Abstract
Background
We investigated whether patients with diabetes who had good control of both low-density lipoprotein cholesterol (LDL-C) and triglycerides (TG) would be associated with better long-term clinical outcomes after percutaneous coronary intervention (PCI).
Methods and Results
Using our PCI registry (Fu-Registry), the 1,006 cases with diabetes were divided into 4 groups: Group 1, LDL-C ≥100 mg/dL and TG ≥175 mg/dL; Group 2, LDL-C <100 mg/dL and TG ≥175 mg/dL; Group 3, LDL-C ≥100 mg/dL and TG <175 mg/dL; and Group 4, LDL-C <100 mg/dL and TG <175 mg/dL. The primary endpoint during the follow-up period (median follow up of 1,984 days) was defined as major adverse cardiac events (MACEs). Additionally, all coronary events were defined as a secondary endpoint. The incidence rates of MACEs were as follows: Group 1, 38%; Group 2, 26%; Group 3, 31%; and Group 4, 27% (P=0.074), and the rates tended to be higher in Group 1. All coronary events were as follows: Group 1, 66%; Group 2, 56%; Group 3, 58%; and Group 4, 51% (P=0.032).
Conclusions
In patients with diabetes who underwent PCI, the LDL-C and TG levels in Group 4 met secondary prevention targets for coronary artery disease and these patients showed better long-term clinical outcomes compared with those in other groups.
Key Words: Diabetes, Low-density lipoprotein cholesterol, Major adverse cardiac events, Percutaneous coronary intervention, Triglycerides
It has been clearly demonstrated that low-density lipoprotein cholesterol (LDL-C) levels are significantly correlated with prevention of both the onset and progression of coronary artery disease (CAD). This finding is consistent across numerous epidemiological surveys conducted in the United States (US) and Europe, including the US Framingham study,1 as well as cohort studies targeting Japanese individuals. In Japan, the Guidelines for the Prevention of Atherosclerotic Cardiovascular Diseases 20222 set a secondary prevention target for CAD at <100 mg/dL for LDL-C levels, recommending aggressive control with statins and other strong medications. In each category of dyslipidemia, target values have been established for management. Achieving the LDL-C management target should be prioritized. Furthermore, actively controlling LDL-C levels is considered to be crucial for improving clinical outcomes after percutaneous coronary intervention (PCI). In contrast, it has been reported3 that the greater the number of risk factors for CAD, the higher the incidence and mortality rates of CAD. Diabetes (DM) is listed as a major risk factor for CAD, along with high LDL-C levels. The probability of the coexistence of DM and high LDL-C levels is high, and their coexistence further increases the incidence of CAD. LDL-C-lowering therapy in DM patients has been shown to have a suppressive effect on CAD, which requires strict control. The aforementioned Guidelines for the Prevention of Atherosclerotic Cardiovascular Diseases 20222 set a strict target LDL-C level of <70 mg/dL for DM patients who also have other risk factors (such as non-cardiogenic stroke, peripheral arterial disease, chronic kidney disease, metabolic syndrome, overlapping major risk factors, and smoking).
Regarding triglycerides (TGs), elevated TG levels have been reported4 to be associated with the risk of CAD. In epidemiological surveys in Japan, the onset of CAD increased with fasting TG levels of ≥150 mg/dL,5 and with TG levels exceeding 167 mg/dL, there is an increased risk of myocardial infarction (MI), effort angina, sudden death,6 and overall CAD risk.7 In the NIPPON DATA80 study,3 the risk of CAD increased when TG levels were more than 210 mg/dL compared with levels of 150–179 mg/dL. In the aforementioned Guidelines for the Prevention of Atherosclerotic Cardiovascular Diseases 2022,2 the target TG control levels were revised to 175 mg/dL (non-fasting) and 150 mg/dL (fasting) for both primary and secondary prevention. It has been reported that lowering TG levels has a suppressive effect on recurrent CAD.8 However, it is also understood that a significant effect in secondary prevention is not necessarily achieved.9 At present, there are many unclear aspects about whether correcting elevated TG levels improves the prognosis in patients with CAD. However, in a subanalysis in the JDCS study,10 it was indicated that LDL-C and TG are strong risk factors for CAD in Japanese patients with type 2 DM.
Thus, controlling not only LDL-C but also TG levels in Japanese DM patients with CAD who have undergone PCI may improve long-term clinical outcomes. We hypothesized that DM patients with good control of both LDL-C and TG would have better long-term clinical outcomes after PCI. Using our department’s PCI registry (FU-Registry), we evaluated the association between the lipid profile (LDL-C and TG levels at the time of PCI) and long-term clinical outcomes over a 5-year follow-up period.
Methods
Patients
We registered 2,394 cases from Fukuoka University Hospital and Fukuoka University Chikushi Hospital where PCI was performed between April 2003 and March 2016. These cases were followed up with coronary angiography at 9–12 months after PCI and were further tracked for long-term clinical outcomes in the FU-Registry. We excluded 23 cases with untraceable follow up, 104 cases who underwent hemodialysis (HD), and 1,261 cases without DM (non-DM), resulting in a study cohort of 1,006 cases eligible for analysis. Based on the results of a blood test at the time of PCI, patients were divided into 4 groups to determine whether the secondary prevention and management targets for CAD according to the secondary prevention target values in the Japan Atherosclerosis Society’s Guidelines for the Prevention of Atherosclerotic Cardiovascular Diseases 2022 (LDL-C <100 mg/dL and TG <175 mg/dL) were achieved: Group 1 (n=125), LDL-C ≥100 mg/dL and TG ≥175 mg/dL; Group 2 (n=102), LDL-C <100 mg/dL and TG ≥175 mg/dL; Group 3 (n=395), LDL-C ≥100 mg/dL and TG <175 mg/dL; and Group 4 (n=384), LDL-C <100 mg/dL and TG <175 mg/dL. Comparative analyses, including patient backgrounds, lesion characteristics (angiographical quantitative and qualitative analyses), and long-term treatment outcomes, were conducted. This study was approved by the Ethics Committee of Fukuoka University Hospital (Reference no. 10-1-08[09-105]), and performed in accordance with the Declaration of Helsinki and the ethical standards of the Independent Review Board of Fukuoka University (Trial registration no. UMIN000005679).
Clinical Outcome
The primary endpoint was defined as major adverse cardiac events (MACEs), which included death from all causes, MI, and any revascularization events related to the target lesion post-PCI, including target lesion revascularization (TLR-PCI) or coronary artery bypass grafting (CABG). Additionally, all coronary events occurring during the follow-up period were defined as ‘all coronary events’, which included MI, TLR-PCI/CABG, target vessel revascularization (TVR)-PCI /CABG, and non-target vessel PCI/CABG. These events were evaluated as one of the assessment criteria. The median duration of follow up for the study cohort was 1,984 days (mean 1,304±839 days). Clinical follow up was conducted for all cases approximately 5 years after PCI, with confirmation of the occurrence and timing of MACEs through the review of the final outpatient records at the respective facilities and communication with primary care physicians. Blood test results included data from fasting samples taken on the morning of scheduled PCI for elective cases and non-fasting data for cases undergoing emergency PCI, collected at the time of the procedure.
Lipid Profile and MI Definition
Blood test results included data from fasting samples collected on the morning of scheduled elective PCI cases, and non-fasting data collected at the time of the procedure for cases undergoing emergency PCI. LDL-C levels were measured directly rather than estimated using the Friedewald equation. Non-HDL-C was calculated by subtracting HDL-C (measured directly) from total cholesterol (TC). MI included both ST-elevation MI and non-ST-elevation MI, and was defined as meeting any of the following criteria: clear ischemic electrocardiography changes, elevated cardiac enzymes (positive high-sensitivity troponin T, creatine kinase (CK) more than twice the upper limit of normal (248 for men and 153 for women), or CK-MB exceeding the upper limit of normal).
PCI and Intravascular Ultrasound (IVUS) Procedure
PCI was performed for cases with ≥50% stenosis on coronary angiography, with chest symptoms, or in cases where cardiac muscle ischemia was demonstrated using non-invasive tests (treadmill stress electrocardiogram, myocardial perfusion imaging) or by fractional flow reserve (FFR). The procedural endpoint was defined as achieving no dissection causing blood flow disturbance, obtaining Thrombolysis in Myocardial Infarction III flow, and ensuring ≤10% stenosis on angiography. IVUS was performed at the discretion of the operator and was used in >90% of all procedures, with analysis focused solely on minimum stent (cross-sectional area [CSA] in mm2) data from nearly all cases where stents were deployed.
Multi-vessel disease was defined as ≥50% stenosis in 2 or more branches on angiography, with involvement of lesions beyond the target vessel, where ischemic findings from symptoms or tests could not be ruled out.
Medications
Regarding antiplatelet medication, aspirin 81 mg, ticlopidine 200 mg, or clopidogrel 75 mg, plus prasugrel 5 mg, was started at least 48 h before stent insertion. In cases of emergency PCI where antiplatelet medication had not been taken, aspirin 162 mg and clopidogrel 300 mg, or prasugrel 20 mg, were administered before the procedure. The discontinuation timing of dual antiplatelet therapy (DAPT) varies depending on the year of PCI, following guidelines such as those outlined in the Guidelines for the Diagnosis and Treatment of Cardiovascular Diseases, including the ‘Guidelines for Interventional Treatment in Coronary Artery Disease (including Coronary Artery Bypass Surgery) – Elective Intervention (2000)’, ‘Guidelines for Elective PCI in Stable Coronary Artery Disease (2011 revised edition)’, ‘Guidelines for the Management of Non-ST-Elevation Acute Coronary Syndromes (2012 revised edition)’, and ‘Guidelines for the Management of ST-Elevation Acute Myocardial Infarction (2013 revised edition)’.
Quantitative Coronary Angiography (QCA)
QCA was performed on all cases where analysis was feasible. Quantitative and qualitative analyses were conducted at the core laboratory of Fukuoka University and Fukuoka University Chikushi Hospital using CMS-GFT and Q-Angio (MEDES, The Netherlands). Analyses were performed for pre-procedural, post-procedural, and follow-up angiograms. All measurements were conducted using angiography after nitroglycerin coronary injection. Segments were defined as in-stent and 5 mm from the proximal and distal edges of the stent.
Statistical Analysis
All statistical data analyses were performed at Fukuoka University Chikushi Hospital using SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA). Categorical variables were expressed as percentages. The differences in categorical variables between/among groups were examined using Chi-square analysis. Continuous variables were expressed as mean±SD. The differences in continuous variables among groups were examined using analysis of variance (ANOVA) and multiple comparisons. A univariate analysis was conducted using logistic regression analysis. Differences among the 4 groups regarding MACEs were visualized using Kaplan-Meier curves, and significance was assessed using the log-rank test. P<0.05 was considered to be statistically significant.
Results
Patient Characteristics at Baseline in Groups 1–4
Table 1 shows patient characteristics at baseline in Groups 1–4. Group 4 had the highest age (70±9 years; P<0.001). There were no significant differences observed in gender, blood pressure, body mass index (BMI), smoking history, family history, cardiac ultrasound-derived ejection fraction, or blood test results including HbA1c and renal function (Cr). Similarly, there were no significant differences in past medical history or comorbidities such as hypertension, arteriosclerotic occlusive disease, or cerebrovascular accidents.
Table 1.
Patient Characteristics at Baseline in Groups 1–4
| Group 1 (n=125) |
Group 2 (n=102) |
Group 3 (n=395) |
Group 4 (n=384) |
P value | |
|---|---|---|---|---|---|
| Age (years) | 63±11 | 65±10 | 68±10 | 70±9 | <0.0001 |
| Sex, male (%) | 82 | 79 | 74 | 79 | 0.119 |
| BMI (%) | 25.1±3.0 | 24.8±3.6 | 24.1±4.3 | 23.8±3.5 | 0.003 |
| SBP (mmHg) | 138±30 | 133±37 | 134±27 | 130±22 | 0.113 |
| DBP (mmHg) | 73±15 | 71±20 | 72±15 | 70±14 | 0.113 |
| Pulse rate (/min) | 75±16 | 66±19 | 73±15 | 73±13 | <0.001 |
| Smoking history (%) | 65 | 67 | 61 | 65 | 0.454 |
| Family history (%) | 18 | 17 | 14 | 13 | 0.493 |
| UCG-LVEF (%) | 59±14 | 60±13 | 59±14 | 60±13 | 0.405 |
| Blood sampling tests | |||||
| TC (mg/dL) | 227±41 | 170±26 | 196±29 | 144±20 | <0.0001 |
| TG (mg/dL) | 241±41 | 288±121 | 106±36 | 100±36 | <0.0001 |
| HDL-C (mg/dL) | 43±11 | 40±10 | 47±12 | 47±13 | <0.0001 |
| LDL-C (mg/dL) | 136±35 | 73±18 | 127±26 | 77±15 | <0.0001 |
| LDL-C/HDL-C ratio | 3.34 | 1.97 | 2.88 | 1.78 | <0.0001 |
| Non-HDL-C (mg/dL) | 184±40 | 131±28 | 149±27 | 97±17 | <0.0001 |
| Cr (mg/dL) | 1.1±0.5 | 1.0±0.3 | 1.0±0.5 | 1.0±0.5 | 0.043 |
| eGFR(mL/min) | 65±34 | 86±25 | 83±33 | 79±27 | 0.063 |
| UA (mg/dL) | 6.0±1.4 | 5.9±1.3 | 5.5±1.5 | 5.6±1.5 | 0.002 |
| HbA1c (%) | 7.5±1.3 | 7.3±1.3 | 7.4±1.3 | 7.3±1.2 | 0.067 |
| Prior diseases/complicated diseases | |||||
| ASO (%) | 12 | 8.8 | 10 | 14 | 0.301 |
| Prior CVA (%) | 17 | 11 | 16 | 14 | 0.468 |
| Hypertension (%) | 72 | 79 | 77 | 80 | 0.354 |
| Dyslipidemia (%) | 95 | 97 | 87 | 88 | <0.0001 |
| SAP/UAP/AMI/OMI/SMI (%) | 36/18/27/2.1/18 | 37/12/22/0/28 | 28/12/46/1.5/13 | 34/10/33/2.4/20 | |
AMI, acute myocardial infarction; ASO, arteriosclerosis obliterans; BMI, body mass index; CABG, coronary artery bypass graft; Cr, creatinine; CVA, cerebrovascular accident; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MI, myocardial infarction; OMI, old myocardial infarction; PCI, percutaneous coronary intervention; SAP, stable angina pectoris; SBP, systolic blood pressure; SMI, subsequent myocardial infarction; TC, total cholesterol; TG, triglycerides; UA, uric acid; UAP, unstable angina pectoris; UCG, ultrasound cardiography.
Medications at Baseline in Groups 1–4
Regarding medications (Table 2), the use of statins was significantly higher in Group 4 (Group 1, 78%; Group 2, 76%; Group 3, 72%; Group 4, 81%; P=0.033). There was no significant difference in the prescription rate of ezetimibe. Regarding ethyl eicosapentaenoic acid, Group 2 had a significantly higher prescription rate, although the overall prescription rates were low (Group 1, 7.2%; Group 2, 9.8%; Group 3, 2.5%; Group 4, 6.8%; P=0.006). Regarding oral hypoglycemic agents, the prescription rate of DPP-4 inhibitors was significantly higher in Group 4 (Group 1, 8.0%; Group 2, 20%; Group 3, 9.1%; Group 4: 21%; P<0.001). However, there were no significant differences in the prescription rates of biguanides, sulfonylureas, α-glucosidase inhibitors, sodium glucose cotransporter 2 inhibitor, or insulin among the groups.
Table 2.
Medications at Baseline in Groups 1–4
| Group 1 (n=125) |
Group 2 (n=102) |
Group 3 (n=395) |
Group 4 (n=384) |
P value | |
|---|---|---|---|---|---|
| CCB (%) | 44 | 50 | 42 | 47 | 0.400 |
| ACEI (%) | 11 | 6.9 | 11 | 12 | 0.415 |
| ARB (%) | 74 | 62 | 67 | 62 | 0.033 |
| β-blocker (%) | 22 | 24 | 17 | 21 | 0.296 |
| Diuretic (%) | 25 | 14 | 26 | 25 | 0.038 |
| Nitrate (%) | 22 | 14 | 23 | 22 | 0.182 |
| Nicorandil (%) | 19 | 20 | 34 | 31 | <0.001 |
| Statin (%) | 78 | 76 | 72 | 81 | 0.033 |
| EPA (%) | 7.2 | 9.8 | 2.5 | 6.8 | 0.006 |
| BG (%) | 11 | 12 | 9.9 | 8.9 | 0.782 |
| SU (%) | 22 | 27 | 24 | 21 | 0.513 |
| α-GI (%) | 22 | 16 | 17 | 21 | 0.267 |
| DPP4I (%) | 8.0 | 17 | 9.1 | 21 | <0.0001 |
| SGLT2I (%) | 0.8 | 0 | 0.5 | 0 | 0.251 |
| Ezetimibe (%) | 1.6 | 4.9 | 3.0 | 4.9 | 0.248 |
| Insulin (%) | 22 | 28 | 31 | 28 | 0.248 |
α-GI, α-glucosidase inhibitor; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BG, biguanides; CCB, calcium channel blocker; DPP4I, dipeptidyl peptidase-4 inhibitor; EPA, eicosapentaenoic acid; SGLT2I, sodium-glucose cotransporter 2 inhibitor; SU, sulfonylureas.
Lesion Characteristics of Coronary Artery, Pre- and Post-Procedural QCA Results in Groups 1–4
For lesion characteristics (Table 3), according to the American College of Cardiology/American Heart Association lesion morphology classification,11 most of the lesions were type B2 and type C. There was a tendency for more triple-vessel disease in Group 4 (Group 1, 12%; Group 2, 22%; Group 3, 18%; Group 4, 23%; P=0.057), along with a tendency for more severe calcified lesions (Group 1, 8.0%; Group 2, 12%; Group 3, 11%; Group 4, 14%; P=0.179). No significant differences were observed among the groups in terms of treatment strategies, device usage, or stent types. In the pre-procedural QCA results, no differences were observed in lesion length or lesion reference diameter. Lesion minimum lumen diameter (MLD) was smaller in Group 3 (P=0.003), but there were no differences in lesion percent diameter stenosis (%DS) among the 4 groups. Similar to the pre-PCI QCA results, there were no significant differences in lesion reference diameter in the post-PCI QCA results. Lesion MLD was smaller in Group 3 (P=0.024), but there were no differences in %DS among the 4 groups. Regarding stent analysis, no significant differences were observed in stent length or stent reference diameter among the 4 groups. Stent MLD was smaller in Group 3 (P=0.048), but there were no differences in stent %DS among the 4 groups. In the post-PCI IVUS findings, the minimum stent CSA was smaller in Group 4 (Group 1, 6.2±3.1; Group 2, 7.5±7.0; Group 3, 6.2±2.4; Group 4, 5.9±2.5; P=0.042).
Table 3.
Lesion Characteristics of Coronary Artery, Pre- and Post-Procedural QCA Results in Groups 1–4
| Group 1 (n=125) |
Group 2 (n=102) |
Group 3 (n=395) |
Group 4 (n=384) |
P value | |
|---|---|---|---|---|---|
| Lesion characteristics | |||||
| RCA/LAD/LCx/LMT (%) | 40/44/10/5.6 | 28/41/24/6.9 | 39/42/14/5.1 | 31/46/19/4.4 | |
| ACC/AHA type B2+C (%) | 60 | 47 | 58 | 52 | 0.178 |
| 3 vessel disease (%) | 12 | 22 | 18 | 23 | 0.057 |
| Severe calcification (%) | 8 | 12 | 11 | 14 | 0.179 |
| BMS/DES/POBA/DCB (%) | 38/52/8.8/0.8 | 24/68/6.9/2.0 | 35/54/10/0.3 | 22/68/8.6/1.6 | |
| 1st/2nd/3rd generation DES (%) | 55/36/9.8 | 48/45/7.3 | 57/36/7.0 | 44/45/11 | |
| QCA result | |||||
| Pre-procedural QCA result | |||||
| Lesion length (mm) | 18.6±10.7 | 15.8±7.7 | 18.6±11.5 | 17.5±10.1 | 0.225 |
| Lesion reference (mm) | 2.65±0.54 | 2.64±0.62 | 2.51±0.61 | 2.54±0.58 | 0.127 |
| Lesion MLD (mm) | 0.77±0.51 | 0.69±0.48 | 0.63±0.43 | 0.77±0.47 | 0.003 |
| Lesion %DS (%) | 71 | 74 | 75 | 70 | 0.010 |
| Post-procedural QCA result | |||||
| Lesion MLD (mm) | 2.1±0.5 | 1.9±0.5 | 1.8±0.6 | 1.9±0.5 | 0.024 |
| Lesion %DS (%) | 27 | 28 | 28 | 30 | 0.423 |
| Stent length (mm) | 23.7±12.6 | 22.7±10.7 | 24.6±12.1 | 23.4±11.9 | 0.608 |
| Stent reference (mm) | 3.0±0.5 | 2.9±0.6 | 2.8±0.6 | 2.9±0.5 | 0.062 |
| Stent MLD (mm) | 2.6±0.6 | 2.5±0.5 | 2.4±0.6 | 2.5±0.5 | 0.048 |
| Stent %DS (%) | 13.8 | 12.2 | 14.4 | 15 | 0.249 |
| IVUS | |||||
| N | 91 | 76 | 265 | 258 | |
| Minimum stent CSA (mm) | 6.2±3.1 | 7.5±6.9 | 6.2±2.4 | 5.9±2.5 | 0.042 |
%DS, percent diameter stenosis; ACC, American College of Cardiology; AHA, American Heart Association; BMS, bare-metal stent; CSA, cross-sectional area; DCB, drug-coated ballon; DES, drug-eluting stent; IVUS, intravascular ultrasound; LAD, left anterior descending coronary artery; LCx, left circumflex branch; LMT, left main trunk; MLD, minimum lumen diameter; POBA, plain old balloon angioplasty; QCA, quantitative coronary angiography; RCA, right coronary artery.
Long-Term Clinical Outcomes in Groups 1–4
Regarding long-term clinical outcomes (Table 4), the mean follow-up periods were as follows: Group 1, 1,064±842 days; Group 2, 1,318±806 days; Group 3, 1,128 ±861 days; and Group 4, 1,328±803 days. Group 1 had a significantly shorter follow-up period (P<0.001). The incidence rates of MACEs were as follows: Group 1, 38%; Group 2, 26%; Group 3, 31%; and Group 4, 27% (P=0.074), showing no significant differences among the 4 groups, although Group 1 tended to be higher. Specifically, there were no significant differences among the groups in terms of death (Group 1, 5.6%; Group 2, 9.8%; Group 3, 8.4%; Group 4, 7.6%; P=0.648). Regarding MI, Group 1 showed a tendency towards a higher incidence (Group 1, 7.2%; Group 2, 2.9%; Group 3, 7.1%; Group 4, 3.4%; P=0.064). For TLR-PCI, Group 1 had a higher incidence rate (Group 1, 28%; Group 2, 13%; Group 3, 19%; Group 4, 17%; P=0.024). Additionally, the rate of TVR-PCI was highest in Group 1 (Group 1, 11%; Group 2, 3.9%; Group 3, 5.1%; Group 4, 9.9%; P=0.011). However, there were no significant differences among the 4 groups in terms of TVR-CABG, non-TVR-PCI, or non-TVR-CABG. All coronary events during the follow-up period were distributed as follows: Group 1, 66%; Group 2, 56%; Group 3, 58%; and Group 4, 51% (P=0.032), indicating that Group 1 had the highest incidence and Group 4 had the lowest.
Table 4.
Long-Term Clinical Outcomes in Groups 1–4
| Group 1 (n=125) |
Group 2 (n=102) |
Group 3 (n=395) |
Group 4 (n=384) |
P value | |
|---|---|---|---|---|---|
| Follow up (days) | 1,064±842 | 1,318±806 | 1,128±860 | 1,328±803 | 0.0007 |
| MACEs (%) | 38 | 26 | 31 | 27 | 0.074 |
| Death (%) | 5.6 | 9.8 | 8.4 | 7.6 | 0.648 |
| MI (%) | 7.2 | 2.9 | 7.1 | 3.4 | 0.064 |
| TLR PCI (%) | 28 | 13 | 19 | 17 | 0.024 |
| TLR CABG (%) | 2.4 | 2 | 1.8 | 0.8 | 0.471 |
| TVR PCI (%) | 11 | 3.9 | 5.1 | 9.9 | 0.011 |
| TVR CABG (%) | 1.6 | 0 | 0.8 | 0.5 | 0.438 |
| Non-TVR PCI (%) | 28 | 28 | 31 | 26 | 0.470 |
| Non-TVR CABG (%) | 3.2 | 1.0 | 1.8 | 0.8 | 0.276 |
| CVA (%) | 3.2 | 5.9 | 3.8 | 5.5 | 0.640 |
| All coronary event (%) | 66 | 56 | 58 | 51 | 0.032 |
MACEs, major adverse cardiac events; TLR, target lesion revascularization; TVR, target vessel revascularization. Other abbreviations as in Table 1.
For both MACE and all coronary events, additional pairwise comparisons were conducted between Group 1 and each of the other groups (Figure 1). Regarding MACE, significant differences were found between Group 1 and Group 2 (P=0.05), as well as between Group 1 and Group 4 (P=0.020). For all coronary events, a significant difference was observed between Group 1 and Group 4 (P=0.001).
Figure 1.
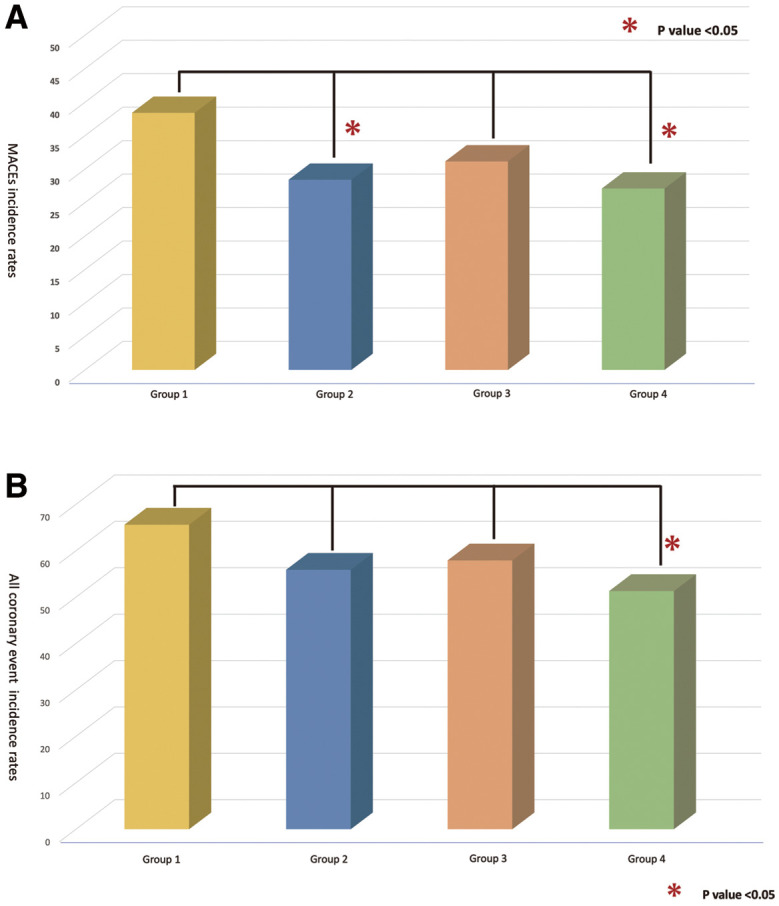
(A) Major adverse cardiac events (MACEs) and (B) all coronary events between Group 1 and each of the other groups using pairwise comparisons.
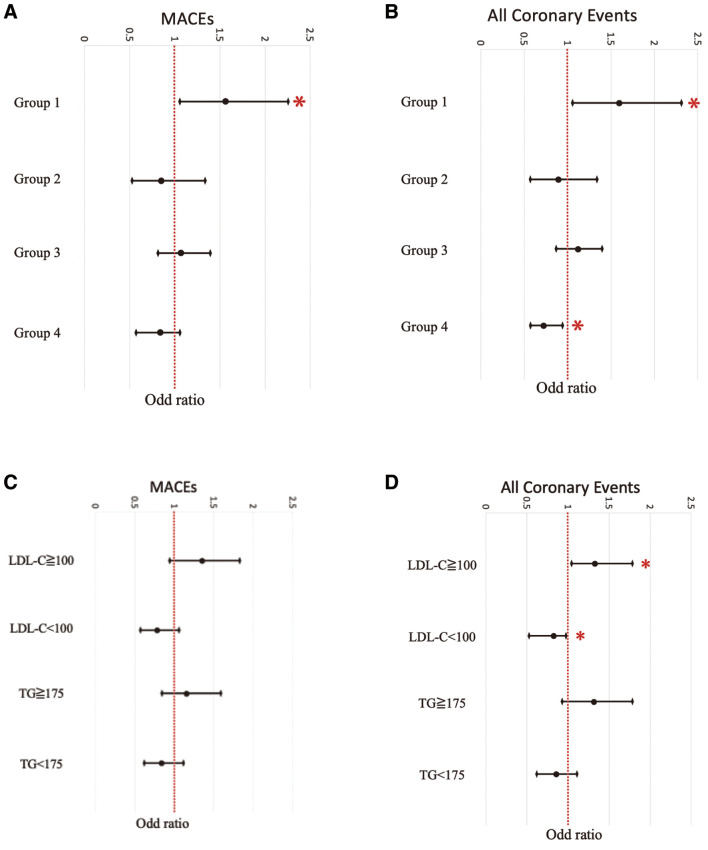
Regarding LDL-C and TG values, which were dummy-coded as composite factors (Groups 1–4) based on whether they met the secondary target values for CAD at the time of PCI, correlations were evaluated with MACEs and all coronary events (Figure 2A,B). Group 1 showed a positive correlation with the occurrence of MACEs (P=0.030) and also demonstrated a positive correlation with all coronary events (P=0.021). In contrast, Group 4 did not show significant differences in the occurrence of MACEs (P=0.111), but did show a significant negative correlation with the occurrence of all coronary events (P=0.019).
Figure 2.
(A) Evaluation of major adverse cardiac events (MACEs) and all (B) coronary events based on whether they met the secondary target values for coronary artery disease at the time of percutaneous coronary intervention by low-density lipoprotein cholesterol (LDL-C) and triglyceride (TG) values, which were dummy-coded as composite factors in each group. Correlation between LDL-C (<100 and ≥100 mg/dL) or TG (<175 mg/dL and ≥175) and (C) MACEs or (D) all coronary events. *P<0.05; †P<0.01; ‡P<0.001.
The correlation between LDL-C levels (<100 mg/dL and ≥100 mg/dL) or TG levels (<175 mg/dL and ≥175 mg/dL) and MACEs or all coronary events was evaluated (Figure 2C,D). LDL-C levels that reached the secondary target for ischemic heart disease showed a tendency to be associated with fewer MACEs (P=0.058), although this was not statistically significant, and a negative correlation with all coronary events (P=0.041). In contrast, TG levels reaching the secondary target for ischemic heart disease did not show a significant inhibitory correlation with either MACEs or all coronary events.
Event-Free Ratios in Groups 1–4
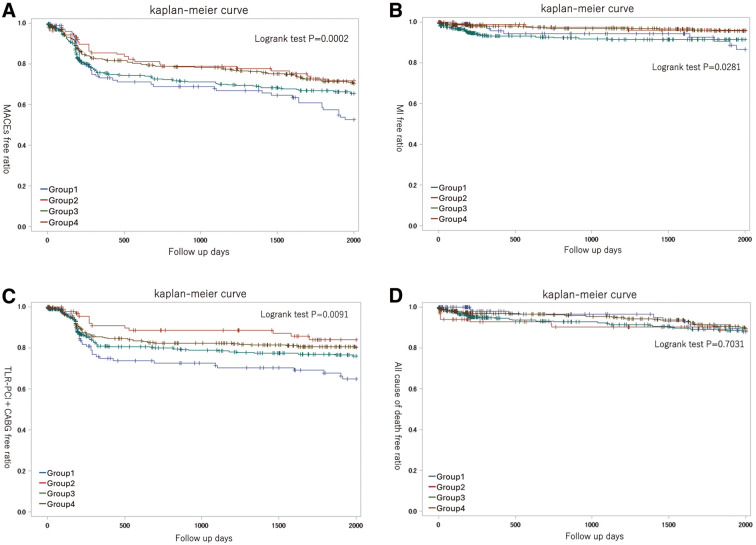
The MACEs-free ratios of each group were visualized and evaluated using Kaplan-Meier curves (Figure 3A). The log-rank test showed a significant difference (P<0.001), indicating markedly lower MACE-free ratios in Group 1. In addition, significant differences were observed among the 4 groups regarding MI-free ratios and TLR-PCI+CABG-free ratios. However, no significant differences were observed in all-cause mortality-free ratios (Figure 3B–D).
Figure 3.
Kaplan-Meier curves of (A) major adverse cardiac event (MACE)-free ratios, (B) myocardial infarction (MI)-free ratios, (C) TLR-PCI+CABG-free ratios, and (D) all cause of death-free ratios of Groups 1, 2, 3 and 4. CABG, coronary artery bypass grafting; TLR-PCI, target lesion revascularization.
Discussion
In the present study, which focused on patients with diabetes, we analyzed 4 groups and found no significant differences in HbA1c levels, BMI, Cr values or the use of antidiabetic medications, particularly insulin. These findings suggest that there were no significant differences in the severity of diabetes among the groups. The assumed Group 4, which we hypothesized would have favorable clinical outcomes post-PCI, was significantly older and showed a tendency towards more cases of triple-vessel disease in the lesion background. Although this result was not statistically significant, this group also included a higher prevalence of severely calcified lesions. The smallest minimum stent CSA observed in the IVUS results post-PCI may be related to the higher incidence of calcified lesions. Conversely, Group 1, which we hypothesized would have worse clinical outcomes post-PCI, was younger compared with Group 4. In terms of the lesion background, this group had a lower rate of triple-vessel disease and severely calcified lesions. The pre- and post-QCA results in Group 1 were similar to those in Group 4, but the minimum stent CSA in IVUS was larger than that in Group 1. However, in terms of long-term clinical outcomes post-PCI, while there was no significant difference in the incidence of MACEs among the 4 groups (P=0.074), significant differences were observed in the incidence of all coronary events (P=0.032). In pairwise comparisons between Group 1 and each group for the incidence of MACEs and all coronary events, as well as from the results of a Kaplan-Meier curve analysis, it was evident that clinical outcomes in Group 1 were worse than those in Group 4. In the univariate analysis, Group 1, which did not reach the secondary prevention target values for LDL-C and TG at the time of PCI, showed a positive correlation with the incidence of MACEs (P=0.030) and also showed a positive correlation with the incidence of all coronary events (P=0.021). In contrast, Group 4, which achieved the secondary prevention target values for both LDL-C and TG at the time of PCI, did not show a significant correlation with the incidence of MACEs (P=0.111), but did show a significant negative correlation with the incidence of all coronary events (P=0.019). These results support the findings from the comparison of the 4 groups mentioned above. Consistent with our hypothesis, although Group 4 had more adverse background factors for post-PCI clinical outcomes compared with Group 1, the clinical outcomes after PCI in Group 4 were better than those in Group 1.
The need for aggressive LDL-C control for patients undergoing PCI due to CAD has been demonstrated in studies such as the PROVE IT study,12 the ESTABLISH study,13 and, in Japan, the MEGA study14 (Pravastatin), and the REAL-CAD study15 (Pitavastatin). It is now widely recognized as common practice to actively control LDL-C, an important risk factor for CAD, to improve clinical outcomes post-PCI. While statin therapy reduces cardiovascular risk by 30–40%, managing LDL-C to target levels still leaves a residual risk of approximately 60–70%. Therefore, there is ongoing interest in studying the cardiovascular event-reducing effects of lipid-lowering agents other than statins to address this remaining risk. Studies on TGs, like JELIS16 and EWTOPIA 75,17 have reported a potential reduction in coronary artery events in CAD patients with the control of TG levels.18 In contrast, reports such as those from the BIP study8 have not reported any significant improvement, suggesting that TGs may not be as strong a risk factor for CAD as LDL-C. However, the results of the present study suggest that viewing TGs as a residual risk factor for CAD after LDL-C has been controlled, and actively intervening and evaluating both factors as composite variables, may potentially have a beneficial impact on clinical outcomes post-PCI, compared with evaluating each factor individually.
There are no data on longitudinal lipid management in this study. Recently, a study by Okada et al. reported that comprehensive achievement and maintenance of early, strong, and sustained LDL-C reduction for 8 months after the onset of acute coronary syndrome (ACS) contributes to the prevention of cardiovascular events (a composite of cardiovascular death, non-fatal MI, angina requiring revascularization, cerebral infarction, and coronary artery bypass surgery).19 The study found that rapid reduction of LDL-C levels in the first 2 months of ACS cases and maintenance of the reduction for the next 6 months (for a total of 8 months) reduced the risk of recurrent cardiovascular events. However, the remaining risk factor, TG >175 mg/dL, was not clearly associated with cardiovascular events. In this study, despite the statin administration rate of approximately 90%, the ezetimibe administration rate was approximately 10%, and only approximately 40% achieved LDL-C<70 mg/dL 2 months after PCI. Therefore, achieving early and vigorous LDL-C management targets, followed by control of TG, may further contribute to the prevention of cardiovascular events, in line with the results of the present study.
In the NIPPON DATA80 study,3 it has been reported that with an increase in the number of risk factors for CAD, the rates of overall mortality and cardiovascular mortality can become significantly higher. In the present study, we focused on patients with abnormal lipid levels (high LDL-C, high TG) in addition to DM. DM is a potent risk factor for CAD. It is well known that PCI in DM patients results in higher rates of stent restenosis and the occurrence of MACEs compared with PCI in non-DM patients, leading to worse short-term and long-term treatment outcomes. Based on the results reported in the JDCS study,10 which stated that ‘LDL-C and TG are potent risk factors for CAD in Japanese type 2 diabetes patients’, we hypothesized that controlling both LDL-C and TG levels in DM patients undergoing PCI would positively impact long-term clinical outcomes. In the same study (i.e., JDCS),10 the evaluation of CAD risk factors in Japanese patients with type 2 diabetes found that LDL-C (P<0.001) was a significant predictor, followed by TG (P=0.005). In the CARDS study,20 the administration of atorvastatin, 10 mg/day, to patients with DM who had high LDL-C reduced ACS by 36%, coronary revascularization by 31%, and mortality by 27% (P=0.05). Furthermore, the FIELD study,21 which investigated the effects of fibrates that lower TG levels on atherosclerotic diseases, showed a significant overall reduction in cardiovascular events. Based on this finding, the same study (i.e., JDCS),10 suggested that TG, comparable with LDL-C, is a significant risk factor for CAD. This supports our hypothesis and is consistent with the results of the present study.
As for TGs, numerous therapeutic options have been shown to be effective, including fibrates, selective peroxisome proliferator-activated receptor-α (PPARα) modulators, nicotinic acid derivatives, statins,22 intestinal cholesterol transporter inhibitors, proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors,23 microsomal TG transfer protein inhibitors, and n-3 polyunsaturated fatty acids. In particular, fibrates are effective for lowering high TG levels, generally reducing TG values by 30–50%, and they are cost-effective, which is beneficial for controlling healthcare costs. In the present study, we found that among DM patients undergoing PCI, those whose LDL-C and TG levels met the secondary prevention targets for ischemic heart disease had better long-term clinical outcomes post-PCI compared with other groups. Controlling TG as an additional residual risk factor for ischemic heart disease, after LDL-C control in DM patients undergoing PCI, is considered to potentially contribute to improving the long-term clinical outcomes of PCI.
Study Limitations
This study was a registry study targeting cases where PCI was performed between April 2003 and March 2016, focusing on patients who were able to undergo follow-up CAG 9–12 months post-PCI and who were also eligible for further long-term follow up. This was not conducted as a cohort study. The blood sampling results from patients undergoing elective PCI were based on fasting samples taken in the morning on the day of the PCI, while samples from patients undergoing emergency PCI due to ACS were collected at the time of the PCI. It is possible that approximately 40% of the data were from non-fasting conditions. In this study, LDL-C and TG were divided into 4 groups based on whether they met the target levels at the time of PCI. The total number of cases analyzed was 1,006. To more accurately support differences in clinical outcomes through multi-group comparisons, it may be necessary to increase the number of cases studied and conduct further long-term follow up. In this analysis, confirmation of the presence and timing of MACEs was conducted through final outpatient records from each relevant facility and verification with primary care physicians. However, due to the nature of evaluating outcomes in long-term follow up after PCI, detailed follow-up data such as lipid-related parameters and medication status at the final follow up were not collected. By obtaining this data, it may be possible to substantiate the results and support discussions of this study. Furthermore, although the intake rates of fibrate drugs and docosahexaenoic acid-containing ω-3 fatty acid mixtures were not available in this dataset, detailed collection could have further substantiated the effectiveness of TG control. Additionally, if information to the date of all coronary events, including TVR-PCI/CABG and non-target vessel PCI/CABG, were collected and evaluated, it might have further demonstrated the significance of controlling both TG and LDL-C together. Last, this study did not examine non-DM patients. This is because numerous studies have already established the importance of lipid control, especially LDL-C control, in improving PCI outcomes in non-DM patients, and statin administration is common practice in PCI.
Conclusions
In DM patients undergoing PCI, those whose LDL-C and TG levels met the secondary prevention targets for ischemic heart disease showed better long-term clinical outcomes compared with other groups. Controlling TG as an additional residual risk factor for CAD after LDL-C control in DM patients undergoing PCI is considered worthwhile, not only from a healthcare-cost perspective because of its cost-effectiveness, but also due to the potential to improve long-term clinical outcomes post-PCI.
Sources of Funding
The authors declare that no funds, grants, or other forms of financial support were received during the preparation of this manuscript.
Disclosures
S.M. has received remuneration from Otsuka Pharmaceutical Co., Ltd., Daiichi Sankyo Co. Ltd., Bayer Yakuhin Ltd., Novartis Pharma, and Boehringer-Ingelheim, research funding from Daiichi Sankyo Co. Ltd., Abbott Medical Japan LLC, Boehringer-Ingelheim, Bayer Yakuhin Ltd., Sumitomo Pharma Co. and JM Medical, and scholarship funds from OMRON Co., SOMPO Japan, and Boston Scientific. S.M. is a member of Circulation Reports’ Editorial Team.
IRB Information
This study was approved by the Ethics Committee of Fukuoka University Hospital (reference number: 10-1-08[09-105]). Trial registration no. UMIN000005679.
Acknowledgments
The authors thank all of the physicians, nurses, heart team members, and patients who were involved in this study.
Data Availability
The deidentified participant data will be shared on a request basis. Please contact the corresponding author directly to request data sharing. All data sets used will be available, including the study protocol. Data will be shared as soon as it is approved by IRB at Fukuoka University School of Medicine, and will be available until the end of March 2026. Access to the data is available to anyone. Any analyses on the data will be approved and data will be shared in an Excel file via email.
References
- 1. Gordon T, Kannel WB, Castelli WP, Dawber TR.. Lipoproteins, cardiovascular disease, and death: The Framingham study. Arch Intern Med 1981; 141: 1128–1131. [PubMed] [Google Scholar]
- 2. Okamura T, Tsukamoto K, Arai H, Fujioka Y, Ishigaki Y, Koba S, et al.; Japan Atherosclerosis Society.. Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2022. J Atheroscler Thromb 2024; 31: 641–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kadowaki S, Okamura T, Hozawa A, Kadowaki T, Kadota A, Murakami Y, et al.. Relationship of elevated casual blood glucose level with coronary heart disease, cardiovascular disease and all-cause mortality in a representative sample of the Japanese population: NIPPON DATA80. Diabetologia 2008; 51: 575–582. [DOI] [PubMed] [Google Scholar]
- 4. Iso H, Sato S, Kitamura A, Imano H, Kiyama M, Yamagishi K, et al.. Metabolic syndrome and the risk of ischemic heart disease and stroke among Japanese men and women. Stroke 2007; 38: 1744–1751. [DOI] [PubMed] [Google Scholar]
- 5. Noda H, Iso H, Saito I, Konishi M, Inoue M, Tsugane S, et al.. The impact of the metabolic syndrome and its components on the incidence of ischemic heart disease and stroke: The Japan public health center-based study. Hypertens Res 2009; 32: 289–298. [DOI] [PubMed] [Google Scholar]
- 6. Iso H, Naito Y, Sato S, Kitamura A, Okamura T, Sankai T, et al.. Serum triglycerides and risk of coronary heart disease among Japanese men and women. Am J Epidemiol 2001; 153: 490–499. [DOI] [PubMed] [Google Scholar]
- 7. Imano H, Noda H, Kitamura A, Sato S, Kiyama M, Sankai T, et al.. Low-density lipoprotein cholesterol and risk of coronary heart disease among Japanese men and women: The Circulatory Risk in Communities Study (CIRCS). Prev Med 2011; 52: 381–386. [DOI] [PubMed] [Google Scholar]
- 8. Bezafibrate Infarction Prevention (BIP) Study.. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease. Circulation 2000; 102: 21–27. [DOI] [PubMed] [Google Scholar]
- 9. O’Brien KD, Hippe DS, Chen H, Neradilek MB, Probstfield JL, Peck S, et al.. Summary of clinical and laboratory data of study subjects with and without DCE-MRI plaque measurements in the AIM-HIGH clinical trial. Data Brief 2016; 6: 476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sone H, Tanaka S, Tanaka S, Iimuro S, Oida K, Yamasaki Y, et al.. Serum level of triglycerides is a potent risk factor comparable to LDL cholesterol for coronary heart disease in Japanese patients with type 2 diabetes: Subanalysis of the Japan Diabetes Complications Study (JDCS). J Clin Endocrinol Metab 2011; 96: 3448–3456. [DOI] [PubMed] [Google Scholar]
- 11. Ryan TJ.. Guidelines for percutaneous transluminal coronary angioplasty: A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transluminal Coronary Angioplasty). JACC 1988; 12: 529–545, doi:10.1016/0735-1097(88)90431-7. [PubMed] [Google Scholar]
- 12. Gibson CM, Pride YB, Hochberg CP, Sloan S, Sabatine MS, Cannon CP, et al.. Effect of intensive statin therapy on clinical outcomes among patients undergoing percutaneous coronary intervention for acute coronary syndrome. PCI-PROVE IT: A PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction 22) Substudy. J Am Coll Cardiol 2009; 54: 2290–2295. [DOI] [PubMed] [Google Scholar]
- 13. Okazaki S, Yokoyama T, Miyauchi K, Shimada K, Kurata T, Sato H, et al.. Early statin treatment in patients with acute coronary syndrome: Demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event; the ESTABLISH study. Circulation 2004; 110: 1061–1068. [DOI] [PubMed] [Google Scholar]
- 14. Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, et al.. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): A prospective randomised controlled trial. Lancet 2006; 368: 1155–1163. [DOI] [PubMed] [Google Scholar]
- 15. Taguchi I, Iimuro S, Iwata H, Takashima H, Abe M, Amiya E, et al.. High-Dose Versus Low-Dose Pitavastatin in Japanese Patients with Stable Coronary Artery Disease (REAL-CAD): A randomized superiority trial. Circulation 2018; 137: 1997–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saito Y, Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Ishikawa Y, et al.. Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: Sub-analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis 2008; 200: 135–140. [DOI] [PubMed] [Google Scholar]
- 17. Ouchi Y, Sasaki J, Arai H, Yokote K, Harada K, Katayama Y, et al.. Ezetimibe Lipid-Lowering Trial on Prevention of Atherosclerotic Cardiovascular Disease in 75 or Older (EWTOPIA 75): A randomized, controlled trial. Circulation 2019; 140: 992–1003. [DOI] [PubMed] [Google Scholar]
- 18. Nordestgaard BG.. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: New insights from epidemiology, genetics, and biology. Circ Res 2016; 118: 547–563. [DOI] [PubMed] [Google Scholar]
- 19. Okada K, Haze T, Kikuchi S, Kirigaya H, Hanajima Y, Tsutsumi K, et al.. Early, intensive and persistent lipid-lowering therapy for secondary prevention of acute coronary syndrome. J Atheroscler Thromb 2024, doi:10.5551/jat.64988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al.. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): Multicentre randomised placebo-controlled trial. Lancet 2004; 364: 685–696. [DOI] [PubMed] [Google Scholar]
- 21. Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, et al.. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet 2005; 366: 1849–1861. [DOI] [PubMed] [Google Scholar]
- 22. Higuma T, Akashi YJ, Fukumoto Y, Obara H, Kakuma T, Asaumi Y, et al.. Residual coronary risk factors associated with long-term clinical outcomes in patients with coronary artery disease treated with high- vs. low-dose statin therapy: REAL-CAD Substudy. Circ J 2024; 88: 995–1003. [DOI] [PubMed] [Google Scholar]
- 23. Kiyosue A, Yasuda S, Tomura A, Usami M, Arai H.. Safety and Effectiveness of alirocumab, a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, in patients with familial or non-familial hypercholesterolemia: A post-marketing survey (J-POSSIBLE). Circ J 2023; 87: 834–846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The deidentified participant data will be shared on a request basis. Please contact the corresponding author directly to request data sharing. All data sets used will be available, including the study protocol. Data will be shared as soon as it is approved by IRB at Fukuoka University School of Medicine, and will be available until the end of March 2026. Access to the data is available to anyone. Any analyses on the data will be approved and data will be shared in an Excel file via email.