Abstract
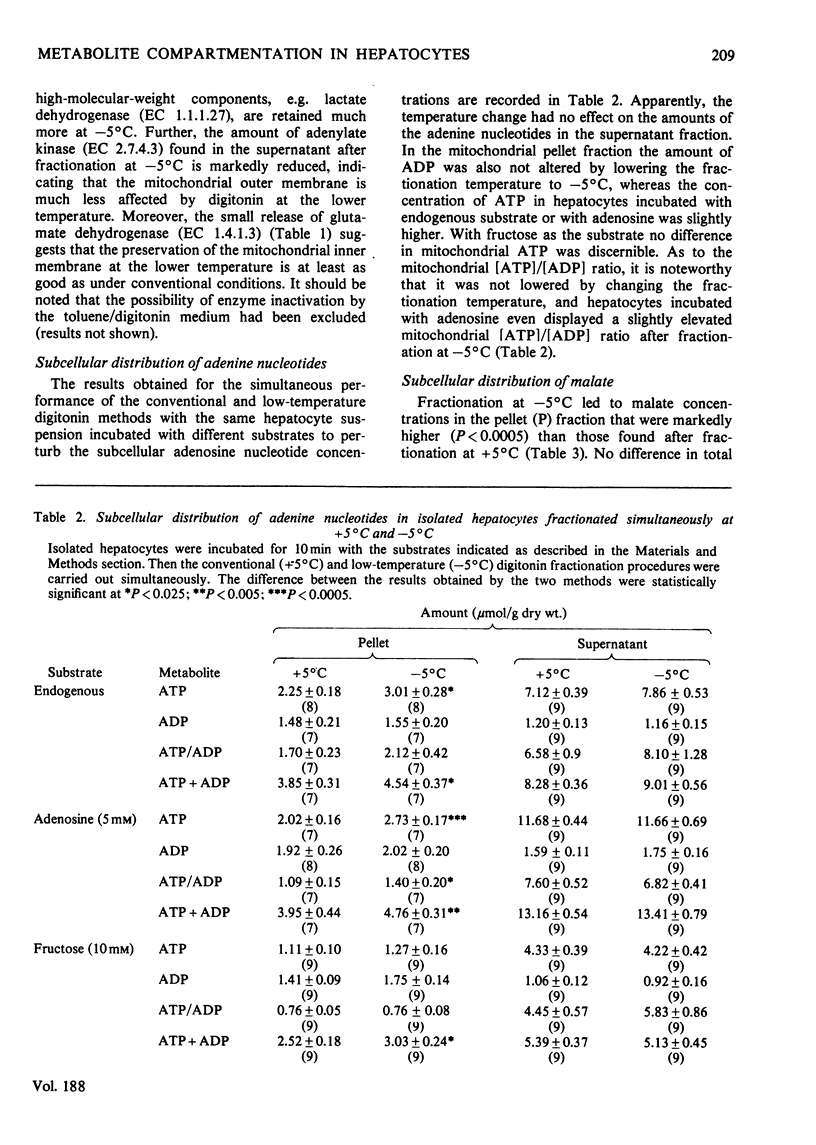
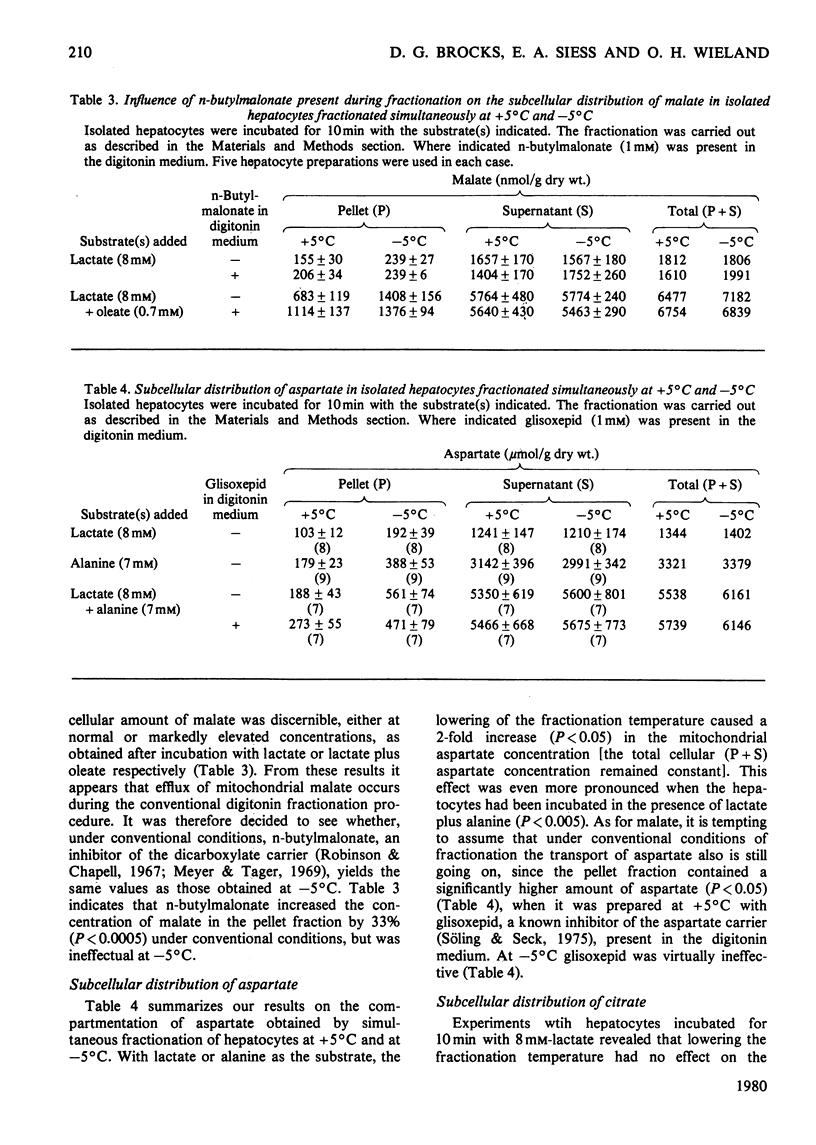
1. A modification of the digitonin method of Zuurendonk & Tager (1974) (Biochim. Biophys. Acta 333, 393-399) (i.e. the 'convaentional' method) was developed that allows the fractionation of isolated hepatocytes at -5 degrees C (i.e. 'low-temperature' method). 2. With respect to compartmentation of adenine nucleotides, glutamate and citrate, the two methods yielded very similar results. 3. In contrast, the mitochondrial amounts of aspartate and malate, as revealed by the low-temperature method, were about twice as high as those found by the conventional procedure. No change in the total cellular content occurred. 4. With n-butylmalonate and glisoxepid present in the conventional digitonin medium, significantly higher amounts of malate and aspartate respectively were found in the mitochondrial pellets. The results obtained by the low-temperature method, however, were not influenced by the these inhibitors. 5. It is concluded that under the conventional conditions of cell fractionation no appreciable redistribution of adenine nucleotides, glutamate and citrate occurs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerboom T. P., Bookelman H., Zuurendonk P. F., van der Meer R., Tager J. M. Intramitochondrial and extramitochondrial concentrations of adenine nucleotides and inorganic phosphate in isolated hepatocytes from fasted rats. Eur J Biochem. 1978 Mar 15;84(2):413–420. doi: 10.1111/j.1432-1033.1978.tb12182.x. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Colbeau A., Nachbaur J., Vignais P. M. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim Biophys Acta. 1971 Dec 3;249(2):462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- Elbers R., Heldt H. W., Schmucker P., Soboll S., Wiese H. Measurement of the ATP/ADP ratio in mitochondria and in the extramitochondrial compartment by fractionation of freeze-stopped liver tissue in non-aqueous media. Hoppe Seylers Z Physiol Chem. 1974 Mar;355(3):378–393. doi: 10.1515/bchm2.1974.355.1.378. [DOI] [PubMed] [Google Scholar]
- Hilderman R. H., Goldblatt P. J., Deutscher M. P. Preparation and characterization of liver cells made permeable to macromolecules by treatment with toluene. J Biol Chem. 1975 Jun 25;250(12):4796–4801. [PubMed] [Google Scholar]
- Meijer A. J., Brouwer A., Reijngoud D. J., Hoek J. B., Tager J. M. Transport of glutamate in rat-liver mitochondria. Biochim Biophys Acta. 1972 Dec 14;283(3):421–429. doi: 10.1016/0005-2728(72)90259-9. [DOI] [PubMed] [Google Scholar]
- Palmieri F., Prezioso G., Quagliariello E., Klingenberg M. Kinetic study of the dicarboxylate carrier in rat liver mitochondria. Eur J Biochem. 1971 Sep 13;22(1):66–74. doi: 10.1111/j.1432-1033.1971.tb01515.x. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Chappell J. B. The inhibition of malate, tricarboxylate and oxoglutarate entry into mitochondria by 2-n-butylmalonate. Biochem Biophys Res Commun. 1967 Jul 21;28(2):249–255. doi: 10.1016/0006-291x(67)90437-8. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Williams G. R., Halperin M. L., Leznoff C. C. The effects of 2-ethylcitrate and tricarballylate on citrate transport in rat liver mitochondria and fatty acid synthesis in rat white adipose tissue. Eur J Biochem. 1970 Aug;15(2):263–272. doi: 10.1111/j.1432-1033.1970.tb01003.x. [DOI] [PubMed] [Google Scholar]
- Siess E. A., Brocks D. G., Lattke H. K., Wieland O. H. Effect of glucagon on metabolite compartmentation in isolated rat liver cells during gluconeogenesis from lactate. Biochem J. 1977 Aug 15;166(2):225–235. doi: 10.1042/bj1660225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess E. A., Brocks D. G., Wieland O. H. Distribution of metabolites between the cytosolic and mitochondrial compartments of hepatocytes isolated from fed rats. Hoppe Seylers Z Physiol Chem. 1978 Jul;359(7):785–798. doi: 10.1515/bchm2.1978.359.2.785. [DOI] [PubMed] [Google Scholar]
- Siess E. A., Brocks D. G., Wieland O. H. Subcellular distribution of key metabolites in isolated liver cells from fasted rats. FEBS Lett. 1976 Oct 15;69(1):265–271. doi: 10.1016/0014-5793(76)80701-6. [DOI] [PubMed] [Google Scholar]
- Siess E. A., Wieland O. H. Phosphorylation state of cytosolic and mitochondrial adenine nucleotides and of pyruvate dehydrogenase in isolated rat liver cells. Biochem J. 1976 Apr 15;156(1):91–102. doi: 10.1042/bj1560091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess E. A., Wieland O. H. Regulation of pyruvate dehydrogenase interconversion in isolated hepatocytes by the mitochondrial ATP/ADP ratio. FEBS Lett. 1975 Apr 1;52(2):226–230. doi: 10.1016/0014-5793(75)80811-8. [DOI] [PubMed] [Google Scholar]
- Soboll S., Scholz R., Heldt H. W. Subcellular metabolite concentrations. Dependence of mitochondrial and cytosolic ATP systems on the metabolic state of perfused rat liver. Eur J Biochem. 1978 Jun 15;87(2):377–390. doi: 10.1111/j.1432-1033.1978.tb12387.x. [DOI] [PubMed] [Google Scholar]
- Söling H. D., Seck A. Precursor specific inhibition of hepatic gluconeogenesis by glisoxepide, an inhibitor of the L-aspartate/L-glutamate antiport system. FEBS Lett. 1975 Mar 1;51(1):52–59. doi: 10.1016/0014-5793(75)80853-2. [DOI] [PubMed] [Google Scholar]
- Tischler M. E., Pachence J., Williamson J. R., La Noue K. F. Mechanism of glutamate-aspartate translocation across the mitochondrial inner membrane. Arch Biochem Biophys. 1976 Apr;173(2):448–461. doi: 10.1016/0003-9861(76)90282-4. [DOI] [PubMed] [Google Scholar]


