Abstract
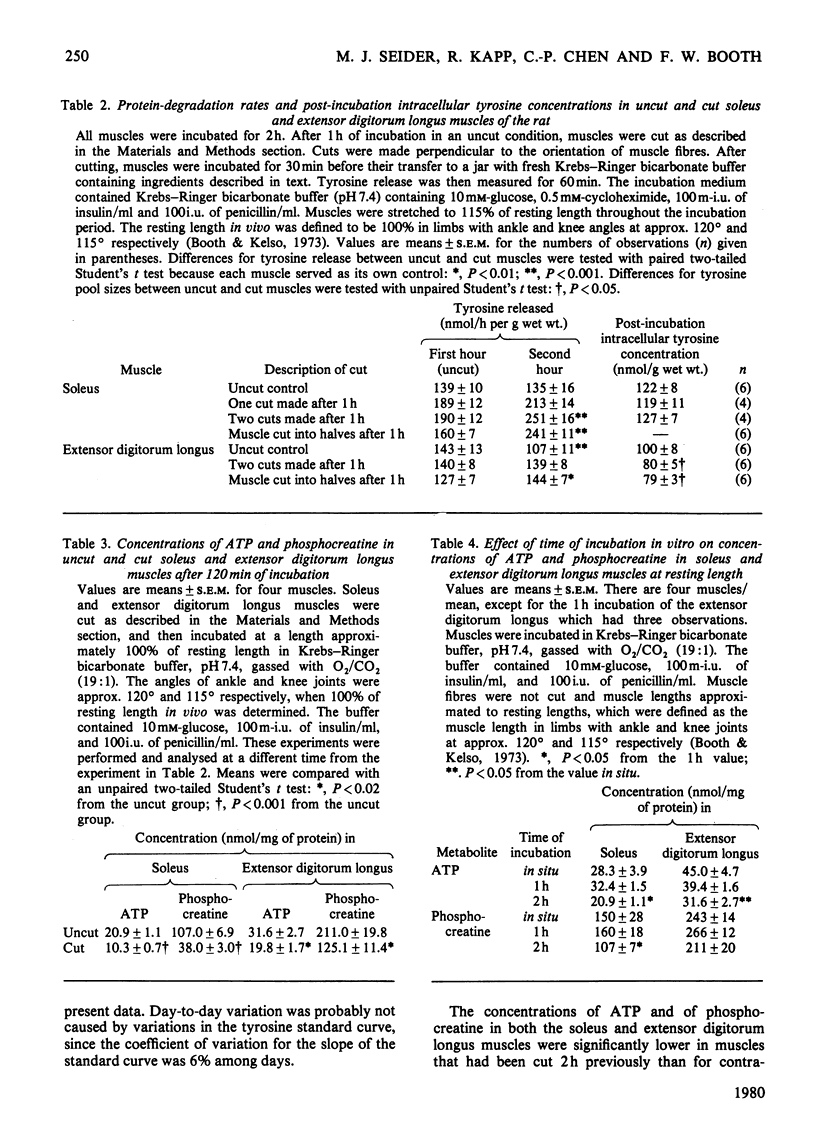
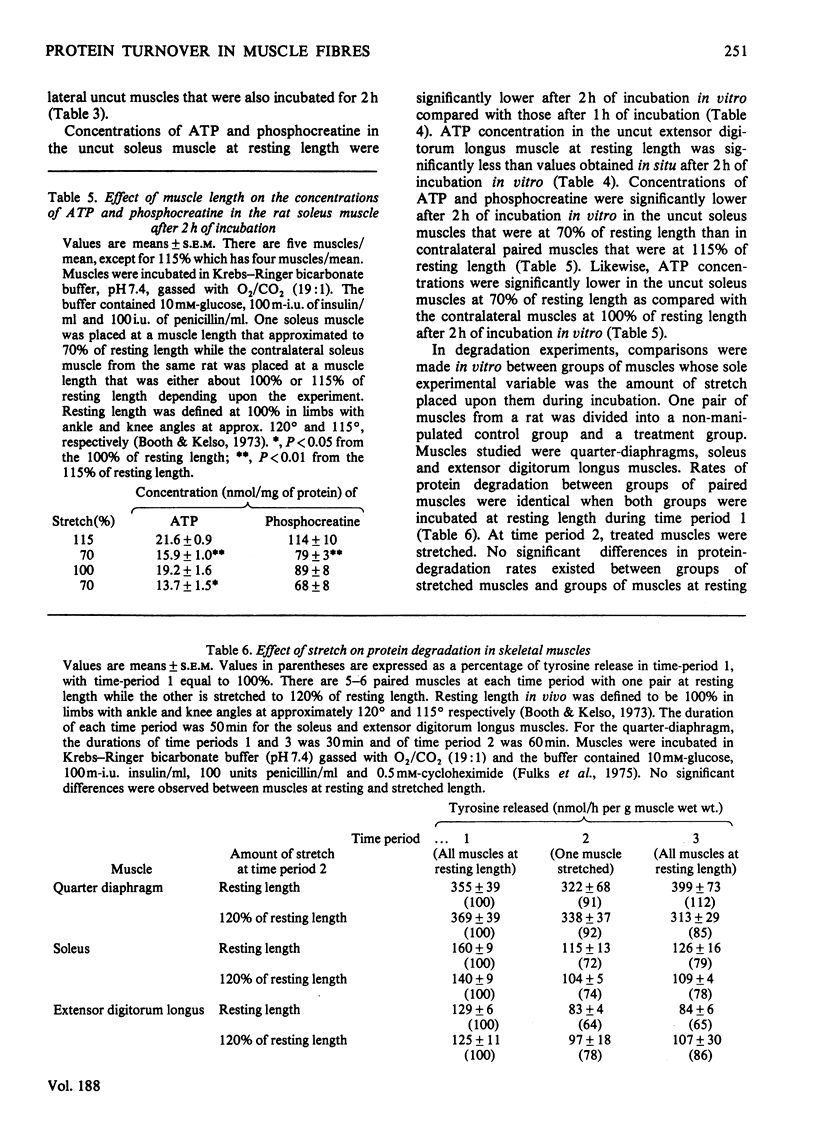
Rates of protein synthesis were significantly lower in the cut soleus and extensor digitorum longus muscles than in their uncut counterparts. Rates of protein degradation were significantly higher in cut soleus muscles, but not in cut extensor digitorum longus muscles as compared with their uncut controls. Concentrations of ATP and phosphocreatine were significantly lower in cut soleus and extensor digitorum longus muscles after incubation in vitro in contrast with respective control uncut muscles. These data indicate that cutting of muscle fibres alters rates of protein synthesis and degradation, in addition to altering concentrations of high-energy phosphates. Since these findings stressed the importance of using intact muscles to study protein metabolism, additional studies were made on intact muscles in vitro. Stretched soleus muscles had higher concentrations of high-energy phosphates at the end of an incubation period than did unstretched muscles. However, the length of the soleus, extensor digitorum longus and diaphragm muscles during incubation did not affect rates of protein degradation.U
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Bergström J., Boström H., Fürst P., Hultman E., Vinnars E. Preliminary studies of energy-rich phosphagens in muscle from severely ill patients. Crit Care Med. 1976 Jul-Aug;4(4):197–204. doi: 10.1097/00003246-197607000-00005. [DOI] [PubMed] [Google Scholar]
- Booth F. W., Kelso J. R. Production of rat muscle atrophy by cast fixation. J Appl Physiol. 1973 Mar;34(3):404–406. doi: 10.1152/jappl.1973.34.3.404. [DOI] [PubMed] [Google Scholar]
- Buse M. G., Biggers J. F., Drier C., Buse J. F. The effect of epinephrine, glucagon, and the nutritional state on the oxidation of branched chain amino acids and pyruvate by isolated hearts and diaphragms of the rat. J Biol Chem. 1973 Jan 25;248(2):697–706. [PubMed] [Google Scholar]
- Chang T. W., Goldberg A. L. The origin of alanine produced in skeletal muscle. J Biol Chem. 1978 May 25;253(10):3677–3684. [PubMed] [Google Scholar]
- Chaudry I. H., Sayeed M. M., Baue A. E. Insulin resistance and its reversal by in vivo infusion of ATP in hermorrhagic shock. Can J Physiol Pharmacol. 1976 Oct;54(5):736–741. doi: 10.1139/y76-102. [DOI] [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- GUROFF G., UNDENFRIEND S. The uptake of tyrosine by isolated rat diaphragm. J Biol Chem. 1960 Dec;235:3518–3522. [PubMed] [Google Scholar]
- Goldberg A. L., Jablecki C., Li J. B. Trophic functions of the neuron. 3. Mechanisms of neurotrophic interactions. Effects of use and disuse on amino acid transport and protein turnover in muscle. Ann N Y Acad Sci. 1974 Mar 22;228(0):190–201. doi: 10.1111/j.1749-6632.1974.tb20510.x. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Goldspink D. F., Goldspink G. Age-related changes in protein turnover and ribonucleic acid of the diaphragm muscle of normal and dystrophic hamsters. Biochem J. 1977 Jan 15;162(1):191–194. doi: 10.1042/bj1620191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink D. F. The influence of immobilization and stretch on protein turnover of rat skeletal muscle. J Physiol. 1977 Jan;264(1):267–282. doi: 10.1113/jphysiol.1977.sp011667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross S. R., Mayer S. E., Longshore M. A. Stimulation of glycogenolysis by beta adrenergic agonists in skeletal muscle of mice with the phosphorylase kinase deficiency mutation (I strain). J Pharmacol Exp Ther. 1976 Sep;198(3):526–538. [PubMed] [Google Scholar]
- Jefferson L. S., Li J. B., Rannels S. R. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem. 1977 Feb 25;252(4):1476–1483. [PubMed] [Google Scholar]
- KIPNIS D. M., CORI C. F. Studies of tissue permeability. III. The effect of insulin on pentose uptake by the diaphragm. J Biol Chem. 1957 Feb;224(2):681–693. [PubMed] [Google Scholar]
- Kameyama T., Etlinger J. D. Calcium-dependent regulation of protein synthesis and degradation in muscle. Nature. 1979 May 24;279(5711):344–346. doi: 10.1038/279344a0. [DOI] [PubMed] [Google Scholar]
- Karlsson J., Willerson J. T., Leshin S. J., Mullins C. B., Mitchell J. H. Skeletal muscle metabolites in patients with cardiogenic shock or severe congestive heart failure. Scand J Clin Lab Invest. 1975 Jan;35(1):73–79. [PubMed] [Google Scholar]
- Laurent G. J., Sparrow M. P., Bates P. C., Millward D. J. Turnover of muscle protein in the fowl. Collagen content and turnover in cardiac and skeletal muscles of the adult fowl and the changes during stretch-induced growth. Biochem J. 1978 Nov 15;176(2):419–427. doi: 10.1042/bj1760419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G. J., Sparrow M. P., Millward D. J. Turnover of muscle protein in the fowl. Changes in rates of protein synthesis and breakdown during hypertrophy of the anterior and posterior latissimus dorsi muscles. Biochem J. 1978 Nov 15;176(2):407–417. doi: 10.1042/bj1760407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. B., Fulks R. M., Goldberg A. L. Evidence that the intracellular pool of tyrosine serves as precursor for protein synthesis in muscle. J Biol Chem. 1973 Oct 25;248(20):7272–7275. [PubMed] [Google Scholar]
- Li J. B., Goldberg A. L. Effects of food deprivation on protein synthesis and degradation in rat skeletal muscles. Am J Physiol. 1976 Aug;231(2):441–448. doi: 10.1152/ajplegacy.1976.231.2.441. [DOI] [PubMed] [Google Scholar]
- Libby P., Goldberg A. L. Leupeptin, a protease inhibitor, decreases protein degradation in normal and diseased muscles. Science. 1978 Feb 3;199(4328):534–536. doi: 10.1126/science.622552. [DOI] [PubMed] [Google Scholar]
- MANCHESTER K. L., KRAHL M. E. Effect of insulin on the incorporation of C14 from C14-labeled carboxylic acids and bicarbonate into the protein of isolated rat diaphragm. J Biol Chem. 1959 Nov;234:2938–2942. [PubMed] [Google Scholar]
- Mallet L. E., Exton J. H., Park C. R. Control of gluconeogenesis from amino acids in the perfused rat liver. J Biol Chem. 1969 Oct 25;244(20):5713–5723. [PubMed] [Google Scholar]
- McGee M. M., Greengard O., Knox W. E. The quantitative determination of phenylalanine hydroxylase in rat tissues. Its developmental formation in liver. Biochem J. 1972 May;127(4):669–674. doi: 10.1042/bj1270669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward D. J. The regulation of muscle-protein turnover in growth and development. Biochem Soc Trans. 1978;6(3):494–499. doi: 10.1042/bst0060494. [DOI] [PubMed] [Google Scholar]
- Odessey R., Khairallah E. A., Goldberg A. L. Origin and possible significance of alanine production by skeletal muscle. J Biol Chem. 1974 Dec 10;249(23):7623–7629. [PubMed] [Google Scholar]
- Palaiologos G., Felig P. Effects of ketone bodies on amino acid metabolism in isolated rat diaphragm. Biochem J. 1976 Mar 15;154(3):709–716. doi: 10.1042/bj1540709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade D. S., Eaton R. P. The regulation of plasma ketone body concentration by counter-regulatory hormones in man. III. Effects of norepinephrine in normal man. Diabetes. 1979 Jan;28(1):5–10. doi: 10.2337/diab.28.1.5. [DOI] [PubMed] [Google Scholar]
- Tarui S., Saito Y., Fujimoto M., Okabayashi T. Effects of insulin on diaphragm muscle independent of the variation of tissue levels of cyclic AMP and cyclic GMP. Arch Biochem Biophys. 1976 May;174(1):192–198. doi: 10.1016/0003-9861(76)90338-6. [DOI] [PubMed] [Google Scholar]
- Turner L. V., Garlick P. J. The effect of unilateral phrenicectomy on the rate of protein synthesis in rat diaphragm in vivo. Biochim Biophys Acta. 1974 Apr 27;349(1):109–113. doi: 10.1016/0005-2787(74)90013-6. [DOI] [PubMed] [Google Scholar]
- WAALKES T. P., UDENFRIEND S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med. 1957 Nov;50(5):733–736. [PubMed] [Google Scholar]
- WOOL I. G., KRAHL M. E. Incorporation of C14-histidine into protein of isolated diaphragms: interaction of fasting, glucose and insulin. Am J Physiol. 1959 Aug;197:367–369. doi: 10.1152/ajplegacy.1959.197.2.367. [DOI] [PubMed] [Google Scholar]


