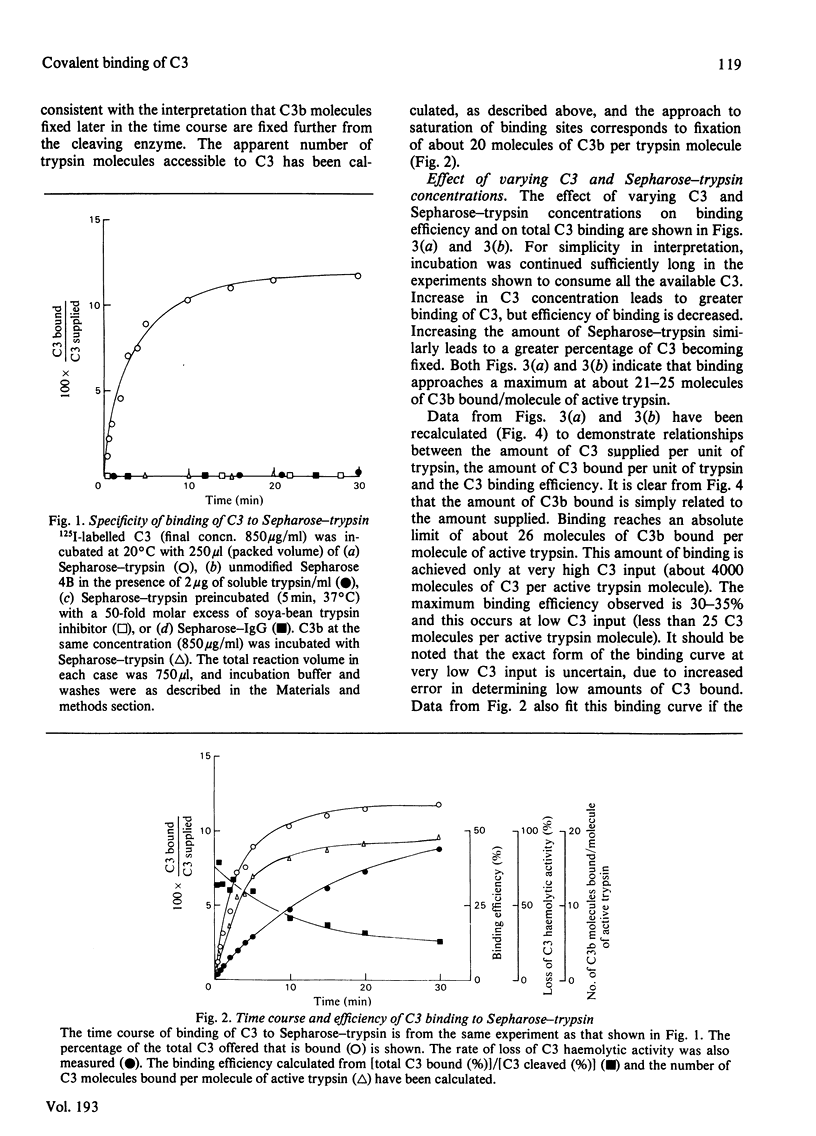
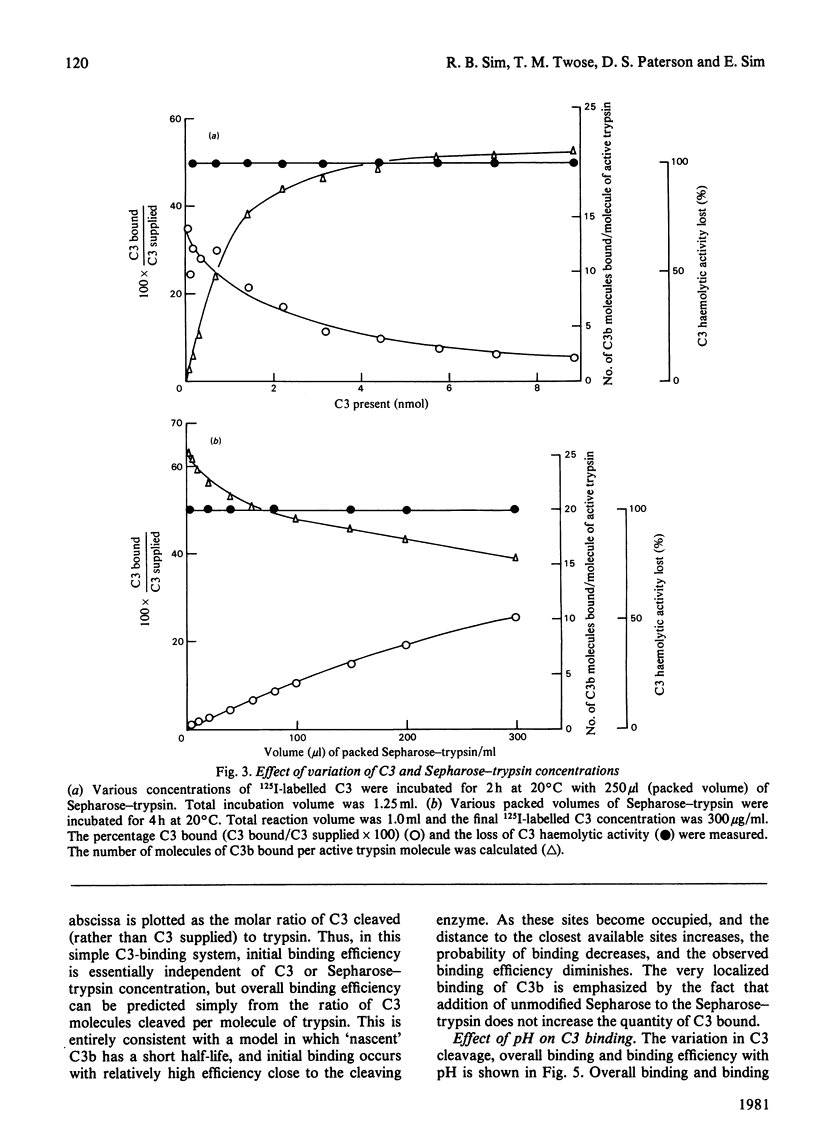
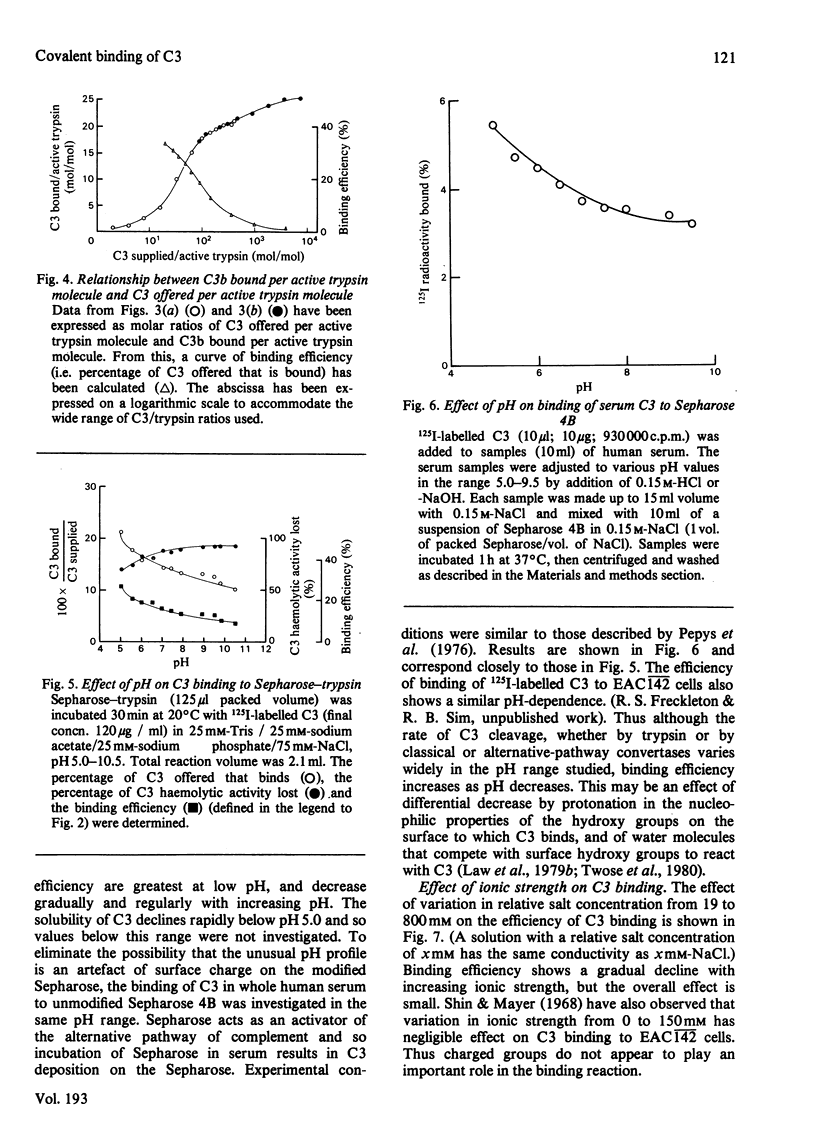
Abstract
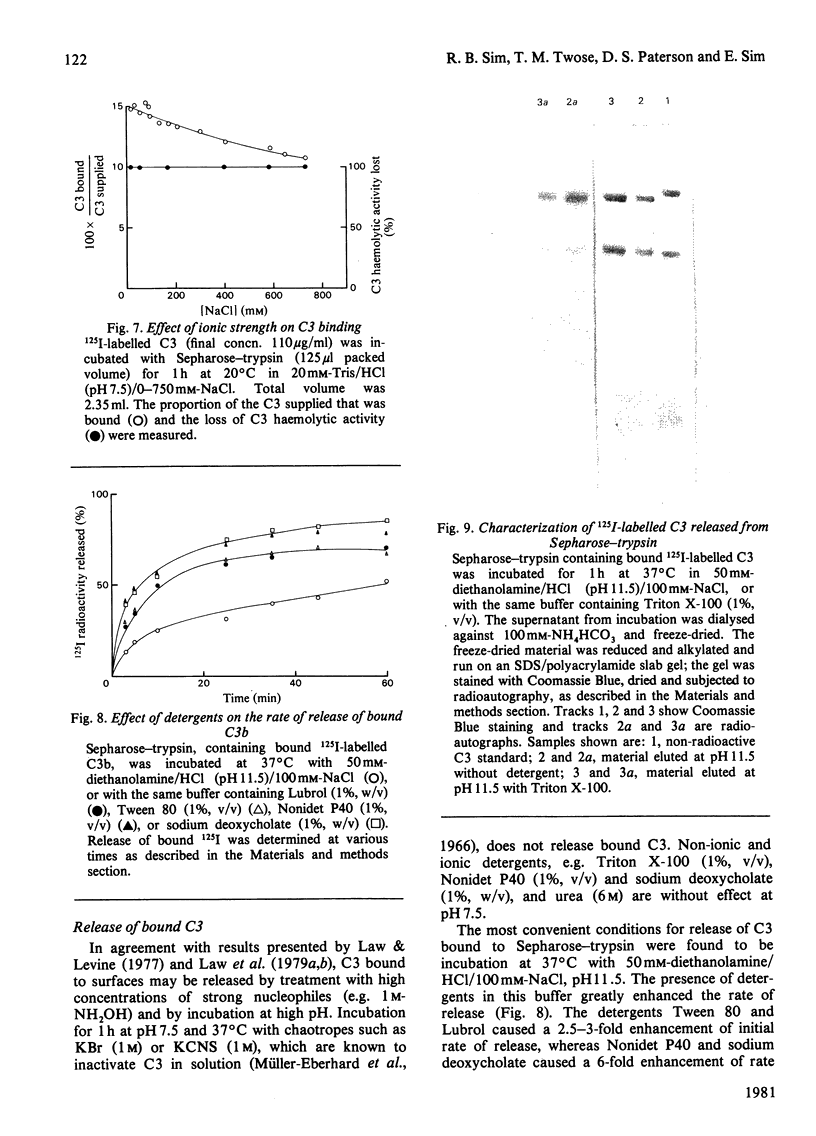
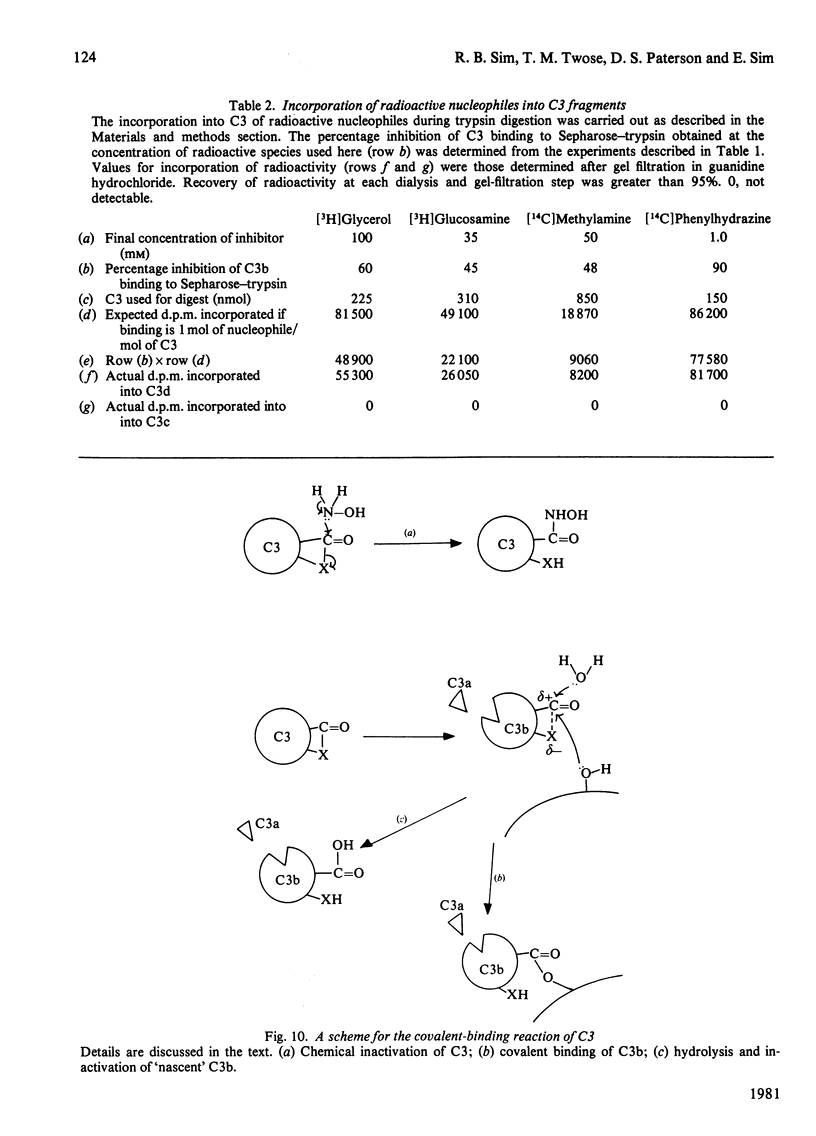
The complement protein C3, when activated by limited proteolysis, forms a short-lived reactive intermediate fragment, 'nascent' C3b, which is known to bind covalently to certain surfaces. The characteristics of the covalent binding reaction have been studied by using Sepharose-trypsin as a combined proteolytic activator and binding surface for C3. Binding of C3 to Sepharose-trypsin is saturable, with a maximum of 25-26 molecules of C3b bound per molecule of trypsin. A minimum life-time of about 60 microseconds for the reactive intermediate has been calculated from binding of C3 at saturation. Initial binding efficiencies of over 30% can be obtained at physiological pH and ionic strength. The efficiency of C3 binding to Sepharose-trypsin decreases as pH increases and also shows a slight decline at high ionic strength. The covalent binding of C3 to Sepharose-trypsin can be inhibited by a range of oxygen and nitrogen nucleophiles. Activation of C3 in the presence of radioactive forms of four such nucleophiles, phenylhydrazine, methylamine, glycerol and glucosamine results in apparent covalent incorporation of the nucleophile into the C3d fragment of C3. The quantity of radioactive nucleophile bound can be predicted from the observed potency of the nucleophile as an inhibitor of the binding of C3 to Sepharose-trypsin. The radioactive nucleophiles may be considered as 'active-site' labels for C3.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaout M. A., Stossel T. P., Rosen F. S., Alper C. A. Solubilization of C3 fragments deposited on cross-linked dextran gel beads. Clin Immunol Immunopathol. 1979 Nov;14(3):384–394. doi: 10.1016/0090-1229(79)90164-8. [DOI] [PubMed] [Google Scholar]
- Axén R., Ernback S. Chemical fixation of enzymes to cyanogen halide activated polysaccharide carriers. Eur J Biochem. 1971 Feb 1;18(3):351–360. doi: 10.1111/j.1432-1033.1971.tb01250.x. [DOI] [PubMed] [Google Scholar]
- BERNFELD P., WAN J. ANTIGENS AND ENZYMES MADE INSOLUBLE BY ENTRAPPING THEM INTO LATTICES OF SYNTHETIC POLYMERS. Science. 1963 Nov 8;142(3593):678–679. doi: 10.1126/science.142.3593.678. [DOI] [PubMed] [Google Scholar]
- Bokisch V. A., Müller-Eberhard H. J., Cochrane C. G. Isolation of a fragment (C3a) of the third component of human complement containing anaphylatoxin and chemotactic activity and description of an anaphylatoxin inactivator of human serum. J Exp Med. 1969 May 1;129(5):1109–1130. doi: 10.1084/jem.129.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Balian G. Cleavage at Asn-Gly bonds with hydroxylamine. Methods Enzymol. 1977;47:132–145. doi: 10.1016/0076-6879(77)47016-2. [DOI] [PubMed] [Google Scholar]
- Byrt P., Ada G. L. An in vitro reaction between labelled flagellin or haemocyanin and lymphocyte-like cells from normal animals. Immunology. 1969 Oct;17(4):503–516. [PMC free article] [PubMed] [Google Scholar]
- Campbell R. D., Dodds A. W., Porter R. R. The binding of human complement component C4 to antibody-antigen aggregates. Biochem J. 1980 Jul 1;189(1):67–80. doi: 10.1042/bj1890067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel P. J., Groeneboer O., Grosveld G., Pondman K. W. The binding of activated C3 to polysaccharides and immunoglobulins. J Immunol. 1978 Dec;121(6):2566–2572. [PubMed] [Google Scholar]
- Crossley L. G., Porter R. R. Purification of the human complement control protein C3b inactivator. Biochem J. 1980 Oct 1;191(1):173–182. doi: 10.1042/bj1910173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Fernandez H. N., Huglij T. E. Chemical evidence for common genetic ancestry of complement components C3 and C5. J Biol Chem. 1977 Mar 10;252(5):1826–1828. [PubMed] [Google Scholar]
- Fersht A. R., Blow D. M., Fastrez J. Leaving group specificity in the chymotrypsin-catalyzed hydrolysis of peptides. A stereochemical interpretation. Biochemistry. 1973 May 22;12(11):2035–2041. doi: 10.1021/bi00735a002. [DOI] [PubMed] [Google Scholar]
- Fontaine M., Joisel F., Lebreton J. P. Evidence that C3d is an amphiphilic protein. Application to its preparation by hydrophobic affinity chromatography. FEBS Lett. 1980 Feb 25;111(1):148–151. doi: 10.1016/0014-5793(80)80780-0. [DOI] [PubMed] [Google Scholar]
- Fontaine M., Rivat C. A study of the breakdown of the third component of human complement (C3). Ann Immunol (Paris) 1979 May-Jun;130C(3):349–366. [PubMed] [Google Scholar]
- Gigli I., Porter R. R., Sim R. B. The unactivated form of the first component of human complement, C1. Biochem J. 1976 Sep 1;157(3):541–548. doi: 10.1042/bj1570541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. Lysis of erythrocytes by complement in the absence of antibody. J Exp Med. 1970 Nov;132(5):898–915. doi: 10.1084/jem.132.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARBOE M., MUELLER-EBERHARD H. J., FUDENBERG H., POLLY M. J., MOLLISON P. L. IDENTIFICATION OF THE COMPONENTS OF COMPLEMENT PARTICIPATING IN THE ANTIGLOBULIN REACTION. Immunology. 1963 Jul;6:412–420. [PMC free article] [PubMed] [Google Scholar]
- Harrison R. A., Lachmann P. J. Novel cleavage products of the third component of human complement. Mol Immunol. 1980 Feb;17(2):219–228. doi: 10.1016/0161-5890(80)90074-7. [DOI] [PubMed] [Google Scholar]
- Kerr M. A. Limited proteolysis of complement components C2 and factor B. Structural analogy and limited sequence homology. Biochem J. 1979 Dec 1;183(3):615–622. doi: 10.1042/bj1830615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Law S. K., Fearon D. T., Levine R. P. Action of the C3b-inactivator on the cell-bound C3b. J Immunol. 1979 Mar;122(3):759–765. [PubMed] [Google Scholar]
- Law S. K., Levine R. P. Interaction between the third complement protein and cell surface macromolecules. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2701–2705. doi: 10.1073/pnas.74.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S. K., Lichtenberg N. A., Levine R. P. Evidence for an ester linkage between the labile binding site of C3b and receptive surfaces. J Immunol. 1979 Sep;123(3):1388–1394. [PubMed] [Google Scholar]
- Lenard J. Rapid and effective removal of sodium dodecyl sulfate from proteins. Biochem Biophys Res Commun. 1971 Nov 5;45(3):662–668. doi: 10.1016/0006-291x(71)90467-0. [DOI] [PubMed] [Google Scholar]
- MUELLER-EBERHARD H. J., BIRO C. E. ISOLATION AND DESCRIPTION OF THE FOURTH COMPONENT OF HUMAN COMPLEMENT. J Exp Med. 1963 Sep 1;118:447–466. doi: 10.1084/jem.118.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardiney M. R., Jr, Müller-Eberhard H. J., Feldman J. D. Ultrastructural localization of the third and fourth components of complement on complement-cell complexes. Am J Pathol. 1968 Aug;53(2):253–261. [PMC free article] [PubMed] [Google Scholar]
- Mezzasoma I., Turano C. Studi su enzimi legati a supporti insolubili: valutazione del metodo della carbodiimide. Boll Soc Ital Biol Sper. 1971 Jul 30;47(14):407–409. [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Complement. Annu Rev Biochem. 1975;44:697–724. doi: 10.1146/annurev.bi.44.070175.003405. [DOI] [PubMed] [Google Scholar]
- Müllerèberhard H. J., Dalmasso A. P., Calcott M. A. The reaction mechanism of beta-1C-globulin (C'3) in immune hemolysis. J Exp Med. 1966 Jan 1;123(1):33–54. doi: 10.1084/jem.123.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa S., Stroud R. M. Mechanism of action of the C3b inactivator: requirement for a high molecular weight cofactor (C3b-C4bINA cofactor) and production of a new C3b derivative (C3b'). Immunochemistry. 1977 Nov-Dec;14(11-12):749–756. doi: 10.1016/0019-2791(77)90345-7. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med. 1977 Jul 1;146(1):257–270. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. R., Reid K. B. Activation of the complement system by antibody-antigen complexes: the classical pathway. Adv Protein Chem. 1979;33:1–71. doi: 10.1016/s0065-3233(08)60458-1. [DOI] [PubMed] [Google Scholar]
- RATNOFF O. D., LEPOW I. H., PILLEMER L. The multiplicity of plasmin inhibitors in human serum, demonstrated by the effect of primary amino compounds. Bull Johns Hopkins Hosp. 1954 Apr;94(4):169–179. [PubMed] [Google Scholar]
- Shin H. S., Mayer M. M. The third component of the guinea pig complement system. II. Kinetic study of the reaction of EAC'4,2a with guinea pig C'3. Enzymatic nature of C'3 comsumption, multiphasic character of fixation, and hemolytic titration of C'3. Biochemistry. 1968 Aug;7(8):2997–3002. doi: 10.1021/bi00848a041. [DOI] [PubMed] [Google Scholar]
- Swenson R. P., Howard J. B. Characterization of alkylamine-sensitive site in alpha 2-macroglobulin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4313–4316. doi: 10.1073/pnas.76.9.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack B. D., Prahl J. W. Third component of human complement: purification from plasma and physicochemical characterization. Biochemistry. 1976 Oct 5;15(20):4513–4521. doi: 10.1021/bi00665a028. [DOI] [PubMed] [Google Scholar]
- Tack B. F., Morris S. C., Prahl J. W. Third component of human complement: structural analysis of the polypeptide chains of C3 and C3b. Biochemistry. 1979 Apr 17;18(8):1497–1503. doi: 10.1021/bi00575a017. [DOI] [PubMed] [Google Scholar]