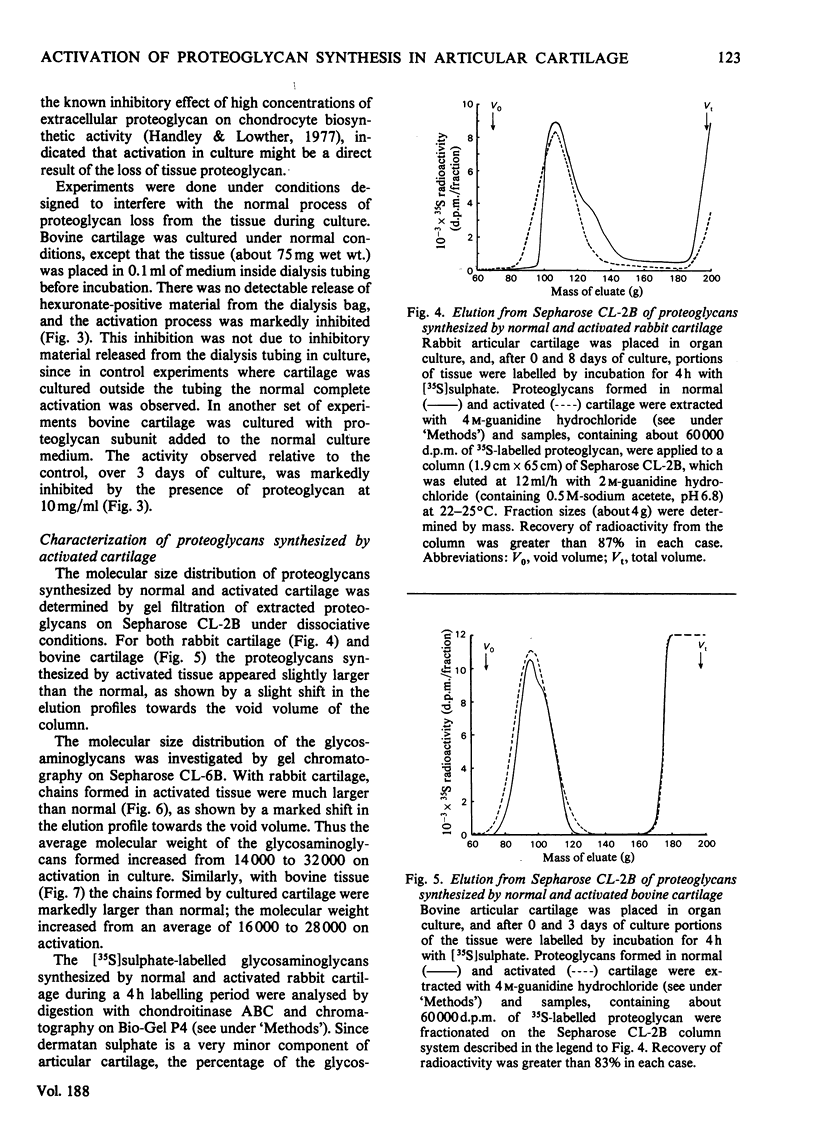
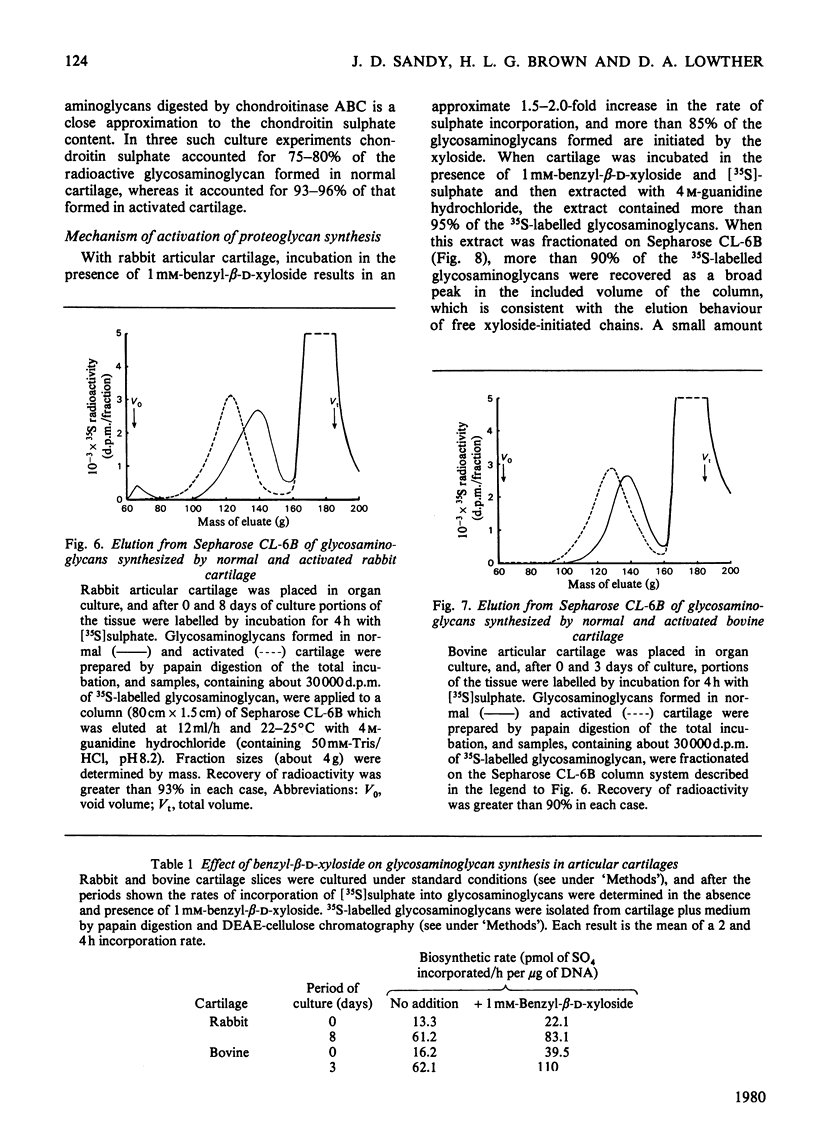
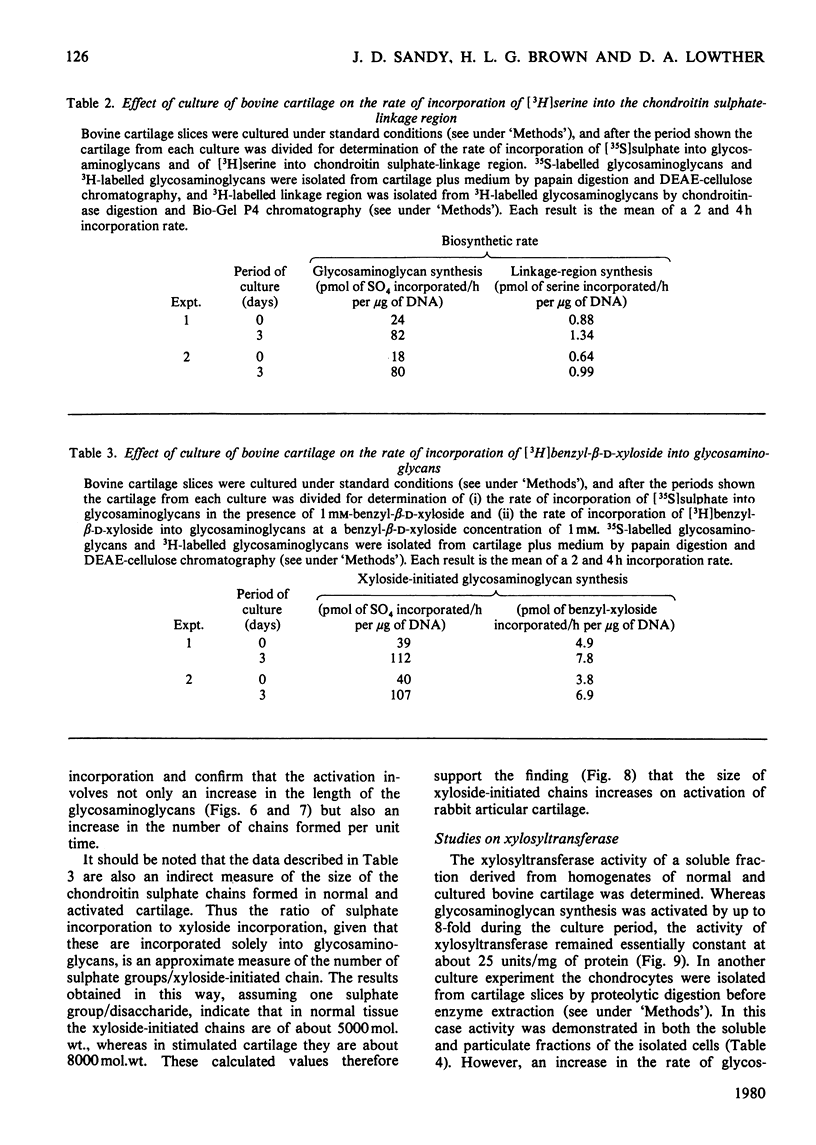
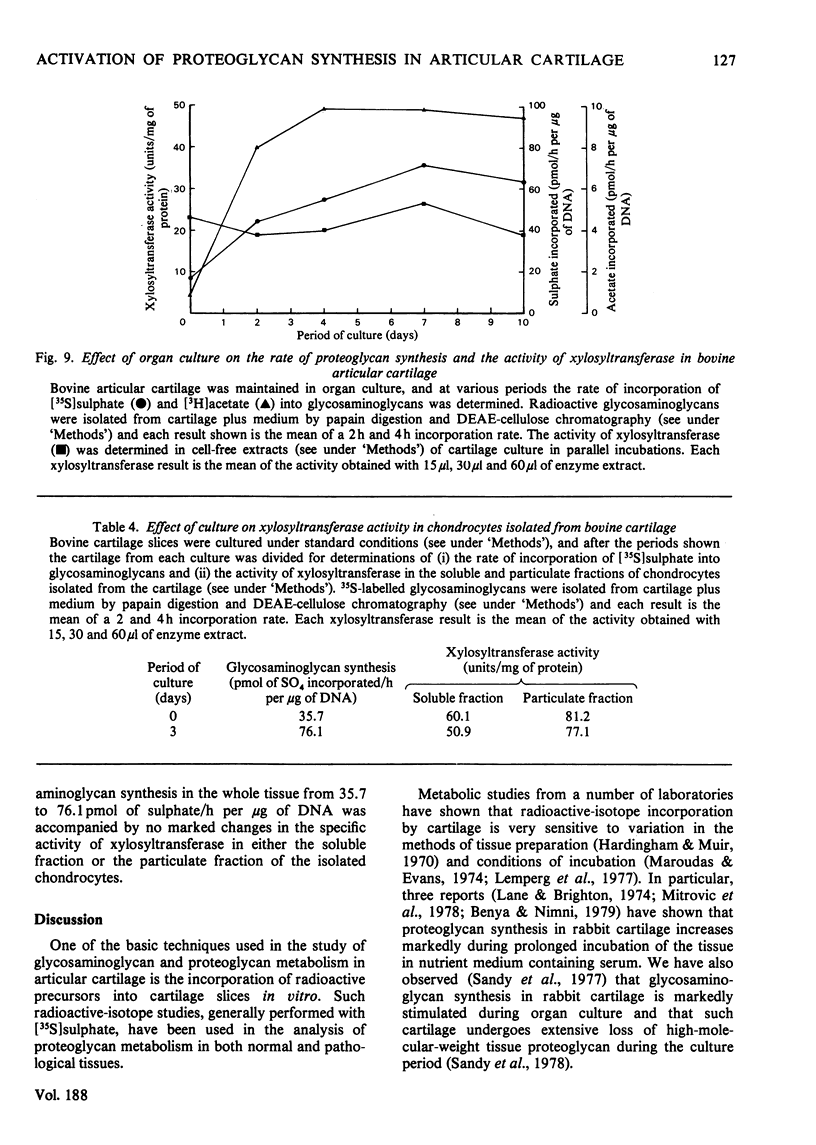
When slices of adult rabbit articular cartilage were incubated in culture medium, the rate of incorporation of [35S]sulphate or [3H]acetate into glycosaminoglycans increased 4-8 fold during the first 5 days of incubation. Similar changes in biosynthetic activity were observed during culture of adult bovine cartilage. The activation of synthesis was not serum-dependent, but appeared to be a result of the depletion of tissue proteoglycan that occurs under these incubation conditions [Sandy, Brown & Lowther (1978) Biochim. Biophys. Acta 543, 536--544]. Thus, although complete activation was observed in serum-free medium, it was not observed if the cartilage was cultured inside dialysis tubing or in medium containing added proteoglycan subunit. The average molecular size of the proteoglycans synthesized by activated tissue was slightly larger than normal, as determined by chromatography on Sepharose CL-2B, and the average molecular size of the glycosaminoglycans synthesized by activated tissue was markedly increased over the normal. The increase in chain size was accompanied by an increase in the proportion of the chains degraded by chondroitinase ABC; these results are consistent with the preferential synthesis by activated chondrocytes of chondroitin sulphate-rich proteoglycans. The increase in glycosaminoglycan chain size was observed whether the chains were formed on endogenous core protein or on exogenous benzyl-beta-D-zyloside. An approximate 4-fold activation in culture of glycosaminoglycan synthesis on protein core was accompanied by a 1.54-fold increase in the rate of incorporation of [3H]serine into the chondroitin sulphate-linkage region of the proteoglycans. A 2.8-fold activation in culture of glycosaminoglycan synthesis on benzyl-beta-D-zyloside was accompanied by a 1.7-fold increase in the rate of incorporation of [3H]benzyl-beta-D-zyloside into glycosaminoglycans. The activation of glycosaminoglycan synthesis was, however, accompanied by no detectable change in the activity of xylosyltransferase (EC 2.4.2.26) in cell-free extracts. These results are discussed in relation to current ideas on the control of proteoglycan synthesis in cartilage.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Audhya T. K., Gibson K. D. Effects of medium composition and metabolic inhibitors on glycosaminoglycan synthesis in chick embryo cartilage and its stimulation by serum and triiodothyronine. Biochim Biophys Acta. 1976 Jul 21;437(2):364–376. doi: 10.1016/0304-4165(76)90006-4. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Baker J. R., Rodén L., Stoolmiller A. C. Biosynthesis of chondroitin sulfate proteoglycan. Xylosyl transfer to Smith-degraded cartilage proteoglycan and other exogenous acceptors. J Biol Chem. 1972 Jun 25;247(12):3838–3847. [PubMed] [Google Scholar]
- Bayliss M. T., Ali S. Y. Isolation of proteoglycans from human articular cartilage. Biochem J. 1978 Jan 1;169(1):123–132. doi: 10.1042/bj1690123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benya P. D., Nimni M. E. The stability of the collagen phenotype during stimulated collagen, glycosaminoglycan, and DNA synthesis by articular cartilage organ cultures. Arch Biochem Biophys. 1979 Feb;192(2):327–335. doi: 10.1016/0003-9861(79)90100-0. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B. Cellular control of macromolecular synthesis: rates of synthesis of extracellular macromolecules during and after depletion by papain. Proc R Soc Lond B Biol Sci. 1968 Mar 26;169(1017):399–425. doi: 10.1098/rspb.1968.0017. [DOI] [PubMed] [Google Scholar]
- COLLINS D. H., McELLIGOTT T. F. Sulphate (35SO4) uptake by chondrocytes in relation to histological changes in osteoarthritic human articular cartilage. Ann Rheum Dis. 1960 Dec;19:318–330. doi: 10.1136/ard.19.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eronen I., Videman T., Friman C., Michelsson J. E. Glycosaminoglycan metabolism in experimental osteoarthrosis caused by immobilization. Acta Orthop Scand. 1978 Aug;49(4):329–334. doi: 10.3109/17453677809050083. [DOI] [PubMed] [Google Scholar]
- Gibson K. D., Segen B. J., Audhya T. K. The effect of beta-D-xylosides on chondroitin sulphate biosynthesis in embryonic chicken cartilage in the absence of protein synthesis inhibitors. Biochem J. 1977 Feb 15;162(2):217–233. doi: 10.1042/bj1620217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley C. J., Bateman J. F., Oakes B. W., Lowther D. A. Characterization of the collagen synthesized by cultured cartilage cells. Biochim Biophys Acta. 1975 Apr 29;386(2):444–450. doi: 10.1016/0005-2795(75)90287-1. [DOI] [PubMed] [Google Scholar]
- Handley C. J., Lowther D. A. Extracellular matrix metabolism by chondrocytes. III. Modulation of proteoglycan synthesis by extracellular levels of proteoglycan in cartilage cells in culture. Biochim Biophys Acta. 1977 Nov 7;500(1):132–139. doi: 10.1016/0304-4165(77)90053-8. [DOI] [PubMed] [Google Scholar]
- Handley C. J., Lowther D. A. Inhibition of proteoglycan biosynthesis by hyaluronic acid in chondrocytes in cell culture. Biochim Biophys Acta. 1976 Aug 24;444(1):69–74. doi: 10.1016/0304-4165(76)90224-5. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Fitton-Jackson S., Muir H. Replacement of proteoglycans in embryonic chick cartilage in organ culture after treatment with testicular hyaluronidase. Biochem J. 1972 Aug;129(1):101–112. doi: 10.1042/bj1290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The effect of temperature on the biosynthesis of chondroitin 4-sulphate in cartilage slices in vitro. FEBS Lett. 1970 Sep 6;9(3):145–148. doi: 10.1016/0014-5793(70)80339-8. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Robinson H. C. The molecular-weight distribution of glycosaminoglycans. Biochem J. 1973 Dec;135(4):631–637. doi: 10.1042/bj1350631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S. F. Environmental control of macromolecular synthesis in cartilage and bone: morphogenetic response to hyaluronidase. Proc R Soc Lond B Biol Sci. 1970 Sep 29;175(1041):405–453. doi: 10.1098/rspb.1970.0029. [DOI] [PubMed] [Google Scholar]
- Lane J. M., Brighton C. T. In vitro rabbit articular cartilage organ model. I. Morphology and glycosaminoglycan metabolism. Arthritis Rheum. 1974 May-Jun;17(3):235–243. doi: 10.1002/art.1780170306. [DOI] [PubMed] [Google Scholar]
- Lane J. M., Brighton C. T., Menkowitz B. J. Anaerobic and aerobic metabolism in articular cartilage. J Rheumatol. 1977 Winter;4(4):334–342. [PubMed] [Google Scholar]
- Lemperg R., Larsson S. E., Jones I. Studies on the in vitro incubation procedure for [35S]sulphate labelling of articular cartilage glycosaminoglycans. Biochim Biophys Acta. 1977 Feb 28;496(2):420–430. doi: 10.1016/0304-4165(77)90324-5. [DOI] [PubMed] [Google Scholar]
- Lowther D. A., Sandy J. D., Santer V. B., Brown H. L. Antigen-induced arthritis. Decreased proteoglycan content and inhibition of proteoglycan synthesis in articular cartilage. Arthritis Rheum. 1978 Jul-Aug;21(6):675–680. doi: 10.1002/art.1780210611. [DOI] [PubMed] [Google Scholar]
- Mankin H. J. Biochemical and metabolic abnormalities in osteoarthritic human cartilage. Fed Proc. 1973 Apr;32(4):1478–1480. [PubMed] [Google Scholar]
- Mankin H. J., Lippiello L. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. J Bone Joint Surg Am. 1970 Apr;52(3):424–434. [PubMed] [Google Scholar]
- Millroy S. J., Poole A. R. Pig articular cartilage in organ culture. Effect of enzymatic depletion of the matrix on response of chondrocytes to complement-sufficient antiserum against pig erythrocytes. Ann Rheum Dis. 1974 Nov;33(6):500–508. doi: 10.1136/ard.33.6.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrovic D., Gruson M., Demignon J., Cohen-Solal L. Increased metabolic activity of rabbit articular cartilage in vitro. Cell Tissue Res. 1978 Jan 9;186(1):149–159. doi: 10.1007/BF00219661. [DOI] [PubMed] [Google Scholar]
- Pearson J. P., Mason R. M. The stability of bovine nasal cartilage proteoglycans during isolation and storage. Biochim Biophys Acta. 1977 Jun 23;498(1):176–188. doi: 10.1016/0304-4165(77)90098-8. [DOI] [PubMed] [Google Scholar]
- Robinson H. C., Brett M. J., Tralaggan P. J., Lowther D. A., Okayama M. The effect of D-xylose, beta-D-xylosides and beta-D-galactosides on chondroitin sulphate biosynthesis in embryonic chicken cartilage. Biochem J. 1975 Apr;148(1):25–34. doi: 10.1042/bj1480025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. C., Dorfman A. The sulfation of chondroitin sulfate in embryonic chick cartilage epiphyses. J Biol Chem. 1969 Jan 25;244(2):348–352. [PubMed] [Google Scholar]
- Robinson H. C., Hopwood J. J. The alkaline cleavage and borohydride reduction of cartilage proteoglycan. Biochem J. 1973 Jul;133(3):457–470. doi: 10.1042/bj1330457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L., Wolfenstein-Todel C., Margolis R., Pal S., Strider W. Proteoglycans from bovine proximal humeral articular cartilage. Structural basis for the polydispersity of proteoglycan subunit. J Biol Chem. 1976 Oct 25;251(20):6439–6444. [PubMed] [Google Scholar]
- Royce P. M., Lowther D. A. Fluorimetric determination of DNA in papain digests of cartilage, using ethidium bromide. Connect Tissue Res. 1979;6(4):215–221. doi: 10.3109/03008207909152323. [DOI] [PubMed] [Google Scholar]
- Sandy J. D., Brown H. L., Lowther D. A. Degradation of proteoglycan in articular cartilage. Biochim Biophys Acta. 1978 Nov 1;543(4):536–544. doi: 10.1016/0304-4165(78)90308-2. [DOI] [PubMed] [Google Scholar]
- Sandy J. D. The assay of xylosyltransferase in cartilage extracts. A modified procedure for preparation of Smith-degraded proteoglycan. Biochem J. 1979 Feb 1;177(2):569–574. doi: 10.1042/bj1770569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz N. B. Biosynthesis of chondroitin sulfate: immunoprecipitation of interacting xylosyltransferase and galactosyltransferase. FEBS Lett. 1975 Jan 1;49(3):342–345. doi: 10.1016/0014-5793(75)80781-2. [DOI] [PubMed] [Google Scholar]
- Schwartz N. B. Chondroitin sulfate glycosyltransferases in cultured chondrocytes. Turnover, oscillatory change during growth, and suppression by 5-bromodeoxyuridine. J Biol Chem. 1976 Jun 10;251(11):3346–3351. [PubMed] [Google Scholar]
- Schwartz N. B., Rodén L. Biosynthesis of chondroitin sulfate. Purification of UDP-D-xylose:core protein beta-D-xylosyltransferase by affinity chromatography. Carbohydr Res. 1974 Oct;37(1):167–180. doi: 10.1016/s0008-6215(00)87072-x. [DOI] [PubMed] [Google Scholar]
- Simůnek Z., Muir H. Changes in the protein-polysaccharides of pig articular cartilage during prenatal life, development and old age. Biochem J. 1972 Feb;126(3):515–523. doi: 10.1042/bj1260515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoolmiller A. C., Horwitz A. L., Dorfman A. Biosynthesis of the chondroitin sulfate proteoglycan. Purification and properties of xylosyltransferase. J Biol Chem. 1972 Jun 10;247(11):3525–3532. [PubMed] [Google Scholar]
- Wiebkin O. W., Muir H. The inhibition of sulphate incorporation in isolated adult chondrocytes by hyaluronic acid. FEBS Lett. 1973 Nov 15;37(1):42–46. doi: 10.1016/0014-5793(73)80422-3. [DOI] [PubMed] [Google Scholar]


