Abstract
Introduction
Echinococcosis is a widespread zoonotic disease caused by tapeworms of the Echinococcus genus, manifesting in mature or larval forms. Cystic echinococcosis (CE) and alveolar echinococcosis (AE) are the primary types affecting humans, linked, respectively, to Echinococcus granulosus and Echinococcus multilocularis. This study is a systematic review and meta-analysis of the risk factors associated with CE and AE in humans.
Methods
Relevant English publications were found through a thorough search of eligible databases. The inclusion criteria focused on cross-sectional and case–control studies investigating risk factors for human echinococcosis. Collected data included author, country, study design, demographics, sample size, literacy, occupation, drinking water source, dog ownership, and hand hygiene.
Results
A total of 1,594 studies were found in the initial search, with only 36 papers (involving 1,207,436 cases) meeting the inclusion criteria. Most of the study population (99.35%) showed no echinococcosis infection, while 0.65% were infected. Of the infected cases, 77.92% had CE, while 22.08% had AE. Among 629,996 (52.18%) females, 4,830 (0.76%) were infected, compared to 2,968 (0.52%) infections among 565,872 (46.86%) males (p < 0.001). Rural areas, low education levels, agricultural/livestock workers, dog owners, water sources, and poor hand hygiene were all significantly associated with the infection (p < 0.05).
Conclusion
Echinococcosis remains a global health concern, particularly among rural residents, those with lower education, agricultural workers, and dog owners. Targeted public health measures, including improved hygiene practices and access to clean water, are essential to reducing its impact.
Keywords: alveolar echinococcosis, cystic echinococcosis, risk factors, Echinococcus granulosus, hydatid cyst, zoonosis
1. Introduction
Echinococcosis is a widespread zoonotic illness caused by the mature or larval forms of tapeworms found in the Echinococcus genus (Taeniidae family). This disease is among the most ancient ones recorded, origin tracing back to the time of Hippocrates (1). The larval infection, known as hydatid disease or hydatidosis, is identified by the gradual development of metacestode (hydatid) cysts within the intermediate host over an extended period (2). The disease occurs worldwide, with a notable prevalence in regions like Eastern Europe, South Africa, the Middle East, South America, Australia, and the Mediterranean, where cattle and sheep farming is common (1). Cystic echinococcosis (CE) and alveolar echinococcosis (AE) represent the predominant types of human disease. They are triggered by the tapeworms Echinococcus granulosus and Echinococcus multilocularis, respectively (3). Typically, CE persists within the domestic cycle involving dogs and domestic ungulates. It remains a prevalent zoonosis in rural regions where humans adopt domesticated dogs. On the other hand, AE is primarily sustained through a wild cycle involving foxes and rodents, which may have connections with domestic dogs and cats (4). Human infections typically occur incidentally through ingesting the parasite eggs from infected definitive hosts or exposure to other sources like consuming contaminated food or water, direct physical contact, or interacting with infected animals like dogs (5, 6). This disease commonly affects the liver (70%) and lungs (20%); however, there are also instances of the disease occurring in less typical locations, such as the neck, kidneys, heart, vascular system, bladder, and thyroid gland (7–9). This study is a systematic review and meta-analysis of the risk factors associated with CE and AE in humans.
2. Method
2.1. Study design
This meta-analysis followed the Preferred Reporting Items for Meta-Analyses (PRISMA) guidelines.
2.2. Data sources and search strategy
A thorough search in “Google Scholar” and “PubMed” was conducted to identify all relevant publications in English. The search combined terms using Boolean operators, and the keywords were echinococcosis OR echinococci OR hydatidosis OR echinococcus AND transmission OR factor OR prevalence.
2.3. Eligibility criteria
The inclusion criteria encompassed cross-sectional and case–control studies concentrating on risk factors linked to human echinococcosis. Studies were excluded if they: (1) were non-English; (2) only had an abstract available; (3) were case reports or case series; (4) were non-articles, review articles, editorials, or opinion pieces lacking primary data; and (5) lacked a control group. All the included studies were assessed for eligibility (10).
2.4. Study selection and data extraction
The titles and abstracts of the identified studies underwent initial screening before a thorough full-text assessment for eligibility. The data gathered from the studies included the first author’s name, country, type of study design, gender, residency, sample size, number of infected and healthy cases, level of education, occupation, drinking water source, dog ownership, and hand hygiene.
2.5. Statistical analysis
Data collection and organization were conducted using Microsoft Excel (2019), while data analysis was performed using Statistical Package for Social Sciences (SPSS) software (v.25). The data were presented in frequency, and percentage. The qualitative analysis was performed utilizing the Chi-squared test, with a significant level determined at a p-value of 0.05 or less.
3. Results
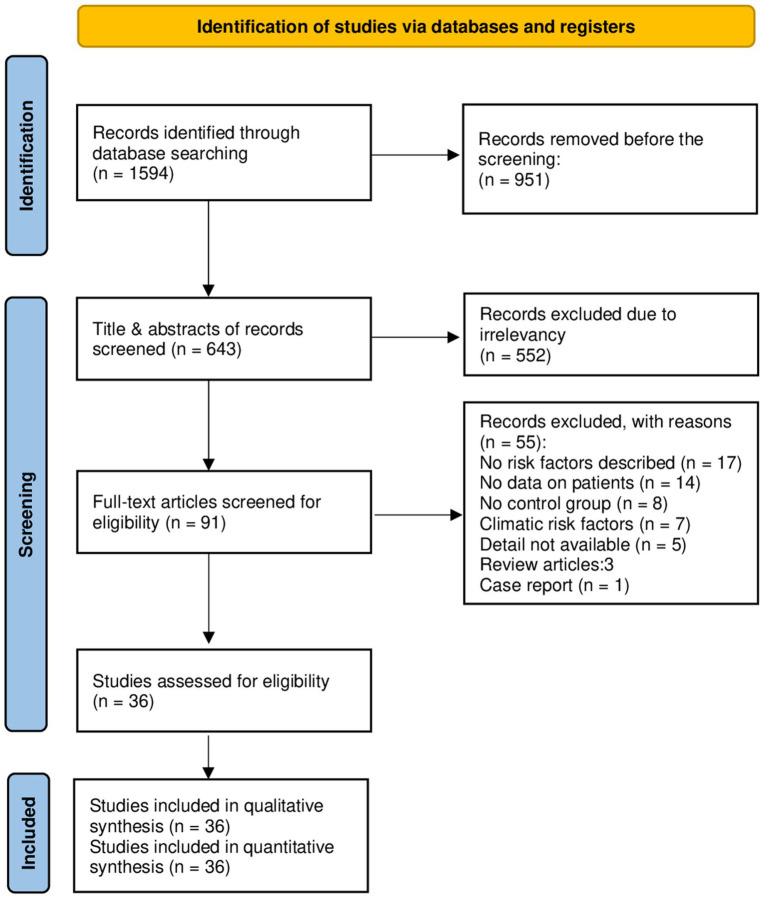
A total of 1,594 studies were found in the initial search, 951 of which were directly removed due to irrelevancy. Then, the titles and abstracts of the remaining 643 papers were screened, of which 552 papers were excluded. The full text of the remaining 91 papers was read, of which 55 papers were excluded, and then finally, 36 papers (3, 6, 11–44) involving 1,207,436 cases were compatible with the inclusion criteria (Figure 1). Among these included studies, 27 (75%) were cross-sectional, while 9 (25%) were case–control. The vast majority of cases (99.35%) were non-infected, while 7,815 cases (0.65%) were identified as infected. Specifically, 6,089 cases (77.92%) presented with CE, while only 1,726 cases (22.08%) had AE (Table 1). China reported the largest number of echinococcosis cases, with 5,748 infections, representing 73.55% of the total cases recorded. Iran ranks next, with 803 cases, accounting for 10.27% (Table 2). The majority of cases, 6,262 (80.13%), were diagnosed using a combination of serology and ultrasound or other imaging methods. Serology alone was used in 917 cases (11.74%), ultrasound alone in 303 (3.87%), and in 333 cases (4.26%), the diagnostic method was unrecorded. In terms of gender distribution, the total number of females was 629,996 (52.18%), with 4,830 (0.77%) being infected, whereas the total number of males was 565,872 (46.86%), with 2,968 (0.52%) infected (p < 0.001). However, the gender of 11,568 individuals (0.96%) was not specified (Table 3). Furthermore, among the rural population totaling 25,369 individuals, 1,594 cases (6.29%) were infected, in contrast to 268 cases (2.70%) among the urban population of 9,893 individuals (p < 0.001). Out of participants with lower educational attainment, 5,392 (0.73%) were infected, whereas among those with higher educational levels, only 912 (0.22%) were infected (p < 0.001). In the occupational context, 5,640 cases (0.62%) of agricultural/livestock workers and 1,483 cases (0.57%) in other occupations were infected, with a statistically significant difference (p = 0.012). Moreover, 1,424 (6.24%) of dog owners were infected, while 682 (3.57%) of non-dog owners had the infection (p < 0.001). The infection rate among participants consuming public water supply was 272 cases (1.60%), whereas among those using alternative water sources, 94 cases (2.54%) were infected (p < 0.001). Participants adhering to proper hand hygiene practices before meals had a lower infection rate (1.14%) compared to those with inadequate hand hygiene (2.80%; p < 0.001; Table 4).
Figure 1.
The PRISMA flow chart detailing the process of study selection.
Table 1.
The raw data of the included studies.
| Author | Study design | Country | Total Cases | Infected | Non-infected | Gender | Resident | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M+ | M- | F+ | F- | NA | Rural + | Rural - | Urban + | Urban - | NA | ||||||
| Acosta-Jamett et al. (11) | Cross-sectional | Chile | 384 | 10 | 374 | 5 | 125 | 5 | 249 | 0 | 10 | 374 | 0 | 0 | 0 |
| Acosta-Jamett et al. (12) | Cross-sectional | Chile | 2,439 | 39 | 2,400 | 17 | 871 | 22 | 1,529 | 0 | 24 | 737 | 15 | 1,663 | 0 |
| Ahmed et al. (13) | Cross-sectional | Sudan | 305 | 20 | 285 | 7 | 105 | 13 | 180 | 0 | NA | NA | NA | NA | 305 |
| Akalin et al. (14) | Cross-sectional | Turkey | 1,133 | 78 | 1,055 | 29 | 501 | 49 | 554 | 0 | 78 | 1,055 | 0 | 0 | 0 |
| Bai et al. (15) | Cross-sectional | China | 882 | 99 | 783 | 33 | NA | 66 | NA | 783 | 99 | 783 | 0 | 0 | 0 |
| Barati et al. (16) | Cross-sectional | Iran | 1,232 | 12 | 1,220 | 4 | 520 | 8 | 700 | 0 | NA | NA | NA | NA | 1,232 |
| Bingham et al. (17) | Cross-sectional | Argentina | 340 | 33 | 307 | 11 | 66 | 22 | 241 | 0 | NA | NA | NA | NA | 340 |
| Bitrus et al. (18) | Cross-sectional | Nigeria | 187 | 6 | 181 | 3 | 108 | 3 | 73 | 0 | NA | NA | NA | NA | 187 |
| Carmona et al. (19) | Cross-sectional | Florida & Uruguay | 9,521 | 156 | 9,365 | 70 | 4,366 | 86 | 4,999 | 0 | NA | NA | NA | NA | 9,521 |
| Craig et al. (20) | Cross-sectional | China | 2,479 | 84 | 2,395 | 32 | 1,370 | 52 | 1,025 | 0 | 84 | 2,395 | 0 | 0 | 0 |
| Dorjsuren et al. (21) | Cross-sectional | Mongolia | 1829 | 87 | 1742 | 30 | 547 | 57 | 1,195 | 0 | NA | NA | NA | NA | 1829 |
| Harandi et al. (22) | Cross-sectional | Iran | 1,062 | 77 | 985 | 4 | 183 | 73 | 802 | 0 | NA | NA | NA | NA | 1,062 |
| Kashinahanji et al. (23) | Cross-sectional | Iran | 400 | 5 | 395 | 3 | 151 | 2 | 244 | 0 | 4 | 188 | 1 | 207 | 0 |
| Khabisi et al. (24) | Cross-sectional | Iran | 551 | 22 | 529 | 7 | 163 | 15 | 366 | 0 | 22 | 529 | 0 | 0 | 0 |
| Ma et al. (3) | Cross-sectional | China | 1,150,680 | 5,216 | 1,145,464 | 2099 | 545,192 | 3,117 | 600,272 | 0 | NA | NA | NA | NA | 1,150,680 |
| Moshfe et al. (25) | Cross-sectional | Iran | 1,005 | 81 | 924 | 11 | 216 | 70 | 708 | 0 | 81 | 924 | 0 | 0 | 0 |
| Muhtarov (26) | Cross-sectional | Bulgaria | 642 | 8 | 634 | 4 | 4 | 298 | 336 | 0 | 8 | 634 | 0 | 0 | 0 |
| Ok et al. (27) | Cross-sectional | Turkey | 6,093 | 9 | 6,084 | NA | NA | NA | NA | 6,093 | 5 | 1848 | 4 | 4,236 | 0 |
| Othieno et al. (28) | Cross-sectional | Uganda | 3,066 | 71 | 2,995 | 24 | 865 | 47 | 2,130 | 0 | 71 | 2,995 | 0 | 0 | 0 |
| Rafiei et al. (29) | Cross-sectional | Iran | 3,446 | 475 | 2,971 | 176 | 1,105 | 299 | 1866 | 0 | 475 | 2,971 | 0 | 0 | 0 |
| Romig et al. (30) | Cross-sectional | Germany | 2,540 | 60 | 2,480 | 31 | 1,161 | 29 | 1,319 | 0 | 60 | 2,480 | 0 | 0 | 0 |
| Safarpour et al. (31) | Cross-sectional | Iran | 1,500 | 131 | 1,369 | 52 | 697 | 79 | 672 | 0 | NA | NA | NA | NA | 1,500 |
| Tamarozzi et al. (32) | Cross-sectional | Peru | 1,304 | 41 | 1,263 | 13 | 365 | 28 | 898 | 0 | NA | NA | NA | NA | 1,304 |
| Uchiumi et al. (6) | Cross-sectional | Argentina | 892 | 42 | 850 | 24 | 354 | 18 | 496 | 0 | 18 | 192 | 24 | 658 | 0 |
| Wang et al. (33) | Cross-sectional | China | 3,676 | 66 | 3,610 | NA | NA | NA | NA | 3,676 | NA | NA | NA | NA | 3,676 |
| Wang et al. (34) | Cross-sectional | China | 705 | 60 | 645 | 25 | 325 | 35 | 320 | 0 | 60 | 645 | 0 | 0 | 0 |
| Wang et al. (35) | Cross-sectional | China | 7,138 | 223 | 6,915 | 91 | 3,334 | 132 | 3,581 | 0 | 164 | 4,614 | 59 | 2,301 | 0 |
| Anuk and Çantay (36), | Case–control | Turkey | 189 | 87 | 102 | 42 | 47 | 45 | 55 | 0 | 66 | 76 | 21 | 26 | 0 |
| Campos-Bueno et al. (37) | Case–control | Spain | 254 | 127 | 127 | 58 | 58 | 69 | 69 | 0 | 91 | 94 | 36 | 33 | 0 |
| Dowling et al. (38) | Case–control | Jordan | 176 | 44 | 132 | 18 | 54 | 26 | 78 | 0 | 0 | 0 | 0 | 0 | 176 |
| Kreidl et al. (39) | Case–control | Austria | 105 | 21 | 84 | 14 | NA | 7 | NA | 84 | NA | NA | NA | NA | 105 |
| Laivacuma et al. (40) | Case–control | Latvia | 92 | 46 | 46 | 14 | 14 | 32 | 32 | 0 | 15 | 2 | 31 | 44 | 0 |
| Larrieu et al. (41) | Case–control | Argentina | 90 | 24 | 66 | NA | NA | NA | NA | 90 | 19 | 34 | 5 | 32 | 0 |
| Moro et al. (42) | Case–control | Peru | 96 | 32 | 64 | NA | NA | NA | NA | 96 | 32 | 64 | 0 | 0 | 0 |
| Piarroux et al. (43) | Case–control | France | 746 | 180 | 566 | NA | NA | NA | NA | 697 | 108 | 141 | 72 | 425 | 0 |
| Schmidberger, et al. (44) | Case–control | Germany | 257 | 43 | 214 | 17 | 37 | 26 | 177 | 0 | NA | NA | NA | NA | 257 |
M+: Infected males, M-: Non-infected males, F+: infected Females, F-: non-infected Females, Rural+: Infected Rural residents, Rural-: Non-infected rural residents, N/A: Not Available Urban+: Infected urban residents, Urban-: Non-Infected urban residents, NA: Not available.
Table 2.
The number of echinococcosis cases identified in the current study across various countries.
| Country | No. of infected cases | Percentage (%) |
|---|---|---|
| China | 5,748 | 73.55% |
| Iran | 803 | 10.27% |
| France | 180 | 2.30% |
| Turkey | 174 | 2.23% |
| Uruguay | 156 | 2.00% |
| Spain | 127 | 1.63% |
| Germany | 103 | 1.32% |
| Argentina | 99 | 1.27% |
| Mongolia | 87 | 1.11% |
| Peru | 73 | 0.93% |
| Uganda | 71 | 0.91% |
| Chile | 49 | 0.63% |
| Latvia | 46 | 0.59% |
| Jordan | 44 | 0.56% |
| Austria | 21 | 0.26% |
| Sudan | 20 | 0.26% |
| Bulgaria | 8 | 0.10% |
| Nigeria | 6 | 0.08% |
Table 3.
Basic characteristics of the included studies.
| Variables | Frequency/Percentage |
|---|---|
| Healthy | 1,199,621 (99.35%) |
| Infected | 7,815 (0.65%) |
| Gender | |
| Male | 565,872 (46.86%) |
| Infected | 2,968 (0.52%) |
| Non-infected | 562,904 (99.48%) |
| Female | 629,996 (52.18%) |
| Infected | 4,830 (0.77%) |
| Non-infected | 625,166 (99.23%) |
| NA | 11,568 (0.96%) |
| Type of echinococcosis | |
| Alveolar echinococcosis | 1,726 (22.08%) |
| Cystic echinococcosis | 6,089 (77.92%) |
| Diagnostic methods | |
| Serology | 917 (11.74%) |
| Ultrasound | 303 (3.87%) |
| Serology + ultrasound or other diagnostic imaging | 6,262 (80.13%) |
| NA | 333 (4.26%) |
| Residency | |
| Rural | 25,369 (2.10%) |
| Infected | 1,594 (6.29%) |
| Non-infected | 23,775 (93.71%) |
| Urban | 9,893 (0.82%) |
| Infected | 268 (2.70%) |
| Non-infected | 9,625 (97.30%) |
| NA | 1,172,174 (97.08%) |
| Literacy | |
| Low education level | 742,535 (61.50%) |
| Infected | 5,392 (0.73%) |
| Non-infected | 737,143 (99.27) |
| High education level | 421,252 (34.89%) |
| Infected | 912 (0.22%) |
| Non-infected | 420,340 (99.78) |
| NA | 43,649 (3.61%) |
| Occupation | |
| Agricultural/livestock worker | 918,773 (76.10%) |
| Infected | 5,640 (0.62%) |
| Non-infected | 913,133 (99.38%) |
| Non-agricultural/livestock worker | 259,799 (21.51%) |
| Infected | 1,483 (0.57%) |
| Non-infected | 258,316 (99.43%) |
| NA | 28,864 (2.39%) |
| Owning dogs | |
| Dog owners | 22,807 (1.89%) |
| Infected | 1,424 (6.24%) |
| Non-infected | 21,383 (93.76%) |
| Non-dog owners | 19,098 (1.58%) |
| Infected | 682 (3.57%) |
| Non-infected | 18,416 (96.42%) |
| NA | 1,165,531 (96.53) |
| Water source | |
| Public water supply | 17,005 (1.41%) |
| Infected | 272 (1.59%) |
| Non-infected | 16,733 (98.40%) |
| Other water sources | 3,706 (0.31%) |
| Infected | 94 (2.54%) |
| Non-infected | 3,612 (97.46%) |
| NA | 1,186,725 (98.28%) |
| Hand hygiene | |
| Handwashing before meals | 6,564 (0.54%) |
| Infected | 75 (1.14%) |
| Non-infected | 6,489 (98.86%) |
| Not handwashing before meals | 2,250 (0.19%) |
| Infected | 63 (2.80%) |
| Non-infected | 2,187 (97.20%) |
| NA | 1,198,622 (99.27%) |
NA: Not available.
Table 4.
The comparison between patient demographics, dog ownership, drinking water sources, and hand hygiene practices with human echinococcosis infection.
| Variables | Total | p-value | |||
|---|---|---|---|---|---|
| Infected | Non-infected | ||||
| Resident | Rural | 25,369 | 1,594 (6.29%) | 23,775 (93.71%) | < 0.001 |
| Urban | 9,893 | 268 (2.70%) | 9,625 (97.30%) | ||
| Gender | Male | 565,872 | 2,968 (0.52%) | 562,904 (99.48%) | < 0.001 |
| Female | 629,996 | 4,830 (0.77%) | 625,166 (99.23%) | ||
| Literacy | Illiterate or primary school education | 742,535 | 5,392 (0.73%) | 737,143 (99.27) | < 0.001 |
| secondary and high school education | 421,252 | 912 (0.22%) | 420,340 (99.78) | ||
| Occupation | Agricultural/livestock workers | 918,773 | 5,640 (0.62%) | 913,133 (99.38%) | 0.012 |
| Non-agricultural/livestock workers | 259,799 | 1,483 (0.57%) | 258,316 (99.43%) | ||
| Owning dogs | Dog owners | 22,807 | 1,424 (6.24%) | 21,383 (93.76%) | < 0.001 |
| Non-dog owners | 19,098 | 682 (3.57%) | 18,416 (96.42%) | ||
| Water source | Public supply | 17,005 | 272 (1.59%) | 16,733 (98.40%) | < 0.001 |
| Other water sources | 3,706 | 94 (2.54%) | 3,612 (97.46%) | ||
| Hand hygiene | Hand washing before meals | 6,564 | 75 (1.14%) | 6,489 (98.86%) | < 0.001 |
| Not handwashing before meals | 2,250 | 63 (2.80%) | 2,187 (97.20%) | ||
4. Discussion
Human echinococcosis, a prevalent parasitic infection, presents a considerable health and economic burden to society, yet it remains largely neglected as a disease (4). This disease typically thrives in nations with extensive livestock farming regions. However, it has become a pressing global health issue, largely attributed to increasing immigration rates and travel activities (45). The present study revealed that China had the highest number of echinococcosis cases, comprising 73.55% of all recorded cases. Iran followed with 10.27%. In contrast, European countries such as France, Turkey, and Germany had lower infection rates, each contributing less than 3%. Other countries, including Mongolia, Peru, and African nations like Uganda and Sudan, accounted for even smaller proportions. This distribution highlights significant regional differences in the incidence of echinococcosis.
The progression of the infection is gradual, with the initial phase of primary infection typically asymptomatic. Small cysts that do not cause significant disease may remain symptomless for extended periods, potentially leading to incidental detection. Parasite eggs can remain viable in various environments for several months to years. The precise duration of the incubation period for CE is uncertain but likely spans many months to years. Symptoms may arise if the cysts rupture or exert pressure due to their size (2, 12). Typically, AE manifests at a later stage than the cystic form. Cases of AE are marked by an initial asymptomatic incubation period lasting 5–15 years, followed by a chronic course. If left untreated or inadequately managed, AE can lead to high fatality rates (2).
Regarding transmission and risk factors, domestic dogs serve as the primary definitive host for both echinococcus species, posing the most significant risk of transmitting both CE and AE to humans (46). In a study by Ahmed et al., individuals in contact with dogs were at least twice as prone to acquiring an echinococcal infection (13). Dogs become infected by consuming offal from livestock that contains cysts. Afterward, they excrete the parasite eggs in their feces, contaminating the environment, including soil, water, and pastures. Livestock get the infection by ingesting these eggs while grazing. Human infection typically arises from consuming contaminated food or water (6). However, a review by Possenti and colleagues indicated that the primary means of transmitting human CE appears to be through the contamination of hands with eggs of E. granulosus excreted by dogs, either directly or indirectly (47). In line with these findings, the current study found that owning a dog significantly increases the likelihood of contracting echinococcosis, and there was also a correlation between inadequate hand hygiene and echinococcosis infection.
Otero-Abad et al. found that females are at a higher risk of echinococcosis than males. This heightened risk is attributed to their greater involvement in household chores, such as food preparation and pet care, which increases their exposure to infected dogs, soil, and vegetables (4). The current study also revealed that females exhibited a significantly higher risk of developing echinococcosis compared to males.
Schantz et al. indicated that individuals who own livestock are three times more likely to be diagnosed with this disease compared to those who do not own livestock (48). Additionally, pastoralism stands out as the occupation carrying the highest risk of contracting both types of echinococcosis. This is attributed to pastoralists’ proximity to livestock, dogs, and wildlife host species (46). The present study indicated that agricultural and livestock workers faced a greater risk of infection with echinococcosis than individuals in other occupations.
Regarding education, Ma et al. demonstrated that lower levels of education are associated with an increased risk of echinococcosis infection (3). In line with this finding, the present study found a significant association between lower education levels and a higher incidence of echinococcosis infection. This may be caused by the fact that educated individuals have higher knowledge about the risk of the disease and the factors that can protect against infection. Additionally, higher-educated individuals typically reside in urban areas and have a lower chance of contact with livestock or hosts.
The overall prevalence of human CE is significantly linked with rural residency, older age, and consumption of non-piped water (12). Othieno et al. indicated that unprotected open spring water sources have been identified as a risk factor in the occurrence of CE. These water sources are often shared with livestock and dogs. The frequency of sharing water increases notably during periods of water scarcity, such as the dry season and drought (28). In this study, the infection rate of echinococcosis was notably higher among participants who consumed water from sources other than public or tap water. Rural residents exhibited a significantly higher incidence of echinococcosis than urban residents.
Early detection of AE and CE can significantly enhance the effectiveness of managing and treating these conditions (49). The definitive diagnosis of AE and CE is typically achieved through physical imaging techniques, including radiology, ultrasonography, computed tomography (CT) scan, and magnetic resonance imaging (MRI) (50, 51). Serological tests, including enzyme-linked immunosorbent assay (ELISA), demonstrate high specificity for diagnosing hydatid disease; however, a positive result does not accurately indicate the cyst’s location within the body. In contrast, imaging modalities provide precise visualization, allowing for the detection of cysts in specific sites. This limitation highlights the necessity of combining serological testing with imaging to confirm both the diagnosis and the exact location of the hydatid cyst (52). In the present study, most cases (80.13%) were diagnosed through imaging techniques and serological testing together.
Case series and small clinical trials indicate a mortality rate of 2–4% for CE, but this rate significantly rises with inadequate treatment and care. Human AE is estimated to cause approximately 18,000 cases annually. Survival analysis indicates that without treatment or with limited access to it, mortality rates exceed 90% within 10–15 years after diagnosis (49). Because the present study aimed solely to shed light on the associated risk factors, it could not provide data regarding the mortality rate among infected cases.
Options for managing CE include surgery, percutaneous sterilization, medication, and observation. Early AE typically requires surgical treatment, but patients unsuitable for surgery or who have undergone surgical removal of parasite lesions must undergo prolonged treatment with benzimidazoles like albendazole or mebendazole (49, 53).
To effectively combat hydatid cyst disease, a comprehensive strategy is essential. This includes interrupting the transmission of Echinococcus from animals to people via deworming initiatives, appropriate disposal of animal remains, and maintaining good hygiene. Raising awareness through community education plays a significant role. Regular monitoring and cooperative actions are key to early detection. Timely intervention, including surgical procedures and medication, is crucial. Sustained control efforts depend on educating the populace and promoting collaboration. An integrated approach that combines prevention, monitoring, treatment, and education is vital for addressing hydatid cyst disease in developing countries (54).
5. Conclusion
Echinococcosis remains a significant public health issue, with higher infection rates linked to rural residency, lower education levels, agricultural work, dog ownership, inadequate hand hygiene, and alternative water sources. These findings highlight the need for targeted public health measures, such as educational initiatives and improved access to clean water and hygiene practices. Strengthened governmental guidelines addressing these risk factors could help reduce the global impact of echinococcosis.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
FK: Conceptualization, Data curation, Validation, Visualization, Writing – review & editing. KA: Investigation, Methodology, Resources, Validation, Writing – review & editing. HAh: Conceptualization, Formal analysis, Methodology, Software, Validation, Writing – review & editing. IH: Conceptualization, Data curation, Methodology, Resources, Writing – review & editing. HeK: Conceptualization, Resources, Validation, Visualization, Writing – review & editing. HN: Validation, Visualization, Writing – original draft, Writing – review & editing. HAb: Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. ST: Investigation, Methodology, Project administration, Visualization, Writing – review & editing. HaK: Conceptualization, Methodology, Resources, Validation, Writing – review & editing. AH: Data curation, Formal analysis, Software, Visualization, Writing – review & editing. DG: Data curation, Formal analysis, Methodology, Software, Writing – review & editing. HAs: Conceptualization, Data curation, Resources, Validation, Writing – review & editing. AM: Formal analysis, Investigation, Methodology, Supervision, Writing – review & editing. BA: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Writing – review & editing. DE: Data curation, Formal analysis, Methodology, Software, Visualization, Writing – review & editing. RR: Data curation, Project administration, Software, Visualization, Writing – review & editing. KH: Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Salih AM, Abdulla ZY, Mohammed DA, Jwamer VI, Ali PG, Hamasaeed AG, et al. Hydatid cyst of thyroid gland, a rare case report with a literature review. Int J Surg Case Rep. (2020) 67:267–70. doi: 10.1016/j.ijscr.2020.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet. (2003) 362:1295–304. doi: 10.1016/S0140-6736(03)14573-4, PMID: [DOI] [PubMed] [Google Scholar]
- 3.Ma T, Wang Q, Hao M, Xue C, Wang X, Han S, et al. Epidemiological characteristics and risk factors for cystic and alveolar echinococcosis in China: an analysis of a national population-based field survey. Parasit Vectors. (2023) 16:181. doi: 10.1186/s13071-023-05788-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Otero-Abad B, Torgerson PR. A systematic review of the epidemiology of echinococcosis in domestic and wild animals. PLoS Negl Trop Dis. (2013) 7:e2249. doi: 10.1371/journal.pntd.0002249, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organisation (2021). Echinococcosis. Who.int. World Health Organization: WHO. Available at: https://www.who.int/news-room/fact-sheets/detail/echinococcosis (Accessed May 5, 2024).
- 6.Uchiumi L, Mujica G, Araya D, Salvitti JC, Sobrino M, Moguillansky Moguillansky S, et al. Prevalence of human cystic echinococcosis in the towns of Ñorquinco and Ramos Mexia in Rio Negro Province, Argentina, and direct risk factors for infection. Parasit Vectors. (2021) 14:262. doi: 10.1186/s13071-021-04753-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baba HO, Salih AM, Abdullah HO, Hassan HA, Ali RK, Salih RQ, et al. Primary hydatid cyst of the posterior neck; a case report with literature review. Int J Surgery Open. (2022) 40:100449. doi: 10.1016/j.ijso.2022.100449, PMID: 37244108 [DOI] [Google Scholar]
- 8.Hussein DM, Kakamad FH, HamaAmin BJ, Baqi DH, Tahir SH, Salih AM, et al. Hydatid cyst in the pulmonary artery; a systematic review. Barw Med J. (2023) 1:8–13. doi: 10.58742/bmj.v1i1.11 [DOI] [Google Scholar]
- 9.Hasan RA, Abdullah F, Saeed BT. Hydatid cysts of the bladder: a systematic review of the literature. Barw Med J. (2023) 1:21–4. doi: 10.58742/bmj.v1i2.46 [DOI] [Google Scholar]
- 10.Abdullah HO, Abdalla BA, Kakamad FH, Ahmed JO, Baba HO, Hassan MN, et al. Predatory publishing lists: a review on the ongoing Battle against fraudulent actions. Barw Med J. (2024) 2:26–30. doi: 10.58742/bmj.v2i2.91 [DOI] [Google Scholar]
- 11.Acosta-Jamett G, Weitzel T, Boufana B, Adones C, Bahamonde A, Abarca K, et al. Prevalence and risk factors for echinococcal infection in a rural area of northern Chile: a household-based cross-sectional study. PLoS Negl Trop Dis. (2014) 8:e3090. doi: 10.1371/journal.pntd.0003090, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acosta-Jamett G, Hernández FA, Castro N, Tamarozzi F, Uchiumi L, Salvitti JC, et al. Prevalence rate and risk factors of human cystic echinococcosis: a cross-sectional, community-based, abdominal ultrasound study in rural and urban north-Central Chile. PLoS Negl Trop Dis. (2022) 16:e0010280. doi: 10.1371/journal.pntd.0010280, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed ME, Abdalla SS, Adam IA, Grobusch MP, Aradaib IE. Prevalence of cystic echinococcosis and associated risk factors among humans in Khartoum state, Central Sudan. Int Health. (2021) 13:327–33. doi: 10.1093/inthealth/ihaa059, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akalin S, Kutlu SS, Caylak SD, Onal O, Kaya S, Bozkurt Aİ. Seroprevalence of human cystic echinococcosis and risk factors in animal breeders in rural communities in Denizli, Turkey. J Infect Dev Count. (2014) 8:1188–94. doi: 10.3855/jidc.4343, PMID: [DOI] [PubMed] [Google Scholar]
- 15.Bai Y, Cheng N, Jiang C, Wang Q, Cao D. Survey on cystic echinococcosis in Tibetans, West China. Acta Trop. (2002) 82:381–5. doi: 10.1016/S0001-706X(02)00038-4, PMID: [DOI] [PubMed] [Google Scholar]
- 16.Barati R, Sharifi-Sarasiabi K, Hamedi Y, Matini M, Shamseddin J. Seroprevalence of Hydatidosis in Kaboodarahang, Hamadan Province, Iran, in 2016-2017. Hormozgan Med J. (2018) 22:e86498. doi: 10.5812/hmj.86498 [DOI] [Google Scholar]
- 17.Bingham GM, Budke CM, Larrieu E, Del Carpio M, Mujica G, Slater MR, et al. A community-based study to examine the epidemiology of human cystic echinococcosis in Rio Negro Province, Argentina. Acta Trop. (2014) 136:81–8. doi: 10.1016/j.actatropica.2014.04.005, PMID: [DOI] [PubMed] [Google Scholar]
- 18.Bitrus D, Weka R, Yakubu R, Ogo IN, Kamani J, Ikeh E. Seroprevalence and associated risk factors of human cystic echinococcosis in some parts of plateau state, Nigeria. Niger J Parasitol. (2020) 41:30–4. doi: 10.4314/njpar.v41i1.5 [DOI] [Google Scholar]
- 19.Carmona C, Perdomo R, Carbo A, Alvarez C, Monti J, Grauert R, et al. Risk factors associated with human cystic echinococcosis in Florida, Uruguay: results of a mass screening study using ultrasound and serology. Am J Trop Med Hyg. (1998) 58:599–605. doi: 10.4269/ajtmh.1998.58.599, PMID: [DOI] [PubMed] [Google Scholar]
- 20.Craig PS, Giraudoux P, Shi D, Bartholomot B, Barnish G, Delattre P, et al. An epidemiological and ecological study of human alveolar echinococcosis transmission in South Gansu, China. Acta Trop. (2000) 77:167–77. doi: 10.1016/S0001-706X(00)00134-0, PMID: [DOI] [PubMed] [Google Scholar]
- 21.Dorjsuren T, Ganzorig S, Dagvasumberel M, Tsend-Ayush A, Ganbold C, Ganbat M, et al. Prevalence and risk factors associated with human cystic echinococcosis in rural areas, Mongolia. PLoS One. (2020) 15:e0235399. doi: 10.1371/journal.pone.0235399, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harandi MF, Moazezi SS, Saba M, Grimm F, Kamyabi H, Sheikhzadeh F, et al. Sonographical and serological survey of human cystic echinococcosis and analysis of risk factors associated with seroconversion in rural communities of Kerman, Iran. Zoonoses Public Health. (2011) 58:582–8. doi: 10.1111/j.1863-2378.2011.01407.x, PMID: [DOI] [PubMed] [Google Scholar]
- 23.Kashinahanji M, Bakhtiari M, Foroughi-Parvar F. Seroprevalence of human cystic echinococcosis and risk factors in Nahavand, Hamadan, Western Iran. Avicenna J f Clin Microbiol Infect. (2023) 10:75–9. doi: 10.34172/ajcmi.3442 [DOI] [Google Scholar]
- 24.Khabisi SA, Khorashad AS, Moghaddasi HA, Almasi Z, Rezaei Z, Etemadi S. Seroprevalence of human hydatid cyst: a cross sectional study in a rural areas of Zahedan, southeastern Iran. Ann Parasitol. (2021) 67:691–6. doi: 10.17420/ap6704.385, PMID: [DOI] [PubMed] [Google Scholar]
- 25.Moshfe A, Sarkari B, Arefkhah N, Nikbakht R, Shahriarirad R, Rezaei Z, et al. Seroepidemiological study of cystic echinococcosis in nomadic communities in the southwest of Iran: a population-based study. J Immunoass Immunochem. (2019) 40:183–92. doi: 10.1080/15321819.2018.1547974, PMID: [DOI] [PubMed] [Google Scholar]
- 26.Muhtarov M. First portable ultrasound based screening study in Bulgaria on the prevalence of cystic echinococcosis in Kardzhali district. Trakia J Sci. (2014) 12:170–4. [Google Scholar]
- 27.Ok ÜZ, Özkol M, Kilimcioğlu AA, Dinç G, Bayındır P, Östan İ, et al. A province-based study using sampling method to investigate the prevalence of cystic echinococcosis among primary school children in Manisa, Turkey. Acta Trop. (2007) 103:116–22. doi: 10.1016/j.actatropica.2007.05.013, PMID: [DOI] [PubMed] [Google Scholar]
- 28.Othieno E, Okwi AL, Mupere E, Zeyhle E, Oba P, Chamai M, et al. Risk factors associated with cystic echinococcosis in humans in selected pastoral and agro-pastoral areas of Uganda (2017) 3:1–6. doi: 10.14202/IJOH.2017.1-6, [DOI] [Google Scholar]
- 29.Rafiei A, Hemadi A, Maraghi S, Kaikhaei B, Craig PS. Human cystic echinococcosis in nomads of south-West Islamic Republic of Iran. East Medit Health J. (2007) 13:41–8. PMID: [PubMed] [Google Scholar]
- 30.Romig T, Kratzer W, Kimmig P, Frosch M, Gaus WI, Flegel WA, et al. An epidemiologic survey of human alveolar echinococcosis in southwestern Germany. Römerstein study group. Am J Trop Med Hyg. (1999) 61:566–73. doi: 10.4269/ajtmh.1999.61.566, PMID: [DOI] [PubMed] [Google Scholar]
- 31.Safarpour AR, Omidian M, Pouryousef A, Fattahi MR, Sarkari B. Serosurvey of cystic echinococcosis and related risk factors for infection in Fars Province, southern Iran: a population-based study. Biomed Res Int. (2022) 2022:1–6. doi: 10.1155/2022/3709694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamarozzi F, Hou A, Morales ML, Giordani MT, Vilca F, Mozo K, et al. Prevalence and risk factors for human cystic echinococcosis in the Cusco region of the Peruvian highlands diagnosed using focused abdominal ultrasound. Am J Trop Med Hyg. (2017) 96:1472–7. doi: 10.4269/ajtmh.16-0882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang YH, Rogan MT, Vuitton DA, Wen H, Bartholomot B, Macpherson CN, et al. Cystic echinococcosis in semi-nomadic pastoral communities in north-West China. Trans R Soc Trop Med Hyg. (2001) 95:153–8. doi: 10.1016/S0035-9203(01)90142-7, PMID: [DOI] [PubMed] [Google Scholar]
- 34.Wang Q, Vuitton DA, Qiu J, Giraudoux P, Xiao Y, Schantz PM, et al. Fenced pasture: a possible risk factor for human alveolar echinococcosis in Tibetan pastoralist communities of Sichuan, China. Acta Trop. (2004) 90:285–93. doi: 10.1016/j.actatropica.2004.02.004, PMID: [DOI] [PubMed] [Google Scholar]
- 35.Wang Q, Qiu J, Yang W, Schantz PM, Raoul F, Craig PS, et al. Socioeconomic and behavior risk factors of human alveolar echinococcosis in Tibetan communities in Sichuan, People's Republic of China. Am J Trop Med Hyg. (2006) 74:856–62. doi: 10.4269/ajtmh.2006.74.856, PMID: [DOI] [PubMed] [Google Scholar]
- 36.Anuk T, Çantay H. Determination of factors affecting human transmission of Echinococcus granulosus parasite: a case-control study, Turkey. Turkiye Parazitol Derg. (2022) 46:201–6. doi: 10.4274/tpd.galenos.2022.73792, PMID: [DOI] [PubMed] [Google Scholar]
- 37.Campos-Bueno AN, López-Abente G, Andrés-Cercadillo AM. Risk factors for Echinococcus granulosus infection: a case-control study. Am J Trop Med Hyg. (2000) 62:329–34. doi: 10.4269/ajtmh.2000.62.329, PMID: [DOI] [PubMed] [Google Scholar]
- 38.Dowling PM, Abo-Shehada MN, Torgerson PR. Risk factors associated with human cystic echinococcosis in Jordan: results of a case-control study. Ann Trop Med Parasitol. (2000) 94:69–75. doi: 10.1080/00034983.2000.11813514, PMID: [DOI] [PubMed] [Google Scholar]
- 39.Kreidl P, Allerberger F, Judmaier G, Auer H, Aspöck H, Hall AJ. Domestic pets as risk factors for alveolar hydatid disease in Austria. Am J Epidemiol. (1998) 147:978–81. doi: 10.1093/oxfordjournals.aje.a009388, PMID: [DOI] [PubMed] [Google Scholar]
- 40.Laivacuma S, Deksne G, Jokelainen P, Ivanovs A, Zaharova L, Zeltiņa I, et al. Risk factors for human cystic echinococcosis in Latvia. Vector-Borne Zoonotic Dis. (2019) 19:430–3. doi: 10.1089/vbz.2018.2354, PMID: [DOI] [PubMed] [Google Scholar]
- 41.Larrieu EJ, Costa MT, Del Carpio M, Moguillansky S, Bianchi G, Yadon ZE. A case-control study of the risk factors for cystic echinococcosis among the children of Rio Negro province, Argentina. Ann Trop Med Parasitol. (2002) 96:43–52. doi: 10.1179/000349802125000501 [DOI] [PubMed] [Google Scholar]
- 42.Moro PL, Cavero CA, Tambini M, Briceño Y, Jiménez R, Cabrera L. Identification of risk factors for cystic echinococcosis in a peri-urban population of Peru. Trans R Soc Trop Med Hyg. (2008) 102:75–8. doi: 10.1016/j.trstmh.2007.09.010 [DOI] [PubMed] [Google Scholar]
- 43.Piarroux M, Piarroux R, Knapp J, Bardonnet K, Dumortier J, Watelet J, et al. Populations at risk for alveolar echinococcosis, France. Emerg Infect Dis. (2013) 19:721–8. doi: 10.3201/eid1905.120867, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidberger J, Uhlenbruck J, Schlingeloff P, Maksimov P, Conraths FJ, Mayer B, et al. Dog ownership and risk for alveolar echinococcosis, Germany. Emerg Infect Dis. (2022) 28:1597–605. doi: 10.3201/eid2808.212514, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abdullah HO, Abdalla BA, Mohammed-Saeed DH, Tahir SH, Fattah FH, Hassan SJ, et al. A comprehensive study of pericardial hydatid cyst: systematic review and Meta-data presentation. Barw Med J. (2023) 1:14–23. doi: 10.58742/bmj.v1i1.12 [DOI] [Google Scholar]
- 46.Zhenghuan W, Xiaoming W, Xiaoqing L. Echinococcosis in China, a review of the epidemiology of Echinococcus spp. EcoHealth. (2008) 5:115–26. doi: 10.1007/s10393-008-0174-0 [DOI] [PubMed] [Google Scholar]
- 47.Possenti A, Manzano-Román R, Sanchez-Ovejero C, Boufana B, La Torre G, Siles-Lucas M, et al. Potential risk factors associated with human cystic echinococcosis: systematic review and meta-analysis. PLoS Negl Trop Dis. (2016) 10:e0005114. doi: 10.1371/journal.pntd.0005114, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schantz PM, Wang H, Qiu J, Liu FJ, Saito E, Emshoff A, et al. Echinococcosis on the Tibetan plateau: prevalence and risk factors for cystic and alveolar echinococcosis in Tibetan populations in Qinghai Province, China. Parasitology. (2003) 127:S109–20. doi: 10.1017/S0031182003004165, PMID: [DOI] [PubMed] [Google Scholar]
- 49.McManus DP, Gray DJ, Zhang W, Yang Y. Diagnosis, treatment, and management of echinococcosis. BMJ. (2012) 344:e3866. doi: 10.1136/bmj.e3866, PMID: [DOI] [PubMed] [Google Scholar]
- 50.Pawłowski ZS, Eckert J, Vuitton DA, Ammann RW, Kern P, Craig PS, et al. (2001). Echinococcosis in humans: clinical aspects, diagnosis and treatment. WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. pp. 20–66.
- 51.Qadir AA, Abdullah IY, Ali MB, Mohammed RO, Dhair HM, Saeed YA, et al. Presentation and Management of Thyroid Hydatid Cyst: a comprehensive systematic review of the literature. Barw Med J. (2024) 2:56–62. doi: 10.58742/mp68zz49 [DOI] [Google Scholar]
- 52.Maleki F, Akhlaghi L, Tabatabaie F. Evaluation of hydatid cyst antigen for serological diagnosis. Med J Islam Repub Iran. (2023):37. doi: 10.47176/mjiri.37.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahmed OF, Hamodat OM, Abdullah F, Ali MH, Kakamad FH, Hussein DM, et al. Capitonnage method for surgical Management of Pulmonary Hydatid Cysts: a case series. Barw Med J. (2023) 1:2–6. doi: 10.58742/bmj.v1i2.26 [DOI] [Google Scholar]
- 54.Hussein DA. What are the strategies for controlling hydatid cysts in developing countries? Barw Med J. (2024) 2:1–2. doi: 10.58742/bmj.v2i1.69 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.