Abstract
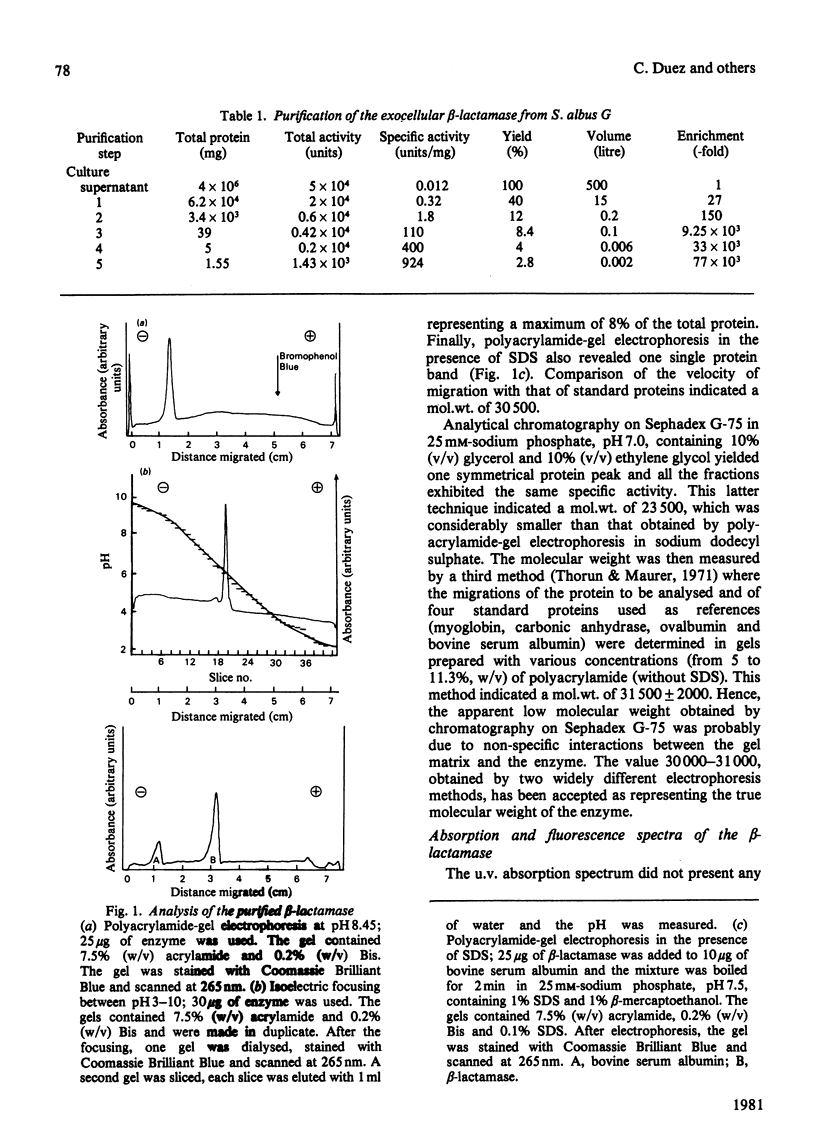
The exocellular beta-lactamase of Streptomyces albus G has been purified to near protein homogeneity. It consists of one single polypeptide chain of mol.wt. 30 000-31 000, has a rather low isoelectric point (at pH 6.0) and contains less lysine (2.1%) and more half-cystine residues than most beta-lactamases from other Gram-positive bacteria. Penicillins are much better substrates than delta 3-cephalosporins; the catalytic-centre activity of good penicillin substrates is 333-500 s-1. The exocellular, mol.wt. 17 000 DD-carboxypeptidase of S. albus G [previously purified to protein homogeneity; Duez, Frère, Geurts, Ghuysen, Dierickx & Delcambe (1978) Biochem. J. 175, 793-800] behaves as an exceedingly poor beta-lactamase, hydrolysing benzylpenicillin into benzylpenicilloate 5 x 10(-6)-fold less rapidly than does the exocellular beta-lactamase. To all appearances, the beta-lactamase has no bivalent cation requirement whereas, as shown elsewhere [Dideberg, Charlier, Dupont, Vermeire, Frère & Ghuysen (1980) FEBS Lett. 117, 212-214, and Dideberg, Joris, Frère, Ghuysen, Weber, Robaye, Delbrouck & Roelands (1980) FEBS Lett. 117, 215-218], the DD-carboxypeptidase possesses one essential Zn2+ ion per molecule. Peptide 'mapping' and immunological studies suggest that the two Streptomyces enzymes probably have very different structural and mechanistic properties.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Corran P. H., Waley S. G. The reaction of penicillin with proteins. Biochem J. 1975 Aug;149(2):357–364. doi: 10.1042/bj1490357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. B., Abraham E. P. Separation, purification and properties of beta-lactamase I and beta-lactamase II from Bacillus cereus 569/H/9. Biochem J. 1974 Oct;143(1):115–127. doi: 10.1042/bj1430115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dideberg O., Charlier P., Dupont L., Vermeire M., Frere J. M., Ghuysen J. M. The 4.5 A resolution structure analysis of the exocellular DD-carboxypeptidase of Streptomyces albus G. FEBS Lett. 1980 Aug 11;117(1):212–214. doi: 10.1016/0014-5793(80)80947-1. [DOI] [PubMed] [Google Scholar]
- Dideberg O., Joris B., Frere J. M., Ghuysen J. M., Weber G., Robaye R., Delbrouck J. M., Roelandts I. The exocellular DD-carboxypeptidase of Streptomyces albus G: a metallo (Zn2+) enzyme. FEBS Lett. 1980 Aug 11;117(1):215–218. doi: 10.1016/0014-5793(80)80948-3. [DOI] [PubMed] [Google Scholar]
- Duez C., Frère J. M., Geurts F., Ghuysen J. M., Dierickx L., Delcambe L. The exocellular DD-carboxypeptidase-endopeptidase from Streptomyces albus G. Purification and chemical properties. Biochem J. 1978 Dec 1;175(3):793–800. doi: 10.1042/bj1750793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J., Belasco J. G., Charnas R. L., Khosla S., Knowles J. R. Beta-lactamase inactivation by mechanism-based reagents. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):309–319. doi: 10.1098/rstb.1980.0048. [DOI] [PubMed] [Google Scholar]
- Frere J., Ghuysen J., Degelaen J., Loffet A., Perkins H. R. Fragmentation of benzylpenicillin after interaction with the exocellular DD-carboxypeptidase-transpeptidases of Streptomyces R61 and R39. Nature. 1975 Nov 13;258(5531):168–170. doi: 10.1038/258168a0. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Geurts F., Ghuysen J. M. The exocellular DD-carboxypeptidase-endopeptidase of Streptomyces albus G. Interaction with beta-lactam antibiotics. Biochem J. 1978 Dec 1;175(3):801–805. doi: 10.1042/bj1750801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frère J. M., Ghuysen J. M., Perkins H. R., Nieto M. Molecular weight and amino acid composition of the exocellular DD-carboxypeptidase-transpeptidase of Streptomyces R61. Biochem J. 1973 Nov;135(3):463–468. doi: 10.1042/bj1350463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frére J. M., Leyh-Bouille M., Ghuysen J. M., Nieto M., Perkins H. R. Exocellular DD-carboxypeptidases-transpeptidases from Streptomyces. Methods Enzymol. 1976;45:610–636. doi: 10.1016/s0076-6879(76)45054-1. [DOI] [PubMed] [Google Scholar]
- Fuad N., Frère J. M., Ghuysen J. M., Duez C., Iwatsubo M. Mode of interaction between beta-lactam antibiotics and the exocellular DD-carboxypeptidase--transpeptidase from Streptomyces R39. Biochem J. 1976 Jun 1;155(3):623–629. doi: 10.1042/bj1550623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuysen J. M., Frère J. M., Leyh-Bouille M., Coyette J., Dusart J., Nguyen-Distèche M. Use of model enzymes in the determination of the mode of action of penicillins and delta 3-cephalosporins. Annu Rev Biochem. 1979;48:73–101. doi: 10.1146/annurev.bi.48.070179.000445. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M., Leyh-Bouille M., Bonaly R., Nieto M., Perkins H. R., Schleifer K. H., Kandler O. Isolation of DD carboxypeptidase from Streptomyces albus G culture filtrates. Biochemistry. 1970 Jul 21;9(15):2955–2961. doi: 10.1021/bi00817a004. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Hammarström S., Strominger J. L. Degradation of penicillin G to phenylacetylglycine by D-alanine carboxypeptidase from Bacillus stearothermophilus. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3463–3467. doi: 10.1073/pnas.72.9.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K., Duez C., Frère J. M., Ghuysen J. M. Beta-lactamases (Actinomycetes species). Methods Enzymol. 1975;43:687–698. doi: 10.1016/0076-6879(75)43134-2. [DOI] [PubMed] [Google Scholar]
- Johnson K., Dusart J., Campbell J. N., Ghuysen J. M. Exocellular beta-lactamases of Streptomyces albus G and strains R39 and K11. Antimicrob Agents Chemother. 1973 Feb;3(2):289–298. doi: 10.1128/aac.3.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott-Hunziker V., Waley S. G., Orlek B. S., Sammes P. G. Penicillinase active sites: labelling of serine-44 in beta-lactamase I by 6beta-bromopenicillanic acid. FEBS Lett. 1979 Mar 1;99(1):59–61. doi: 10.1016/0014-5793(79)80248-3. [DOI] [PubMed] [Google Scholar]
- Le Goffic F., Andrillon-Spiegel J., Letarte R. Purification des beta-lactamases par chromatographie d'affinité. Biochimie. 1975;57(1):29–34. doi: 10.1016/s0300-9084(75)80106-4. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Proceedings of the biochemical society. Biochem J. 1966 Jan;98(1):1–16P. [PMC free article] [PubMed] [Google Scholar]
- Schneider C. H., de Weck A. L. Studies on the direct neutral penicilloylation of functional groups occurring on proteins. Biochim Biophys Acta. 1968 Sep 10;168(1):27–35. doi: 10.1016/0005-2795(68)90230-4. [DOI] [PubMed] [Google Scholar]


