Abstract
Catheter-related bloodstream infections (CRBSIs) add to the morbidity and mortality of hemodialysis patients. Stenotrophomonas maltophilia is an extremely resistant, gram-negative, non-lactose-fermenting nosocomial bacterium that contributes significantly to mortality and morbidity. This bacterium is predominantly associated with community-acquired pneumonia, bacteremia, eye afflictions, biliary sepsis, urinary tract infection, skin and soft tissue infection, and very rarely chronic enteritis with colonic ulcers. Here, we present two cases that presented indolently and exhibit strikingly contrasting behaviors. The first case was a patient with primary hyperoxaluria on maintenance hemodialysis, who presented with CRBSI due to S. maltophilia, which responded appropriately to catheter removal and levofloxacin. The second patient, a case of diabetic nephropathy on maintenance hemodialysis, developed CRBSI due to S. maltophilia, which initially responded to catheter removal and levofloxacin but was later complicated dramatically by pneumonia and enteritis with colonic ulcers. These cases highlight the variable clinical presentation of this organism and emphasize the need for active surveillance in the dialysis unit.
Keywords: catheter-related bloodstream infection (crbsi), colonic ulcers, hemodialysis, resistance, stenotrophomonas maltophilia
Introduction
Stenotrophomonas maltophilia is a multidrug-resistant, gram-negative, non-lactose-fermenting, and non-hemolytic nosocomial pathogen that is responsible for in-hospital mortality of 14%-69% and is associated predominantly with bacteremia, pneumonia, biliary sepsis, meningitis, urinary tract infection, dacryocystitis, and infection of the bones/joints [1]. Catheter-related bloodstream infection (CRBSI) due to S. maltophilia usually responds to timely catheter removal and appropriate antibiotic treatment [2]. The treatment algorithm for S. maltophilia is complicated due to poor susceptibility to existing antibiotic regimens and diverse mechanisms for acquired resistance to antibiotics [3]. Here, we describe two cases of CRBSI due to this nosocomial organism with variable clinical presentation and contrary outcomes.
Case presentation
Case 1
A 38-year-old female patient with chronic kidney disease due to primary hyperoxaluria on maintenance hemodialysis twice weekly for seven years via a left tunneled internal jugular catheter presented with a low-grade fever for one week. She had a significant history of CRBSI due to different pathogens and multiple vascular access failures. On evaluation, she was febrile with a temperature of 101 degrees Fahrenheit and a respiratory rate of 24/min with normotension. Clinical examination revealed a few scattered crepitations in the right lower zone and decreased air entry in the right base of the lung. She had new-onset absence seizures during admission. Contrast-enhanced computerized tomography of the brain revealed normal study, and electroencephalogram revealed generalized spike-wave discharges of 2-4 Hz suggestive of metabolic etiology. She was started on levetiracetam (1,000 mg/day) for the seizures. Labs revealed hemoglobin (Hb) of 7.3 g/dL, white blood cell (WBC) count of 6,210/mm3, platelet of 169,000/mm3, normal liver function tests, creatinine of 6.9 mg/dL, urea of 57 mg/dL, and normal electrolytes (Table 1).
Table 1. Lab parameters of Case 1 on admission.
g/dL: grams/deciliter; mg/dL: milligrams/deciliter; meq/L: milliequivalents/liter; mm3: cubic millimeter; U/L: units/liter
| Parameter | Values | Reference range |
| Hemoglobin (g/dL) | 7.3 | 12-16 |
| White blood cell count (/mm3) | 6,210 | 4,500-11,000 |
| Platelet count (/mm3) | 169,000 | 150,000-370,000 |
| Creatinine (mg/dL) | 6.9 | 0.6-1.1 |
| Urea (mg/dL) | 57 | 15-40 |
| Sodium (meq/L) | 136 | 135-145 |
| Potassium (meq/L) | 4.9 | 3.5-5 |
| Chloride (meq/L) | 104 | 96-106 |
| Bicarbonate (meq/L) | 22 | 22-26 |
| Total bilirubin (mg/dL) | 0.4 | 0.2-1.2 |
| Alanine transaminase (U/L) | 22 | 19-40 |
| Aspartate transaminase (U/L) | 16 | 10-36 |
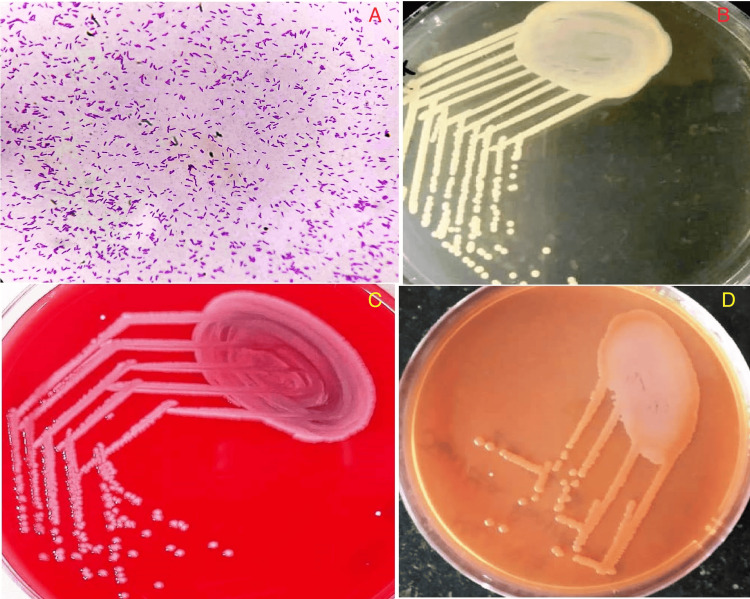
Chest X-ray revealed small right lower zone consolidation, and she was empirically started on injection of cefoperazone with sulbactam and azithromycin. However, the fever did not remit, and a blood culture drawn through a catheter revealed aerobic, gram-negative, motile, polar flagellated (Figure 1A) and catalase-positive organisms with small circular, raised, yellowish pigmented colonies on nutrient agar (Figure 1B) suggestive of S. maltophilia. A clinical decision was taken to remove the dialysis catheter with the insertion of a temporary left femoral dialysis catheter, and an injection of levofloxacin was initiated as per antibiotic susceptibility. The organism’s antibiotic susceptibility pattern demonstrated only sensitivity to levofloxacin with resistance to minocycline and cotrimoxazole. The patient had a good clinical response in terms of fever defervescence with three weeks of exposure to levofloxacin.
Figure 1. (A) Hematoxylin and eosin stain showing gram-negative, motile, polar flagellated bacterium suggestive of Stenotrophomonas maltophilia (magnification: 600 DPI; 400X). (B) Nutrient agar showing small circular, raised, yellowish pigmented colonies suggestive of Stenotrophomonas maltophilia (magnification: 600 DPI). (C) Blood agar classically showing non-hemolytic colonies of Stenotrophomonas maltophilia (magnification: 600 DPI). (D) MacConkey agar showing non-lactose-fermenting colonies of Stenotrophomonas maltophilia (magnification: 600 DPI).
DPI: dots per inch
Case 2
A 65-year-old female patient with diabetic nephropathy on maintenance hemodialysis twice weekly in our unit for 1.5 years via a right internal jugular tunneled dialysis catheter presented with intermittent high-grade fever and nausea with decreased appetite for 10 days. On evaluation, she had a significant history of chills during hemodialysis sessions, and there was no systemic abnormality on clinical examination. Labs revealed Hb of 7.5 g/dL, WBC of 10,680/mm3, platelet of 231,000/mm3, creatinine of 6.6 mg/dL, urea of 62 mg/dL, normal serum electrolytes, and normal liver function tests (Table 2).
Table 2. Lab parameters of Case 2 on admission.
mg/dL: milligrams/deciliter; meq/L: milliequivalents/liter; mm3: cubic millimeter; U/L: units/liter
| Parameter | Values | Reference range |
| Hemoglobin (g/dL) | 7.5 | 12-16 |
| White blood cell count (/mm3) | 10,680 | 4,500-11,000 |
| Platelet count (/mm3) | 231,000 | 150,000-370,000 |
| Creatinine (mg/dL) | 6.6 | 0.6-1.1 |
| Urea (mg/dL) | 62 | 15-40 |
| Sodium (meq/L) | 135 | 135-145 |
| Potassium (meq/L) | 4.3 | 3.5-5 |
| Chloride (meq/L) | 106 | 96-106 |
| Bicarbonate (meq/L) | 21 | 22-26 |
| Total bilirubin (mg/dL) | 0.7 | 0.2-1.2 |
| Alanine transaminase (U/L) | 32 | 19-40 |
| Aspartate transaminase (U/L) | 24 | 10-36 |
The chest radiograph was normal. Blood cultures were drawn from the dialysis catheter, which revealed the growth of S. maltophilia. Blood agar classically showed non-hemolytic colonies of S. maltophilia (Figure 1C) and non-lactose fermentation on MacConkey agar (Figure 1D). The organism was susceptible to levofloxacin, and initiation of levofloxacin with the removal of the dialysis catheter resulted in an initial clinical response. Despite initial clinical improvement, she developed right-sided chest pain with desaturation on the sixth day of admission. She developed increasing desaturation, and clinical examination revealed harsh crepitations bilaterally with right-sided predominance. She developed spontaneous hemoptysis and hematochezia within two hours of ICU admission. Computerized tomography of the chest revealed patchy areas of consolidation with diffuse areas of atelectatic changes involving all segments of the right upper and lower lobes along with a few areas of consolidation in the apico-posterior segment of the left upper lobe. Repeat labs revealed Hb of 5 g/dL, WBC of 18,980/mm3, platelet of 195,000/mm3, prothrombin time of 13 seconds, international normalized ratio (INR) of 1.08, and activated partial thromboplastin time (aPTT) of 45 seconds (Table 3).
Table 3. Lab parameters of Case 2 on the sixth day after admission.
g/dL: grams/deciliter; mg/dL: milligrams/deciliter; meq/L: milliequivalents/liter; mm3: cubic millimeter; U/L: units/liter
| Parameter | Values | Reference range |
| Hemoglobin (g/dL) | 5 | 12-16 |
| White blood cell count (/mm3) | 18,980 | 4,500-11,000 |
| Platelet count (/mm3) | 195,000 | 150,000-370,000 |
| Creatinine (mg/dL) | 7.8 | 0.6-1.1 |
| Urea (mg/dL) | 102 | 15-40 |
| Sodium (meq/L) | 134 | 135-145 |
| Potassium (meq/L) | 5.1 | 3.5-5 |
| Chloride (meq/L) | 104 | 96-106 |
| Bicarbonate (meq/L) | 19 | 22-26 |
| Total bilirubin (mg/dL) | 0.6 | 0.2-1.2 |
| Alanine transaminase (U/L) | 38 | 19-40 |
| Aspartate transaminase (U/L) | 32 | 10-36 |
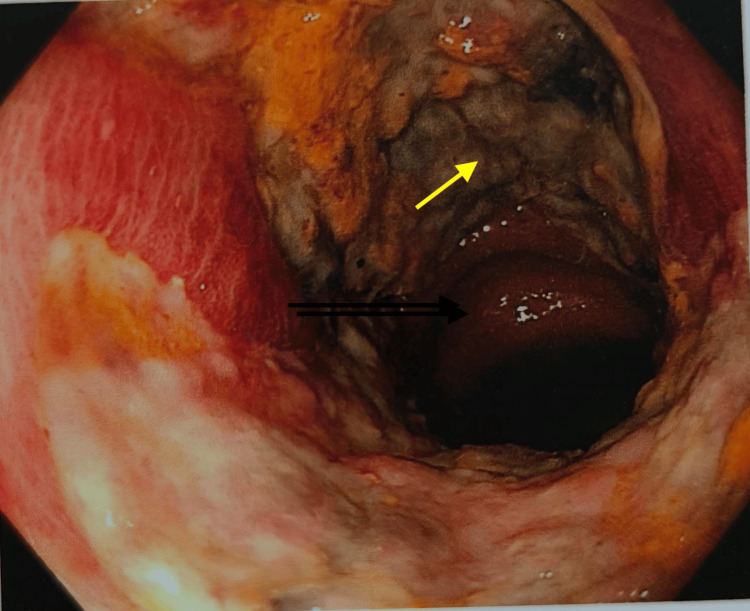
She was immediately dialyzed with optimal ultrafiltration along with packed red blood cell transfusion. Repeat blood cultures were sterile, and sputum culture was non-contributory. She was continued on levofloxacin along with the addition of meropenem. Colonoscopy for hematochezia was suggestive of a large circumferential friable ulcer with areas of necrosis and slough with ileitis (Figure 2).
Figure 2. Colonoscopy showing a large circumferential friable ulcer (yellow arrow) with areas of necrosis and slough (magnification: 600 DPI).
DPI: dots per inch
Amoeba serology was negative. The histology of the ulcer showed only a necrotic ulcer with mild granulation tissue, which was non-contributory to the etiology. The fecal culture was negative. She was gradually weaned off from non-invasive ventilation with appropriate renal dosing of levofloxacin and meropenem. After three weeks of dual antibiotics, her respiratory and intestinal symptoms completely subsided, and she is continuing her thrice weekly hemodialysis via a new right femoral dialysis catheter.
Discussion
S. maltophilia is a gram-negative, nosocomial, multidrug-resistant pathogen that has recently wreaked havoc on the health system with a wide affliction on all organ systems [1]. They have been retrieved from soil, wastewater, hemodialysis water distribution systems, dialysate samples, contaminated chlorhexidine antiseptics, and plastics, with a predilection to form biofilms [1,2]. A Greek multicenter study evaluating common gram-negative bacteria and antimicrobial resistance patterns in hemodialysis units found a prevalence of 13.5% for S. maltophilia, which is a testament to its presence and contamination of dialysate and water distribution systems in developing countries [4]. The source of infection in hemodialysis units due to this unusual organism is attributed to the contaminated hemodialysis water distribution system, contaminated dialysate water, O rings of reprocessed dialyzers, contaminated water for reprocessing dialyzers, adulterated chlorhexidine antiseptics, and contaminated handwashing agents [1,2,5].
S. maltophilia, previously known as Pseudomonas maltophilia, is an opportunistic pathogen that causes dacryocystitis, endophthalmitis, community-acquired pneumonia, biliary sepsis, meningitis, brain abscess, hepatic abscess, enterocolitis, arthritis, osteomyelitis, urinary tract infection, bacteremia, and implant/catheter infection [1,6]. The cause of pneumonia in our second case is due to this malevolent organism since there was no other documented culture positivity in our patient. The risk factors for mortality due to this organism include an elevated Charlson comorbidity index and sequential organ failure assessment (SOFA) score, septic shock, hypoalbuminemia, prior antibiotic use, quinolone resistance, need for invasive ventilation, presence of central venous or hemodialysis catheters, need for continuous renal replacement therapy, hematological malignancies, and chemotherapy [6]. S. maltophilia is a perfect example of a multi-drug-resistant bacteria, and this microbiological characteristic is conferred by the expression of multi-drug efflux pumps, alteration of bacterial lipopolysaccharide and target sites, acquisition of gene resistance by plasmid transfer, and antibiotic-inactivating enzymes [1,3]. The conventional antibiotics of choice for this organism include cotrimoxazole and fluoroquinolones [1,2,7]. However, in recent years, cotrimoxazole- and fluoroquinolone-resistant mutants have emerged due to the overexpression of SmeDEF or SmeVWX efflux pumps, which also confer resistance to second-line antibiotics, such as tigecycline [3]. The intrinsic resistance of this organism to all major conventional antibiotics is due to antibiotic-inactivating enzymes (L1/L2 β-lactamases and aminoglycoside-inactivating enzymes), chromosomally encoded "Qnr" pentapeptide repeat proteins, and the expression of multidrug efflux pumps, making it challenging in terms of treatment and outcome [7]. The higher rate of biofilm formation in this bacterium makes it unamenable to attack by the host immune system, chemical compounds, and susceptible antibiotics, which contribute to the increased clinical load of hemodialysis catheter-related bacteremia [2,8]. Traditionally, it is thought that Stenotrophomonas is intrinsically resistant to carbapenems; however, studies have shown that not all clinical isolates are resistant to carbapenems [9], which was proven by the fact that our second case had a good clinical response to meropenem.
Life-threatening colonic involvement is very rare in S. maltophilia, and it is due to the major extracellular protease (called StmPr1) produced by this bacterium, which is responsible for this severe hemorrhagic process and extensive tissue lesions in the form of colonic ulcer with ileitis [10]. Fecal carriage of this organism ranges between 10% and 30% [10], and this would have contributed to the negative fecal culture report in the background of susceptible antibiotics in our second case. However, by the principle of causality in infectious disease [11], we attribute that these colonic ulcers may have been caused by this unscrupulous organism considering the antecedent clinical events and treatment response. We could not ascertain the source of S. maltophilia contamination, and we postulate that some contamination might have occurred during the reprocessing of the dialyzer in the first case.
Conclusions
Both cases highlight that clinical scenarios can dynamically vary in infections caused by S. maltophilia due to the acquisition of antimicrobial resistance, which is influenced by external environmental factors, leading to contrary clinical outcomes. These cases highlight the importance of active surveillance, refinement of standard hygiene practices, and universal precautions in hemodialysis units.
Acknowledgments
The authors acknowledge the wholehearted encouragement of Dr. Tanuj Moses Lamech for this case report.
Disclosures
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Mathew Gerry George, Tamilarasan V, Jagan V, Leela KV, Arunkumar Asokan, Varadharajan Jayaprakash
Acquisition, analysis, or interpretation of data: Mathew Gerry George, Tamilarasan V
Drafting of the manuscript: Mathew Gerry George, Tamilarasan V, Jagan V, Leela KV, Arunkumar Asokan, Varadharajan Jayaprakash
Critical review of the manuscript for important intellectual content: Mathew Gerry George, Tamilarasan V
Supervision: Varadharajan Jayaprakash
References
- 1.Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Brooke JS. Clin Microbiol Rev. 2012;25:2–41. doi: 10.1128/CMR.00019-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hemodialysis catheter-related bacteremia caused by Stenotrophomonas maltophilia. Kataria A, Lata S, Khillan V. Indian J Nephrol. 2015;25:318–319. doi: 10.4103/0971-4065.157425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mechanisms of antimicrobial resistance in Stenotrophomonas maltophilia: a review of current knowledge. Gil-Gil T, Martínez JL, Blanco P. Expert Rev Anti Infect Ther. 2020;18:335–347. doi: 10.1080/14787210.2020.1730178. [DOI] [PubMed] [Google Scholar]
- 4.Occurrence and antimicrobial resistance of Gram-negative bacteria isolated in haemodialysis water and dialysate of renal units: results of a Greek multicentre study. Arvanitidou M, Vayona A, Spanakis N, Tsakris A. J Appl Microbiol. 2003;95:180–185. doi: 10.1046/j.1365-2672.2003.01966.x. [DOI] [PubMed] [Google Scholar]
- 5.An outbreak of gram-negative bacteremia traced to contaminated O-rings in reprocessed dialyzers. Flaherty JP, Garcia-Houchins S, Chudy R, Arnow PM. Ann Intern Med. 1993;119:1072–1078. doi: 10.7326/0003-4819-119-11-199312010-00003. [DOI] [PubMed] [Google Scholar]
- 6.Advances in the microbiology of Stenotrophomonas maltophilia. Brooke JS. Clin Microbiol Rev. 2021;34:0. doi: 10.1128/CMR.00030-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antimicrobial resistance in Stenotrophomonas spp. Wang Y, He T, Shen Z, Wu C. Microbiol Spectr. 2018;6 doi: 10.1128/microbiolspec.arba-0005-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antimicrobial susceptibility pattern of Stenotrophomonas species isolated from Mexico. Elufisan TO, Luna IC, Oyedara OO, et al. Afr Health Sci. 2020;20:168–181. doi: 10.4314/ahs.v20i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Susceptibility testing of Stenotrophomonas maltophilia to carbapenems. Howe RA, Wilson MP, Walsh TR, Millar MR. J Antimicrob Chemother. 1997;40:13–17. doi: 10.1093/jac/40.1.13. [DOI] [PubMed] [Google Scholar]
- 10.Life-threatening chronic enteritis due to colonization of the small bowel with Stenotrophomonas maltophilia. Hellmig S, Ott S, Musfeldt M, et al. Gastroenterology. 2005;129:706–712. doi: 10.1016/j.gastro.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 11.How to gain evidence for causation in disease and therapeutic intervention: from Koch's postulates to counter-counterfactuals. Evans DW. Med Health Care Philos. 2022;25:509–521. doi: 10.1007/s11019-022-10096-x. [DOI] [PMC free article] [PubMed] [Google Scholar]