Abstract
Purpose
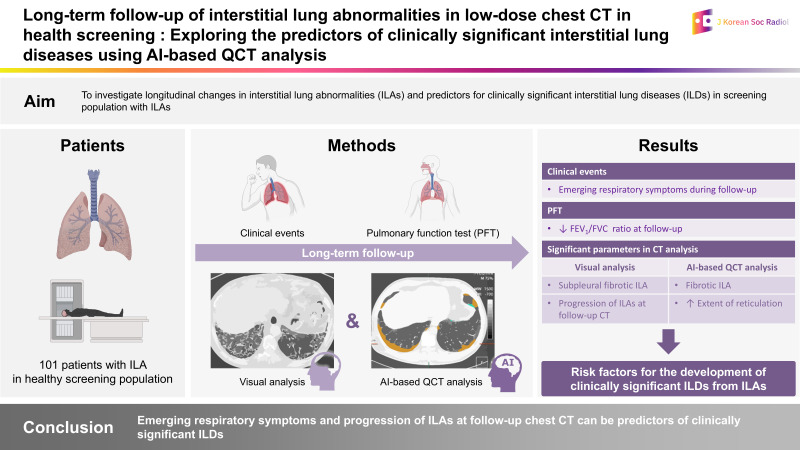
This study examined longitudinal changes in interstitial lung abnormalities (ILAs) and predictors of clinically significant interstitial lung diseases (ILDs) in a screening population with ILAs.
Materials and Methods
We retrieved 36891 low-dose chest CT records from screenings between January 2003 and May 2021. After identifying 101 patients with ILAs, the clinical findings, spirometry results, and initial and follow-up CT findings, including visual and artificial intelligence-based quantitative analyses, were compared between patients diagnosed with ILD (n = 23, 23%) and those who were not (n = 78, 77%). Logistic regression analysis was used to identify significant parameters for the clinical diagnosis of ILD.
Results
Twenty-three patients (n = 23, 23%) were subsequently diagnosed with clinically significant ILDs at follow-up (mean, 8.7 years). Subpleural fibrotic ILAs on initial CT and signs of progression on follow-up CT were common in the ILD group (both p < 0.05). Logistic regression analysis revealed that emerging respiratory symptoms (odds ratio [OR], 5.56; 95% confidence interval [CI], 1.28–24.21; p = 0.022) and progression of ILAs at follow-up chest CT (OR, 4.07; 95% CI, 1.00–16.54; p = 0.050) were significant parameters for clinical diagnosis of ILD.
Conclusion
Clinically significant ILD was subsequently diagnosed in approximately one-quarter of the screened population with ILAs. Emerging respiratory symptoms and progression of ILAs at follow-up chest CT can be predictors of clinically significant ILDs.
Keywords: Interstitial Lung Abnormalities; Fibrosis; Interstitial Lung Disease; Tomography, X-Ray Computed; Lung
Graphical Abstract
Abstract
목적
본 연구는 일반검진 집단에서 간질성 폐 이상(interstitial lung abnormality; 이하 ILA)의 장기적 변화를 확인하고 임상적으로 유의미한 간질성 폐질환(interstitial lung disease; 이하 ILD)으로 진행하는 예측 인자를 탐색하였다.
대상과 방법
2003년 1월부터 2021년 5월까지 검진 CT 검사에서 36891개의 저선량 흉부 CT 기록을 검색하였다. ILA 환자 101명을 선정하여, 추적검사에서 ILD를 진단받은 환자(23명, 23%)와 진단받지 않은 환자(78명, 77%)를 대상으로 임상 자료, 폐활량측정검사(spirometry), 시각 및 인공지능 기반 정량 분석을 포함한 최초 및 추적 CT 결과를 비교하였고, 로지스틱 회귀 분석을 통해 ILD의 임상 진단에 중요한 인자를 확인하였다.
결과
23명의 환자(23%)가 추적기간(평균 추적 기간, 8.7년) 동안 임상적으로 의미 있는 ILD로 진단되었다. 초기 CT의 흉막하 섬유성 ILA와 추적 CT에서의 진행이 ILD 환자에서 자주 관찰되었다(p < 0.05). 로지스틱 회귀 분석 결과, 새로운 호흡기 증상 발현(odds ratio [이하 OR] 5.56, 95% confidence interval [이하 CI] 1.28–24.21, p = 0.022)과 추적 흉부 CT 검사에서 간질성 폐 이상의 진행 소견(OR 4.07, 95% CI 1.00–16.54, p = 0.050)이 ILD의 임상 진단에 유의미한 예측 인자였다.
결론
ILA 환자의 약 4분의 1에서 이후 임상적으로 의미 있는 ILD로 진단되었다. 새로운 호흡기 증상의 발현과 추적 흉부 CT 검사에서 간질성 폐 이상의 진행 소견은 임상적으로 중요한 ILD의 예측 인자가 될 수 있다.
INTRODUCTION
Interstitial lung abnormality (ILA) refers to the presence of lung abnormalities on chest CT that are potentially associated with underlying interstitial lung disease (ILD) in patients without a prior clinical diagnosis (1). With the increasing number of chest CT scans for lung cancer screening and other diagnostic purposes, ILAs have been more frequently identified. Previous studies have reported varying prevalence and progression of ILAs (2,3). Although not all cases of ILA exhibit progression, in some cases, it can represent an early stage of clinically significant ILD (subclinical or preclinical). ILA is also associated with increased mortality (4,5). Several studies have supported the hypothesis that ILA may not solely be an imaging abnormality but may indicate an undiagnosed form of ILD; however, limited studies have established a direct relationship between ILA and ILD.
Considering the subtle and variable characteristics of ILAs, long-term follow-up is required for many patients. Assessment of subsequent changes using automated computer-based quantitative analysis can be helpful for regular monitoring. Studies have used texture-based and automated quantitative CT (QCT) analysis in patients with ILDs, which has been shown to be beneficial for early diagnosis and prognostication (6,7,8,9). However, to the best of our knowledge, few studies have assessed the risk factors for the diagnosis of clinically significant ILDs in patients with ILAs and the long-term follow-up data, particularly using automated artificial intelligence (AI)-based QCT analysis. Therefore, this study aimed to examine the longitudinal changes in ILAs during long-term follow-up using AI-based QCT analysis and to explore the predictors for the diagnosis of clinically significant ILDs in a general screening cohort with ILAs on initial low-dose chest CT.
MATERIALS AND METHODS
STUDY SAMPLE
This retrospective study was approved by the Institutional Review Board of Soonchunhyang University Seoul Hospital (IRB No. 2022-10-005), and the requirement for informed consent was waived. We retrieved 36891 low-dose chest CT scans from the general screening population at our institution between January 2003 and May 2021. Our health screening center offers general screenings for various purposes and is intended for the asymptomatic healthy population; it is not specifically dedicated to lung cancer screening in high-risk patient groups. Low-dose chest CT scans were included as part of a comprehensive medical checkup after counseling between the patients and the professionally trained paramedical staff at our health-screening center. Electronic medical database and radiology reports were reviewed using search terms suggesting the presence of ILAs such as “interstitial,” “ILD,” “DILD,” “IPF,” “UIP,” “NSIP,” “reticul-,” and a combination of “dependent” and “fibrosis.” A total of 119 (0.3%) CT scans that met the aforementioned criteria were identified. We retrospectively reviewed CT findings for the presence of ILAs according to the criteria suggested by the Fleischner Society (1). Among the 119 patients, 18 did not fulfill the ILA criteria and were excluded for the following reasons: suboptimal inspiration or dependent atelectasis (n = 13); prior ILD diagnosis (n = 3); asbestosis (n = 2). Ultimately, 101 patients (0.3%) were included in this study (Fig. 1).
Fig. 1. The flowchart presents the selection of the study population.
*The search terms suggesting the presence of ILAs were “interstitial,” “ILD,” “DILD,” “IPF,” “UIP,” “NSIP,” “reticul-,” and “dependent+fibrosis.”
ILA = interstitial lung abnormality, ILD = interstitial lung disease
CLINICAL DATA
Clinical characteristics, including age, sex, height, weight, smoking history, comorbidities (hypertension, diabetes mellitus, cardiovascular disease, and cancer), and respiratory symptoms (dyspnea, cough, and sputum), were recorded at baseline and during follow-up. We collected the results of pulmonary function tests (PFTs), including initial and follow-up data on forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), ratio of FEV1 to FVC (FEV1/FVC), and diffusing capacity of the lungs for carbon monoxide (DLCO). Only PFT data with an interval of less than 1 month between PFTs and CT were included in our study (mean interval, 0.5 ± 2.9 days; range, 0–20 days). Significant FVC decline was defined as a decrease in FVC of 5% or more during follow-up. Significant clinical events, including newly diagnosed clinically significant ILD, lung cancer, and death, were identified through a meticulous review of electronic medical records. The diagnosis of clinically significant ILD was made by respiratory clinicians and was defined as having at least two of the following criteria: identification of relevant imaging findings on CT scan; clinical assessment of patients with significant respiratory symptoms, including those that persisted or worsened over a period of time, decreased pulmonary function, and impaired gas exchange (defined as a decline in FVC by 5% or DLCO by 10%); establishment of the final diagnosis through a comprehensive clinical evaluation, including histopathologic examination and multidisciplinary discussion (5,10,11).
CHEST CT SCANNING
Low-dose chest CT was performed without contrast medium using multidetector CT scanners (Discovery CT750 HD; GE Healthcare, Milwaukee, WI, USA; SOMATOM Definition Edge, Sensation 64, and Sensation 4; Siemens Medical Solutions, Erlangen, Germany). All images were obtained in the caudocranial direction from the lung bases through the thoracic inlet during a single inspiratory breath-hold. CT images were reconstructed with a section thickness of 3.0 mm using a soft tissue algorithm, and lung window (window width, 1500 Hounsfield unit [HU]; window level, -700 HU) images were available for analysis.
Follow-up CT scans were available for 63 patients (62%) and comprised 34 low-dose chest CT scans, 10 standard-dose chest CT scans, and 19 high-resolution CT scans with or without supplementary prone position scans with a 1-mm section thickness. Initial and follow-up CT intervals were reviewed (mean, 83.2 ± 57.1 months; range, 2–196 months).
VISUAL CT EVALUATION
The CT scans were reviewed by two thoracic radiologists (B.D.N. and J.H.H. with 11 and 30 years of experience in thoracic imaging, respectively) who were blinded to the clinical information and reached a consensus through a joint review. The presence of ILA was determined as previously described (1). ILAs affecting less than 5% of any lung zone or those with unilateral lung abnormalities were defined as equivocal ILAs. The ILA patterns were classified as subpleural fibrotic, subpleural nonfibrotic, and nonsubpleural nonfibrotic. CT extensiveness was defined as disease involvement of all three lung zones. We also evaluated concurrent lung diseases, including emphysema and tuberculosis (TB). On comparing the initial and follow-up CT scans, the disease course was classified as progression, stability, or resolution. Progression on follow-up CT was defined as an increase in the extent and coarseness of the interstitial abnormalities or the appearance of a new abnormality in a patient without a previous ILA. Stability was defined as no change in extent or coarseness. Resolution was defined as a decrease in the extent or disappearance of the ILA-related imaging findings.
QCT EVALUATION
The CT data were transferred to a post-processing workstation for AI-based QCT analysis by an investigator (with 11 years of experience in thoracic imaging). Dedicated semiautomatic software for ILA (AVIEW Lung Texture ILA version 1.1.42.12; Coreline Soft, Seoul, Korea) was used for quantitative analysis. Quantitative analysis of CT parenchymal patterns was performed using content-based image retrieval (CBIR). Lung segmentation and disease pattern classification were performed using a two-dimensional U-Net architecture with a deep convolutional neural network, as previously described (12). Automated parenchymal pattern analysis was performed, and classified digital imaging and communication in medical images were successfully received by the AI platform. The percentage of lung texture was analyzed for each morphology, including ground-glass opacity, reticulation, consolidation, honeycombing, and emphysema. Total lung volume was estimated using a validated CBIR system at the pixel level (Supplementary Fig. 1). The performance of this program has been validated using low-dose CT in recently published studies (13,14).
STATISTICAL ANALYSES
Data are presented as means with standard deviations for continuous variables and numbers with percentages for categorical variables. The clinical characteristics, PFT data, and CT findings were compared between the two groups with and without clinically significant ILD using Student’s t-test for continuous variables and Fisher’s exact test or Pearson’s chi-square test for categorical variables as well as between smokers and nonsmokers. Pearson’s correlation coefficients were used to determine the relationship between the PFT results and QCT parameters. A paired t-test was used to compare the initial and follow-up QCT analyses. Factors associated with a subsequent clinical diagnosis of ILD were analyzed using univariate and multivariate logistic regression analyses. Variables with a p-value less than 0.05 on the univariate analysis were included in the multivariate logistic regression model. All statistical analyses were performed using R version 3.6.1 (The R Project, Vienna, Austria) and Rex version 3.0.3 (RexSoft Inc., Seoul, Korea).
RESULTS
PATIENT CHARACTERISTICS AND CLINICAL EVENTS
A total of 101 patients were included in this study; their demographic and clinical characteristics are summarized in Table 1. The mean age was 62.3 ± 9.5 years, and 88 (87%) were men. Eighteen patients (18%) presented with respiratory symptoms during the follow-up period (mean interval between the symptom onset and CT detection of ILA, 36.6 ± 39.2 months; range, 0–129 months), with cough (60%) as the most common symptom, followed by dyspnea (20%) and sputum (20%). Twenty-three patients (23%) were subsequently diagnosed with clinically significant ILDs during the follow-up period (mean, 104.2 ± 50.3 months; range, 13–208 months), which consisted of idiopathic pulmonary fibrosis (n = 22) and rheumatoid arthritis-related ILD (n = 1). ILD was diagnosed by histologic evaluation (n = 3) or a clinical–radiologic–pathologic multidisciplinary discussion (n = 20). Lung cancer was diagnosed in 4 patients (4%) during follow-up, with a higher incidence in patients with ILD than in those without ILD (13% vs. 1%; p = 0.035).
Table 1. Baseline and Follow-Up Characteristics of Study Participants according to the Clinical Diagnosis of ILD.
| Total (n = 101) | No Clinical Diagnosis of ILD (n = 78) | Clinical Diagnosis of ILD (n = 23) | p-Value | ||
|---|---|---|---|---|---|
| Age (years) | 62.3 ± 9.5 | 61.6 ± 10.1 | 64.5 ± 6.6 | 0.1130 | |
| Sex (M:F) | 88:13 | 69:9 | 19:4 | 0.7022 | |
| Height (cm) | 165.7 ± 12.8 | 166.7 ± 7.7 | 162.4 ± 22.4 | 0.3800 | |
| Weight (kg) | 70.5 ± 13.1 | 69.7 ± 9.3 | 73.2 ± 21.0 | 0.4448 | |
| Smoking history | |||||
| Unknown | 17 (16.8) | 15 (19.2) | 2 (8.7) | 0.0668 | |
| Current smoker | 23 (22.8) | 19 (24.4) | 4 (17.4) | ||
| Ex-smoker | 19 (18.8) | 10 (12.8) | 9 (39.1) | ||
| Non-smoker | 42 (41.6) | 34 (43.6) | 8 (34.8) | ||
| Comorbidity | |||||
| HTN | 36 (37.9) | 26 (36.1) | 10 (43.5) | 0.6986 | |
| DM | 25 (26.3) | 19 (26.4) | 6 (26.1) | 0.9999 | |
| CVD | 9 (9.5) | 8 (11.1) | 1 (4.4) | 0.5787 | |
| Cancer | 6 (6.3) | 4 (5.6) | 2 (8.7) | 0.9628 | |
| Emerging respiratory symptoms during follow-up (n) | 18 (17.8) | 10 (12.8) | 8 (34.8) | <0.001* | |
| Cough | 12 | 5 | 7 | ||
| Sputum | 4 | 2 | 2 | ||
| Dyspnea | 4 | 0 | 4 | ||
| Symptom duration (month)† | 19.5 ± 37.5 | 19.0 ± 27.8 | 19.7 ± 44.3 | 0.9745 | |
| Interval between symptom onset and ILA on CT (month) | 36.6 ± 39.2 | 18.6 ± 16.3 | 48.1 ± 45.6 | 0.0710 | |
| Clinical events | |||||
| Diagnosis with lung cancer | 4 (4.0) | 1 (1.3) | 3 (13.0) | 0.0352* | |
| Death | 2 (2.0) | 1 (1.3) | 1 (4.4) | 0.9395 | |
| Initial PFT (%) | |||||
| FVC | 91.1 ± 19.2 | 92.7 ± 17.6 | 85.0 ± 23.4 | 0.1509 | |
| FEV1 | 93.7 ± 18.5 | 95.0 ± 17.5 | 88.9 ± 21.0 | 0.2166 | |
| FEV1/FVC | 87.2 ± 14.2 | 86.6 ± 14.7 | 89.1 ± 12.6 | 0.4427 | |
| DLCO | 82.9 ± 24.9 | 74.5 ± 26.2 | 91.3 ± 24.0 | 0.3822 | |
| Last PFT (%)‡ | |||||
| FVC | 96.0 ± 17.0 | 97.4 ± 14.1 | 92.9 ± 22.2 | 0.4501 | |
| FEV1 | 99.2 ± 17.5 | 100.5 ± 15.8 | 96.2 ± 20.8 | 0.4538 | |
| FEV1/FVC | 83.9 ± 17.8 | 87.8 ± 18.1 | 76.5 ± 14.9 | 0.0250* | |
| DLCO | 90.3 ± 15.2 | 92.5 ± 15.1 | 89.3 ± 16.2 | 0.7418 | |
| Significant FVC decline (n)§ | 23 (39.7) | 12 (33.3) | 11 (50.0) | 0.3259 | |
*Statistically significant results (p < 0.05).
†Eighteen patients developed respiratory symptoms, and 11 of 18 were able to determine the duration of their respiratory symptoms (7 with clinically significant ILD and 4 without clinically significant ILD).
‡Last PFTs were performed in 58 patients (22 with clinically significant ILD and 36 without clinically significant ILD).
§Significant FVC decline was defined as a decrease in FVC of 5% or more during follow-up.
CVD = cardiovascular disease, DLCO = diffusing capacity of the lung for carbon monoxide, DM = diabetes mellitus, FEV1 = forced expiratory volume in one second, FVC = forced vital capacity, HTN = hypertension, ILA = interstitial lung abnormality, ILD = interstitial lung disease, PFT = pulmonary function test
PFT ANALYSIS
There were no significant differences in the initial PFT results between patients with and without a subsequent ILD diagnosis. However, the percentages of the mean FVC and FEV1 were lower in patients with a diagnosis of ILD (FVC, 85.0% ± 23.4% vs. 92.7% ± 17.6%; FEV1, 88.9% ± 21.0% vs. 95.0% ± 17.5%, all p > 0.050). Fifty-eight patients (57%) underwent PFT during the study period. In the last follow-up PFT analysis, the FEV1/FVC ratio was significantly decreased in patients with clinically significant ILDs (76.5% ± 14.9% vs. 87.8% ± 18.1%; p = 0.025). Twenty-three patients showed significant FVC decline, 11 of whom were in the clinically significant ILD group (50% vs. 33%; p = 0.326) (Table 1).
VISUAL CT ANALYSIS
Among the 101 patients, subpleural nonfibrotic ILA was the most common subtype (39%), followed by subpleural fibrotic (25%), nonsubpleural (18%), and equivocal (18%) ILAs. Comparisons of visual CT analyses between patients with and without clinically significant ILD are summarized in Table 2. Compared with the initial CT, there was a significant difference in the ILA subtypes between the two groups (p = 0.008). Subpleural fibrotic ILAs were more frequently identified in patients with clinically significant ILD than in those without clinically significant ILD (10/23, 44% vs. 15/78, 19%; p = 0.036) (Fig. 2). Subpleural nonfibrotic ILAs also tended to be more frequent in patients with clinically significant ILD than in those without clinically significant ILD (11/23, 48% vs. 28/78, 36%; p = 0.430) (Fig. 3). CT findings of equivocal ILAs were observed only in patients without clinically significant ILD (19/78, 24%; p = 0.020). 24 patients (24%) showed concurrent emphysema, and 16 (16%) had concomitant sequelae of TB on chest CT. In our study, follow-up CT scans were available for 63 patients (62%; 22 patients with clinically significant ILD and 41 patients without clinically significant ILD), and disease progression was more frequently identified in patients with a subsequent clinical diagnosis of ILD than in those without (16/22, 73% vs. 11/41, 27%; p = 0.001).
Table 2. Comparison of Visual CT Analysis between Patients with and without a Clinical Diagnosis of ILD.
| Total (n = 101) | No Clinical Diagnosis of ILD (n = 78) | Clinical Diagnosis of ILD (n = 23) | p-Value | |||
|---|---|---|---|---|---|---|
| CT interval (month) | 87.5 ± 56.8 | 79.9 ± 58.5 | 104.2 ± 50.3 | 0.0960 | ||
| Number of CTs | 4.1 ± 4.1 | 3.4 ± 3.9 | 6.5 ± 4.0 | 0.0022* | ||
| Initial CT | ||||||
| ILA subtype | 0.0078* | |||||
| Subpleural fibrotic | 25 (24.8) | 15 (19.2) | 10 (43.5) | 0.0363* | ||
| Subpleural nonfibrotic | 39 (38.6) | 28 (35.9) | 11 (47.8) | 0.4302 | ||
| Nonsubpleural | 18 (17.8) | 16 (20.5) | 2 (8.7) | 0.3215 | ||
| Equivocal | 19 (18.8) | 19 (24.4) | 0 (0) | 0.0202* | ||
| Extensiveness (>3 lung zones) | 17 (16.8) | 10 (12.8) | 7 (30.4) | 0.0955 | ||
| Concurrent lung disease | ||||||
| Emphysema | 24 (23.8) | 17 (21.8) | 7 (30.4) | 0.5641 | ||
| TB sequelae | 16 (15.8) | 10 (12.8) | 6 (26.1) | 0.2277 | ||
| Follow-up CT† | ||||||
| ILA subtype‡ | 0.0088* | |||||
| Subpleural fibrotic | 29 (51.8) | 12 (29.3) | 17 (30.4) | 0.0061* | ||
| Subpleural nonfibrotic | 17 (30.4) | 12 (29.3) | 5 (21.7) | 0.3983 | ||
| Nonsubpleural | 3 (5.4) | 3 (7.3) | 0 (0) | 0.4608 | ||
| Equivocal | 7 (12.5) | 7 (16.7) | 0 (0) | 0.1290 | ||
| Progression | 27 (42.9) | 11 (26.8) | 16 (72.7) | 0.0012* | ||
| Stability | 29 (46.0) | 23 (56.1) | 6 (27.3) | |||
| Resolution | 7 (11.1) | 7 (17.1) | 0 (0) | |||
*Statistically significant results (p < 0.05).
†Follow-up CT scans were performed in 63 patients (22 with clinically significant ILD and 41 without clinically significant ILD).
‡Interstitial lung abnormalities were resolved in seven patients on follow-up CT, and the remaining 56 patients were classified as subtype.
ILA = interstitial lung abnormality, ILD = interstitial lung disease, TB = tuberculosis
Fig. 2. These are CT images of a 59-year-old man with a subpleural fibrotic ILA. Idiopathic pulmonary fibrosis was diagnosed in this patient with a usual interstitial pneumonia pattern on lung biopsy performed 1 year after the initial low-dose CT scan.
A, B. Axial low-dose chest CT scan (A) shows subpleural reticulation with traction bronchiolectasis in both basal lungs, which is classified as a subpleural fibrotic ILA by visual analysis. On QCT analysis (B), the extent of the disease is 5.6% of the total lungs (yellow, fibrotic ILA; green, nonfibrotic ILA).
C, D. Axial high-resolution CT scan with prone positioning (C) performed at the 11-year follow-up shows an increased progression of the lung fibrosis with increased honeycombing. Traction bronchiectasis is also progressing with decreased lung volume. On QCT analysis (D), the extent of disease has increased to 25.0% of the total lungs (yellow, ground-glass opacity; orange, reticulation; red, honeycombing or traction bronchiectasis/bronchiolectasis).
ILA = interstitial lung abnormality, QCT = quantitative CT
Fig. 3. These are CT images of a 63-year-old man with subpleural nonfibrotic ILA and subsequent clinicoradiologic diagnosis of interstitial lung disease with a radiologic pattern of fibrotic nonspecific interstitial pneumonia.
A, B. Axial low-dose chest CT scan (A) shows mild ground-glass opacities with subtle reticulation (arrows) in the subpleural and nonsubpleural areas of both basal lungs. No evidence of honeycombing or architectural distortion is noted. This case is classified as a subpleural nonfibrotic ILA by visual analysis. On QCT analysis (B), the extent of disease is 0.6% of the total lungs (yellow, fibrotic ILA; green, nonfibrotic ILA).
C, D. Axial high-resolution CT with prone positioning (C) taken after 5 years reveals increased fibrosis with subpleural reticulation and traction bronchiolectasis in both basal lungs (arrows). On QCT analysis (D) the extent of disease has increased to 7.0% of the total lungs (yellow, ground-glass opacity; orange, reticulation; red, honeycombing or traction bronchiectasis/bronchiolectasis).
ILA = interstitial lung abnormality, QCT = quantitative CT
QCT ANALYSIS
The comparison of the QCT analyses between patients with and without clinically significant ILD is summarized in Table 3. Initial QCT analysis was performed for all 101 patients, and follow-up QCT analysis was performed for 63 patients. In the AI-based QCT analysis of the initial CT scans, the extent of consolidation was significantly greater in patients with a subsequent clinical diagnosis of ILD (p = 0.036). The extent of the ILA showed a tendency to be greater in patients with clinically significant ILD than in those without (fibrotic ILA, 0.8% ± 1.2% vs. 0.4% ± 0.6%; p = 0.203; nonfibrotic ILA, 2.6% ± 6.4% vs. 1.7% ± 3.8%; p = 0.552; total ILA, 3.3% ± 6.7% vs. 2.2% ± 4.0%; p = 0.432).
Table 3. Comparison of AI-Based QCT Analysis between Patients with and without a Clinical Diagnosis of ILD.
| Total (n = 101) | No Clinical Diagnosis of ILD (n = 78) | Clinical Diagnosis of ILD (n = 23) | p-Value | ||
|---|---|---|---|---|---|
| Initial CT | |||||
| Fibrotic ILA (%) | 0.5 ± 0.8 | 0.4 ± 0.6 | 0.8 ± 1.2 | 0.2025 | |
| Nonfibrotic ILA (%) | 1.9 ± 4.5 | 1.7 ± 3.8 | 2.6 ± 6.4 | 0.5515 | |
| Total ILA (%) | 2.4 ± 4.7 | 2.2 ± 4.0 | 3.3 ± 6.7 | 0.4323 | |
| Total lung volume (cc) | 4486.1 ± 1241.0 | 4578.7 ± 1116.3 | 4172.1 ± 1582.9 | 0.2595 | |
| Emphysema (%) | 0.8 ± 2.3 | 0.6 ± 1.4 | 1.2 ± 4.2 | 0.4995 | |
| Consolidation (%) | 0.0 ± 0.1 | 0.0 ± 0.1 | 0.1 ± 0.1 | 0.0364* | |
| GGO (%) | 2.4 ± 5.5 | 1.7 ± 3.8 | 4.8 ± 9.0 | 0.1276 | |
| Reticulation (%) | 0.6 ± 1.0 | 0.5 ± 0.7 | 1.1 ± 1.7 | 0.0791 | |
| Honeycombing (%) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.1 | 0.2944 | |
| Follow-up CT† | |||||
| Fibrotic ILA (%) | 2.4 ± 5.2 | 1.0 ± 2.2 | 5.1 ± 7.8 | 0.0259* | |
| Nonfibrotic ILA (%) | 2.3 ± 6.8 | 2.4 ±7.8 | 2.2 ± 4.7 | 0.9218 | |
| Total ILA (%) | 4.7 ± 9.3 | 3.3 ± 8.6 | 7.4 ± 10.3 | 0.1317 | |
| Total lung volume (cc) | 4645.7 ± 1253.2 | 4709.1 ± 1243.9 | 4518.7 ± 1292.8 | 0.5799 | |
| Emphysema (%) | 0.9 ± 2.1 | 1.0 ± 2.3 | 0.8 ± 1.9 | 0.7742 | |
| Consolidation (%) | 0.3 ± 1.2 | 0.2 ± 0.9 | 0.6 ± 1.6 | 0.2220 | |
| GGO (%) | 2.3 ± 6.9 | 2.4 ± 7.8 | 2.2 ± 4.7 | 0.9282 | |
| Reticulation (%) | 2.0 ± 4.3 | 0.9 ± 2.2 | 4.1 ± 6.3 | 0.0356* | |
| Honeycombing (%) | 0.3 ± 1.7 | 0.02 ± 0.1 | 0.8 ± 2.9 | 0.2052 | |
*Statistically significant results (p < 0.05).
†QCT analysis was performed in 63 patients (22 with clinically significant ILD and 41 without clinically significant ILD).
AI = artificial intelligence, GGO = ground-glass opacity, ILA = interstitial lung abnormality, ILD = interstitial lung disease, QCT = quantitative CT
In the analysis of the relationship between QCT parameters and PFTs, reticulation and consolidation on initial CT correlated well with FVC and FEV1 (reticulation: FVC, rho = -0.232; p = 0.025; reticulation: FEV1, rho = -0.232; p = 0.026; consolidation: FVC, rho = -0.221; p = 0.033; consolidation: FEV1, rho = -0.186; p = 0.075). DLCO showed no significant correlation with the QCT parameters (Supplementary Table 1).
AI-based QCT analysis of follow-up CT scans revealed a significant increase in the extent of CT abnormalities in all patients with fibrotic ILAs on the initial CT and a significant increase in the extent of total ILAs compared with those in the initial study (fibrotic ILA, 0.5% ± 0.8% and 2.4% ± 5.2%; p = 0.006; total ILA, 2.4% ± 4.7% and 4.7% ± 9.3%; p = 0.024). The extent of CT abnormalities in patients with nonfibrotic ILAs and total lung volume were not significantly changed on follow-up (nonfibrotic ILA, 2.3% ± 6.8% and 1.9% ± 4.5%; p = 0.370; total lung volume, 4727.5 ± 962.4 and 4776.7 ± 1068.7; p = 0.711) (Supplementary Fig. 2).
RISK FACTORS FOR THE DIAGNOSIS OF CLINICALLY SIGNIFICANT ILDS
In the univariate logistic regression analysis, the emerging respiratory symptoms during the follow-up period (odds ratio [OR], 9.43; 95% confidence interval [CI], 3.05–29.12; p < 0.001), progression of CT findings on follow-up (OR, 7.77; 95% CI, 2.39–25.25; p = 0.001), increased extent of reticulation (OR, 1.80; 95% CI, 1.09–2.96; p = 0.022), and fibrotic ILA pattern on QCT analysis of the initial CT scans (OR, 1.65; 95% CI, 1.01–2.71; p = 0.046) were significant predictive parameters for the subsequent clinical diagnosis of ILD. In the multivariate logistic regression analysis, emerging respiratory symptoms during follow-up (OR, 5.56; 95% CI, 1.28–24.21; p = 0.022) and progression of CT findings on follow-up (OR, 4.07; 95% CI, 1.00–16.54; p = 0.050) were significant predictors of clinically significant ILD (Table 4).
Table 4. Univariate and Multivariate Analyses for Factors Associated with a Subsequent Clinical Diagnosis of ILD in Patients with ILA.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Age | 0.0313 | 0.0011–0.8801 | 0.0418* | 0.2639 | 0.9096–1.0842 | 0.8764 |
| Sex | 0.6107 | 0.1694–2.2015 | 0.4510 | 0.8216 | 0.0592–11.4123 | 0.8837 |
| Emerging respiratory symptoms during follow-up | 9.4286 | 3.0531–29.1176 | <0.0001* | 5.5587 | 1.2761–24.2131 | 0.0223* |
| Interval between symptom onset and ILA on CT | 1.0359 | 0.9468–1.1334 | 0.4417 | NA | NA | NA |
| ILA subtype, visual (nonfibrotic) | 0.1875 | 0.0352–0.9997 | 0.0050* | 0.2841 | 0.0169–4.6803 | 0.3767 |
| Imaging progression on follow-up CT | 7.7714 | 2.3918–25.2514 | 0.0006* | 4.0689 | 1.0009–16.5405 | 0.0498* |
| Reticulation extent by AI-based QCT | 1.7959 | 1.0892–2.9613 | 0.0217* | 1.4016 | 0.6986–2.8122 | 0.3420 |
| Fibrotic ILA extent by AI-based QCT | 1.6540 | 1.0097–2.7092 | 0.0457* | 1.3547 | 0.8710–2.1070 | 0.1779 |
*Statistically significant results (p < 0.05).
AI = artificial intelligence, CI = confidence interval, ILA = interstitial lung abnormality, ILD = interstitial lung disease, NA = not applicable, OR = odds ration, QCT = quantitative CT
ANALYSIS OF ILA ASSOCIATED WITH SMOKING
Of the 101 patients with ILA, 42 (42%) were nonsmokers, 42 (42%) were current or ex-smokers, and 17 had an unknown smoking history. Comparing of the initial clinical characteristics of the nonsmoker and smoker groups showed that the nonsmoker group was significantly older (64.7 ± 10.0 vs. 60.5 ± 8.5 years; p = 0.040) and had a lower proportion of men (76% vs. 95%; p = 0.029). Notably, the initial visual CT analysis revealed a significantly lower prevalence of concurrent emphysema among nonsmokers (11.9% vs. 38.1%; p = 0.012), with no discernible discrepancy in ILA subtype or extensiveness between the two groups. Furthermore, on initial QCT analysis, the nonsmoker group exhibited a lesser extent of fibrotic ILA than the smoker group (0.3% ± 0.4% vs. 0.7% ± 1.1%; p = 0.020). Among the follow-up analyses, PFT results showed a significantly higher FEV1/FVC ratio in the nonsmoker group (90.0% ± 20.7% vs. 75.5% ± 11.5%; p = 0.006), which contrasts with the initial PFT results that did not differ between the two groups. No other visual or QCT analyses revealed significant differences between the two groups (Supplementary Tables 2, 3).
DISCUSSION
In this study, we explored the predictors of clinically significant ILD by recruiting more than 30000 low-dose chest CT scans from the general screening population over a long-term follow-up period. Our study demonstrated that approximately one-quarter of patients with ILA on initial low-dose chest CT were subsequently diagnosed with clinically significant ILD during a mean follow-up period of 8.7 years. Furthermore, the appearance of respiratory symptoms during the follow-up period (OR, 5.56; 95% CI, 1.28–24.21; p = 0.022) and the progression of ILA on follow-up chest CT (OR, 4.07; 95% CI, 1.00–16.54; p = 0.049) were significant predictors of clinically significant ILD.
ILAs are associated with respiratory symptoms, functional impairment, increased risk of lung cancer, and increased all-cause mortality (15,16,17,18,19). However, few studies have investigated the relationship between ILAs and ILD diagnosis. Furthermore, the characterization of ILAs and suspected ILDs in diverse populations is necessary to determine proper estimates of population-specific prevalence. We found that emerging respiratory symptoms during follow-up were associated with ILA progression and the subsequent diagnosis of ILD in our general screening population. Considering that there was no significant difference in the initial PFT results between the two groups, the findings of our study could have important implications for the risk stratification of ILAs, enabling early detection of ILDs before a substantial decline in lung function occurs. Based on our results, we recommend further clinical evaluation, including PFTs, for the general screening of patients with incidental detection of ILAs and respiratory symptoms. The combination of radiologic and physiologic abnormalities enables a more comprehensive and proper assessment of individuals at high risk of ILD, including subclinical or preclinical ILD. Our screening cohort was not limited to lung cancer screening but included a wide range of individual screening purposes, which may have better implications for the generalization of the study results. However, further validation in larger and more ethnically diverse populations is required.
Our study also identified disease progression on follow-up CT with visual analysis as a crucial predictive factor for subsequent clinical diagnosis of ILD. Approximately 43% of our patients with ILAs initially detected on low-dose CT showed progression during follow-up (mean period, 7.3 years), and the majority (70%) of our patients with progressive ILAs were subsequently diagnosed with clinically significant ILD. Moreover, the increased extent of fibrotic ILAs and reticulation in AI-based QCT analysis were significant predictors of clinically diagnosed ILD, providing more objective and robust evidence for the results of our study. These findings demonstrate that ILA progression on follow-up CT is comparable to progressive pulmonary fibrosis and can independently contribute to the diagnosis of clinically significant ILD (10,19,20).
In our review of more than 30000 low-dose chest CT scans, the proportion of ILAs in the general screening cohort was 0.3%, which is relatively low compared with that of previous studies, which reported prevalence rates ranging from 0.8% to 22.8% (4,5,15,21,22,23,24). In a study of an Asian population-based cohort, the prevalence of ILAs was approximately 3% (25). The low proportion of ILAs observed in the present study can be attributed to several factors. First, the study cohort comprised a general screening population with diverse screening purposes and no known risk factors. Unlike many previous studies that focused on lung cancer screening in heavy smokers, our study included an equal ratio of ever- and never-smokers. This may explain the low ILA prevalence observed in our study, given the widely recognized association between cigarette smoking and ILAs (5,23). Moreover, our study was not originally designed to determine the prevalence of ILAs. Instead, we retrospectively collected relevant data by searching the radiologic reports of our general screening cohort. Nevertheless, we found that a significant proportion (43%) of patients with incidental ILA findings on an initial CT and evidence of disease progression on follow-up were subsequently diagnosed with clinically relevant ILD, which is consistent with the findings of previous studies (23,25).
On the other hand, when we compared the clinical and imaging features according to smoking history in our study, the frequency of concomitant emphysema and the extent of fibrotic ILA on QCT analysis were significantly higher in the smoking group, consistent with previous studies (13,14,25,26). Nonsmokers were older, which may reflect the relationship between ILA and aging, which is considered part of the normal spectrum of senescent lungs (11). Although spirometry at follow-up showed better lung function in the nonsmoker group, visual and QCT analyses did not reveal any differences between the two groups, and no significant differences were identified in the diagnosis of clinically significant ILD. Prospective studies with longer follow-up periods are essential to elucidate the pathophysiology of ILA in nonsmokers and to identify progression and prognostic factors beyond the well-established association between smoking and ILA observed in previous studies.
Quantitative analysis of CT parenchymal patterns allows for the objective recognition of regional disease patterns and extent of pulmonary parenchymal disease, ensuring reproducibility (7,27,28). Several studies on QCT analysis of ILA or ILD have demonstrated its sensitivity for detecting and scoring pulmonary fibrosis, offering a more reliable assessment than visual analysis (6,29). In our study, we found that the extent of reticulation and fibrotic ILA estimated using AI-based QCT analysis were significant predictors of clinically significant ILD. Our results suggest the possibility of a wider implementation of quantitative tools in the assessment of imaging progression and phenotypic subgrouping of detected ILAs. There have been studies with similar findings (30) and suggestions to adjust the current threshold value of the CT extent (<5%) for ILA diagnosis owing to the lower sensitivity of QCT.
Our study has several limitations. First, this was a single-center, retrospective study with a relatively small cohort. Our study relied on a review of radiology reports written by multiple radiologists with varying levels of experience, and there may have been missing data before the era of ILA conceptualization. Consequently, we selected patients using search terms that implied the presence of ILA. However, this approach is subject to potential selection bias, which prevented us from obtaining accurate ILA prevalence rates. Additionally, the incidence of clinically significant ILD may be higher because we included only overt ILA. Second, the follow-up interval, follow-up CT scanners, and protocols varied owing to the retrospective design, and follow-up CT was not performed in all enrolled patients; in particular, thin-section and prone-positioning images were not available for some patients. However, we made substantial efforts to detect ILAs by applying the current ILA criteria and conducting an accurate visual analysis to assess their extent and patterns. To overcome this variability, we used a more objective method through QCT. Third, we did not perform an interobserver agreement analysis in the visual analysis. Finally, data regarding the severity and duration of patients’ respiratory symptoms, diagnosis of clinically significant ILD and lung cancer, and mortality rates were extracted from electronic medical records, which could limit data accessibility and potentially affect accurate assessments. Further studies are needed to overcome these limitations.
In conclusion, in this study, approximately one-quarter of the general screening population with ILAs detected on initial low-dose CT were subsequently diagnosed with clinically significant ILD. In patients with ILAs, emerging respiratory symptoms and the progression of ILAs on follow-up chest CT are significant predictors for the diagnosis of clinically significant ILD. Delayed diagnosis is common in ILDs, and most patients with advanced ILDs show decreased quality of life and poor prognosis, indicating the importance of early diagnosis and proper management to improve patient outcomes. Given the relationship between ILAs and well-established ILD revealed in our study, appropriate evaluation and surveillance are required for the general screening population with incidental findings of ILAs on low-dose chest CT and a subsequently high likelihood of progression to clinically significant ILD.
Footnotes
- Conceptualization, N.B.D., H.J.H.
- data curation, J.W.J., N.B.D.
- formal analysis, N.B.D.
- funding acquisition, N.B.D.
- investigation, J.W.J., N.B.D., Y.H., L.E.J.
- methodology, O.E., J.J., B.S.H.
- project administration, N.B.D., H.J.H.
- resources, N.B.D., H.J.H., L.C.H.
- software, N.B.D.
- supervision, N.B.D., H.J.H.
- validation, all authors.
- visualization, all authors.
- writing—original draft, all authors.
- writing—review & editing, all authors.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding: This research was supported by the Soonchunhyang University Research Fund. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2021R1I1A3049835).
Availability of Data and Material
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
Supplementary Materials
The Supplement is available with this article at http://doi.org/10.3348/jksr.2024.0032.
Analysis of the Relationships between PFTs and Q-CT Parameters
Baseline and Follow-up Characteristics of Nonsmokers and Smokers
Comparison of Visual and AI-based QCT Analysis between Nonsmokers and Smokers
User interface of the automated quantitative tool for interstitial lung abnormality (ILA).
Comparative analysis of interstitial lung abnormalities between the initial and last follow-up CT.
References
- 1.Hatabu H, Hunninghake GM, Richeldi L, Brown KK, Wells AU, Remy-Jardin M, et al. Interstitial lung abnormalities detected incidentally on CT: a position paper from the Fleischner Society. Lancet Respir Med. 2020;8:726–737. doi: 10.1016/S2213-2600(20)30168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araki T, Putman RK, Hatabu H, Gao W, Dupuis J, Latourelle JC, et al. Development and progression of interstitial lung abnormalities in the Framingham Heart Study. Am J Respir Crit Care Med. 2016;194:1514–1522. doi: 10.1164/rccm.201512-2523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Putman RK, Gudmundsson G, Axelsson GT, Hida T, Honda O, Araki T, et al. Imaging patterns are associated with interstitial lung abnormality progression and mortality. Am J Respir Crit Care Med. 2019;200:175–183. doi: 10.1164/rccm.201809-1652OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoyer N, Wille MMW, Thomsen LH, Wilcke T, Dirksen A, Pedersen JH, et al. Interstitial lung abnormalities are associated with increased mortality in smokers. Respir Med. 2018;136:77–82. doi: 10.1016/j.rmed.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Putman RK, Hatabu H, Araki T, Gudmundsson G, Gao W, Nishino M, et al. Association between interstitial lung abnormalities and all-cause mortality. JAMA. 2016;315:672–681. doi: 10.1001/jama.2016.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SM, Seo JB, Oh SY, Kim TH, Song JW, Lee SM, et al. Prediction of survival by texture-based automated quantitative assessment of regional disease patterns on CT in idiopathic pulmonary fibrosis. Eur Radiol. 2018;28:1293–1300. doi: 10.1007/s00330-017-5028-0. [DOI] [PubMed] [Google Scholar]
- 7.Weatherley ND, Eaden JA, Stewart NJ, Bartholmai BJ, Swift AJ, Bianchi SM, et al. Experimental and quantitative imaging techniques in interstitial lung disease. Thorax. 2019;74:611–619. doi: 10.1136/thoraxjnl-2018-211779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humphries SM, Yagihashi K, Huckleberry J, Rho BH, Schroeder JD, Strand M, et al. Idiopathic pulmonary fibrosis: data-driven textural analysis of extent of fibrosis at baseline and 15-month follow-up. Radiology. 2017;285:270–278. doi: 10.1148/radiol.2017161177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes H, Humphries SM, George PM, Assayag D, Glaspole I, Mackintosh JA, et al. Machine learning in radiology: the new frontier in interstitial lung diseases. Lancet Digit Health. 2023;5:e41–e50. doi: 10.1016/S2589-7500(22)00230-8. [DOI] [PubMed] [Google Scholar]
- 10.Raghu G, Remy-Jardin M, Richeldi L, Thomson CC, Inoue Y, Johkoh T, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205:e18–e47. doi: 10.1164/rccm.202202-0399ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hata A, Schiebler ML, Lynch DA, Hatabu H. Interstitial lung abnormalities: state of the art. Radiology. 2021;301:19–34. doi: 10.1148/radiol.2021204367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choe J, Hwang HJ, Seo JB, Lee SM, Yun J, Kim MJ, et al. Content-based image retrieval by using deep learning for interstitial lung disease diagnosis with chest CT. Radiology. 2022;302:187–197. doi: 10.1148/radiol.2021204164. [DOI] [PubMed] [Google Scholar]
- 13.Chae KJ, Chung MJ, Jin GY, Song YJ, An AR, Choi H, et al. Radiologic-pathologic correlation of interstitial lung abnormalities and predictors for progression and survival. Eur Radiol. 2022;32:2713–2723. doi: 10.1007/s00330-021-08378-8. [DOI] [PubMed] [Google Scholar]
- 14.Kim MS, Choe J, Hwang HJ, Lee SM, Yun J, Kim N, et al. Interstitial lung abnormalities (ILA) on routine chest CT: comparison of radiologists’ visual evaluation and automated quantification. Eur J Radiol. 2022;157:110564. doi: 10.1016/j.ejrad.2022.110564. [DOI] [PubMed] [Google Scholar]
- 15.Hunninghake GM, Hatabu H, Okajima Y, Gao W, Dupuis J, Latourelle JC, et al. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med. 2013;368:2192–2200. doi: 10.1056/NEJMoa1216076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whittaker Brown SA, Padilla M, Mhango G, Powell C, Salvatore M, Henschke C, et al. Interstitial lung abnormalities and lung cancer risk in the national lung screening trial. Chest. 2019;156:1195–1203. doi: 10.1016/j.chest.2019.06.041. [DOI] [PubMed] [Google Scholar]
- 17.Axelsson GT, Putman RK, Aspelund T, Gudmundsson EF, Hida T, Araki T, et al. The associations of interstitial lung abnormalities with cancer diagnoses and mortality. Eur Respir J. 2020;56:1902154. doi: 10.1183/13993003.02154-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buendía-Roldán I, Fernandez R, Mejía M, Juarez F, Ramirez-Martinez G, Montes E, et al. Risk factors associated with the development of interstitial lung abnormalities. Eur Respir J. 2021;58:2003005. doi: 10.1183/13993003.03005-2020. [DOI] [PubMed] [Google Scholar]
- 19.Rose JA, Menon AA, Hino T, Hata A, Nishino M, Lynch DA, et al. Suspected interstitial lung disease in COPDGene study. Am J Respir Crit Care Med. 2023;207:60–68. doi: 10.1164/rccm.202203-0550OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tzilas V, Tzouvelekis A, Ryu JH, Bouros D. 2022 update on clinical practice guidelines for idiopathic pulmonary fibrosis and progressive pulmonary fibrosis. Lancet Respir Med. 2022;10:729–731. doi: 10.1016/S2213-2600(22)00223-5. [DOI] [PubMed] [Google Scholar]
- 21.Doyle TJ, Hunninghake GM, Rosas IO. Subclinical interstitial lung disease: why you should care. Am J Respir Crit Care Med. 2012;185:1147–1153. doi: 10.1164/rccm.201108-1420PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin GY, Lynch D, Chawla A, Garg K, Tammemagi MC, Sahin H, et al. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology. 2013;268:563–571. doi: 10.1148/radiol.13120816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sverzellati N, Guerci L, Randi G, Calabrò E, La Vecchia C, Marchianò A, et al. Interstitial lung diseases in a lung cancer screening trial. Eur Respir J. 2011;38:392–400. doi: 10.1183/09031936.00201809. [DOI] [PubMed] [Google Scholar]
- 25.Lee JE, Chae KJ, Suh YJ, Jeong WG, Lee T, Kim YH, et al. Prevalence and long-term outcomes of CT interstitial lung abnormalities in a health screening cohort. Radiology. 2023;306:e221172. doi: 10.1148/radiol.221172. [DOI] [PubMed] [Google Scholar]
- 26.Lee TS, Jin KN, Lee HW, Yoon SY, Park TY, Heo EY, et al. Interstitial lung abnormalities and the clinical course in patients with COPD. Chest. 2021;159:128–137. doi: 10.1016/j.chest.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Chen A, Karwoski RA, Gierada DS, Bartholmai BJ, Koo CW. Quantitative CT analysis of diffuse lung disease. Radiographics. 2020;40:28–43. doi: 10.1148/rg.2020190099. [DOI] [PubMed] [Google Scholar]
- 28.Raghunath S, Rajagopalan S, Karwoski RA, Maldonado F, Peikert T, Moua T, et al. Quantitative stratification of diffuse parenchymal lung diseases. PLoS One. 2014;9:e93229. doi: 10.1371/journal.pone.0093229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kliment CR, Araki T, Doyle TJ, Gao W, Dupuis J, Latourelle JC, et al. A comparison of visual and quantitative methods to identify interstitial lung abnormalities. BMC Pulm Med. 2015;15:134. doi: 10.1186/s12890-015-0124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chae KJ, Lim S, Seo JB, Hwang HJ, Choi H, Lynch D, et al. Interstitial lung abnormalities at CT in the Korean national lung cancer screening program: prevalence and deep learning-based texture analysis. Radiology. 2023;307:e222828. doi: 10.1148/radiol.222828. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Analysis of the Relationships between PFTs and Q-CT Parameters
Baseline and Follow-up Characteristics of Nonsmokers and Smokers
Comparison of Visual and AI-based QCT Analysis between Nonsmokers and Smokers
User interface of the automated quantitative tool for interstitial lung abnormality (ILA).
Comparative analysis of interstitial lung abnormalities between the initial and last follow-up CT.
Data Availability Statement
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.