Abstract
Active surveillance (AS) has been widely adopted as an alternative to immediate surgery owing to the indolent nature and favorable outcomes of papillary thyroid microcarcinoma (PTMC). AS is generally recommended for tumors measuring ≤1 cm without aggressive cytological subtypes, risk of gross extrathyroidal extension (ETE), lymph node metastasis (LNM), or distant metastasis. AS requires careful patient selection based on various patient and tumor characteristics, and ultrasound (US) findings. Moreover, during AS, regular US is performed to monitor any signs of tumor progression, including tumor growth, new US features of potential gross ETE, and LNM. Therefore, appropriate imaging-based assessment plays a crucial role in determining whether AS or surgery should be pursued. However, detailed recommendations concerning US evaluation are currently insufficient, necessitating the formulation of this guideline. The Korean Society of Thyroid Radiology has developed a consensus statement for low-risk PTMC, covering US assessment methods when considering AS as a management option and conducting follow-up imaging tests during AS. This guideline aims to provide optimal scientific evidence and expert opinion consensus regarding a standardized US-based assessment protocol for low-risk PTMC.
Keywords: Active Surveillance, Consensus, Papillary Thyroid Cancer, Practice Guideline, Recommendation, Thyroid Neoplasms, Watchful Waiting
Abstract
갑상선의 미세 유두암(papillary thyroid microcarcinoma; 이하 PTMC)는 병의 진행이 더디고 및 예후가 좋아 적극적 감시(active surveillance)가 즉각적인 수술의 대안으로 고려되고 있다. 적극적 감시는 일반적으로 공격적인 세포학적 아형이 없고, 종양 크기가 1 cm 이하이며, 육안적 갑상선 외 침범(extrathyroidal extension; 이하 ETE), 림프절 전이(lymph node metastasis) 또는 원격 전이의 위험이 없는 종양에서 권장된다. 적극적 감시를 고려할 때는 환자와 종양의 다양한 특성 및 초음파 소견을 바탕으로 한 신중한 환자 선별이 필요하다. 또한 적극적 관찰 중에는 종양의 성장, 잠재적인 육안적 ETE 징후, 림프절 전이 등 종양 진행의 징후를 모니터링하기 위해 정기적으로 초음파 검사를 시행하게 되므로, 적극적 관찰 시작하는 시점과 적극적 관찰 도중에는 적절한 영상 기반 평가가 적극적 관찰을 지속할지 수술을 시행할지를 결정하는 데 중요한 역할을 한다. 그러나 현재까지는 초음파 평가에 대한 구체적인 권장사항이 불충분하여 영상 소견 중심의 가이드라인의 필요성이 대두되었다. 이에 대한갑상선영상의학회의 진료지침위원회는 저위험 PTMC에 대한 초음파 평가에 대한 합의 성명을 개발하였으며, 이 성명에서는 적극적 관찰을 고려할 때와 적극적 관찰 중 추적 영상 검사를 수행할 때의 초음파 평가 방법을 다루고 있다. 이 가이드라인은 저위험 PTMC에 대한 표준화된 초음파 기반 평가 프로토콜에 대한 최적의 과학적 증거와 전문가 의견 합의를 제공하는 것을 목표로 한다.
INTRODUCTION
Thyroid cancer is a common cancer with a global incidence of 10.1 per 100000 in women and 3.1 per 100000 in men (1). The incidence of thyroid cancer dramatically increased in the 1990s and the early 2000s (2). This increase has mainly been attributed to the detection of indolent small thyroid cancers using medical imaging, especially ultrasound (US) (3,4). Papillary thyroid microcarcinoma (PTMC) is a tumor measuring ≤1 cm in size, with an excellent prognosis (5,6). Due to the indolent nature of PTMC, a management strategy called “active surveillance” (AS), which involves watchful waiting rather than immediate surgery, has emerged. Japanese studies (7,8) on AS have raised concerns about the overtreatment of thyroid cancer and reported favorable outcomes for low-risk PTMC; consequently, several prospective studies on AS have been conducted in various countries (7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39). The insights from these studies have influenced thyroid cancer management guidelines worldwide. In 2015, the American Thyroid Association considered AS an alternative to immediate surgery for PTMC (40). Various international guidelines provide recommendations on AS for treating thyroid cancer (40,41,42,43,44,45,46,47,48,49,50,51,52).
AS for small thyroid cancers involves careful imaging assessment of tumors upon initiation of AS for the careful selection of appropriate candidates. While most PTMCs tend to be indolent, some tumors may exhibit aggressive behaviors such as advanced local invasion or lymph node metastasis (LNM) and may progress during AS. Thus, the careful selection of appropriate candidates and timely management of AS are important clinical issues.
Serial high-quality imaging, such as US, is crucial for assessing the possibility of tumor progression and the need for deferred treatment (53). Given the central role of US in AS, standardization of the US technique and image interpretation is critical during AS for PTMC (53). These backgrounds have necessitated the development of clinical guidelines on US for AS of PTMC from radiologists’ perspectives. The task force of the Korean Society of Thyroid Radiology (KSThR) has reached a consensus on the need for detailed radiological practice guidelines that provide specific recommendations to maximize effectiveness and enhance physician confidence for the widespread adoption of AS in clinical practice. The goal is to achieve expert consensus to develop a detailed practice guideline for US evaluation in patients with thyroid microcarcinoma.
METHODOLOGY
Two authors searched MEDLINE via PubMed for articles (original studies and guidelines) published until October 2023 using keywords provided by two members of the KSThR Task Force (J.Y.L. and M.K.L.). The members reviewed the retrieved articles and proposed tentative key questions based on these articles. Five in-person meetings, six online meetings, and e-mail communications were conducted to formulate key questions and recommendations and to refine the detailed content (Table 1). A consensus of 13 panels (comprising experts on thyroid radiology) was reached using the modified Delphi method, especially regarding the benefits (median value ≥7: significant net benefits) and harms (median value ≤3: harms outweigh benefits). All suggestions were regularly reviewed by all the panel members through tracked changes and online meetings. The draft document was revised until no further revisions were requested by any of the panel members. After a draft of the guidelines was finalized, an internal peer review was performed and finally approved by the board members of the KSThR. The draft was made available for a month on the KSThR website (http://www.thyroidimaging.kr). Supplementary Tables 1, 2, 3 summarize the scope of the guideline, methodology, and identifying information and availability, respectively.
Table 1. Summary of Key Questions and Recommendations.
| Key Question | Recommendation |
|---|---|
| 1. What are the indications for AS in thyroid cancer? | [1-1] When AS is considered as a management strategy for thyroid cancer, the appropriateness of AS should be evaluated |
| [1-2] AS can be considered for adult patients with low-risk PTMC (≤1 cm) as an alternative to immediate surgery. Immediate surgery is recommended for high-risk PTMCs | |
| [1-3] AS is primarily considered for Bethesda V or VI thyroid nodules on FNA or CNB, without suspicious imaging features of gross ETE (particularly the trachea and RLN), LNM, and distant metastasis. However, taking patient preference into account, AS can also be considered for highly suspicious (K-TIRADS 5) thyroid nodules on US without biopsy | |
| 2. What is the appropriate US evaluation for patients with PTMC when considering the initiation of AS? | [2-1] Prior to the initiation of AS for thyroid cancer, high-quality US of the thyroid and neck should be performed by experts in thyroid and neck US imaging |
| [2-2] CT of the neck may play a supplementary role in the detection of additional LNM in low-risk PTMC | |
| [2-3] Chest CT is not routinely indicated before AS of low-risk PTMC | |
| 3. How should US evaluation of ETE and LNM be performed? | [3-1] Paratracheal tumors abutting the trachea or posteromedial subcapsular tumors should be carefully evaluated for the risk of gross ETE, especially with respect to the trachea and RLN |
| [3-2] Anterior subcapsular tumors with strap muscle replacement, paratracheal tumors abutting ≥90° to the trachea, posterior paratracheal tumors without normal intervening parenchyma, and posterior subcapsular tumors with protrusion pose a high risk of gross ETE and immediate surgery should be considered | |
| [3-3] FNA with thyroglobulin testing should be performed for any suspicious LNs | |
| 4. What is the appropriate US imaging technique for evaluating thyroid nodules under AS? | [4-1] We recommend using consistent measurement methods throughout the initial and follow-up examinations during AS |
| [4-2] Measurement for three axes should be performed during AS using the same imaging plane and slice as previously measured | |
| 5. How should tumor progression be evaluated by US imaging? | [5-1] Tumor progression can be assessed by evaluating tumor size enlargement and new US features of potential gross ETE or LNM |
| [5-2] Tumor size enlargement can be assessed by measuring the maximal tumor diameter increase of ≥3 mm or increase of ≥2 mm in at least two dimensions | |
| 6. What is the appropriate interval between US examinations during AS? | [6] US evaluations of changes in tumor size and the appearance of novel potential gross ETE and LNM are recommended every 6 months for the first 1–2 years after the initiation of AS and once a year thereafter if no tumor progression is detected |
| 7. When should a conversion to surgery be considered during follow-up? | [7] Surgical conversion can be considered when the PTMC grows to 13 mm (or 12 mm in two dimensions) or when new features inappropriate for AS appear |
AS = active surveillance, ETE = extrathyroidal extension, FNA = fine-needle aspiration, K-TIRADS = Korean Thyroid Imaging Reporting and Data System, LNM = lymph node metastasis, PTMC = papillary thyroid microcarcinoma, RLN = recurrent laryngeal nerve, US = ultrasound
RECOMMENDATIONS
INDICATIONS FOR AS AND IMMEDIATE SURGERY FOR THYROID CANCER
KEY QUESTION 1: WHAT ARE THE INDICATIONS FOR AS IN THYROID CANCER?
[RECOMMENDATIONS]
1-1: When AS is considered as a management strategy for thyroid cancer, the appropriateness of AS should be evaluated
1-2: AS can be considered for adult patients with low-risk PTMC (≤1 cm) as an alternative to immediate surgery. Immediate surgery is recommended for high-risk PTMCs
1-3: AS is primarily considered for Bethesda V or VI thyroid nodules on FNA or CNB, without suspicious imaging features of gross ETE (particularly the trachea and RLN), LNM, and distant metastasis. However, taking patient preference into account, AS can also be considered for highly suspicious (K-TIRADS 5) thyroid nodules on US without biopsy
Patients with PTMC (≤1 cm) who present with high-risk features such as LNM, distant metastasis, and invasion of adjacent organs should undergo immediate surgery (40). In Japan, Kuma Hospital conducted AS for PTMC ≤1 cm, excluding cases in which the tumor was located adjacent to the trachea or on the dorsal surface of the thyroid lobe, potentially invading the recurrent laryngeal nerve (RLN), or in which LNM was present (7,15). Most guidelines recommend that AS should be considered for cases with tumor size ≤1 cm, no aggressive subtype on cytology, no risk of gross extrathyroidal extension (ETE), and no clinical LNM or distant metastasis (46,47,51,54). Nonetheless, the debate over the indications for AS continues (40,41,42,43,44,45,46,47,48,49,50,51,52). Initially, Japanese groups selected the safest candidates for AS. Previous reports demonstrated that compared with immediate surgery, delayed surgery did not worsen overall survival even with tumor growth or LNM (15,55). Consequently, expansion of criteria for AS has been suggested (31,32,56,57). In contrast, some researchers have advocated the implementation of stringent criteria to exclude patients with potential risk factors, such as multiple unconfirmed nodules, pregnancy, and hyperthyroidism (29).
With the accumulation of more experience and research findings pertaining to AS, Brito et al. (58) proposed a comprehensive framework categorizing the characteristics of tumors, patients, and medical teams into ideal, appropriate, or inappropriate for AS consideration and decision-making. When considering the initiation of AS, it is recommended that the appropriateness of the tumor for AS be confirmed using a checklist. In this guideline, we enumerate detailed US-based appropriateness criteria for AS in patients with PTMC and introduce a detailed US-based checklist for AS candidacy in subsequent key questions. Imaging findings allow for the classification of patients into one of the following three categories, expressing the suitability of AS management (Table 2).
Table 2. US Based Appropriateness Criteria for AS in PTMC.
| Risk of Tumor | Appropriateness for AS | US Feature |
|---|---|---|
| Low-risk | Ideal | Confined to the thyroid |
| No contact with the thyroid capsule and adjacent organs No suspicious feature of LN metastasis* or distant metastasis | ||
| Appropriate | Anterior subcapsular tumors with a capsular abutment, capsular disruption or protrusion | |
| Paratracheal tumors with acute angle abutment to the trachea | ||
| Posteromedial subcapsular tumors showing preserved thyroid parenchyma between tumor and TEG | ||
| Posterolateral subcapsular tumors with capsular abutment | ||
| Tumors with ill-defined margin | ||
| High-risk | Inappropriate (candidates for immediate surgery) | Anterior subcapsular tumors with replacement of strap muscle |
| Paratracheal tumors with right- or wide-angle abutment to trachea | ||
| Posteromedial tumors with loss of normal parenchyma between TEG and tumor, or obvious protrusion | ||
| Posterolateral subcapsular tumors with obvious protrusion | ||
| Presence of biopsy proven or clinical lymph node metastasis or distant metastasis |
*Cortical hyperechogenicity, cystic change, echogenic foci (calcification) or abnormal vascularity on US.
AS = active surveillance, LN = lymph node, PTMC = papillary thyroid microcarcinoma, TEG = tracheoesophageal groove, US = ultrasound
IDEAL—Classic ideal patients with a tumor suitable for AS are those with probable or proven solitary PTMC that is not adjacent to the thyroid capsule and is confined to the thyroid parenchyma.
APPROPRIATE—Compared with ideal patients, patients classified as appropriate candidates possess specific characteristics that render observations more technically difficult to follow-up (e.g., ill-defined nodule margin, US-diffuse thyroid disease) or have a subcapsular tumor without definite evidence of gross ETE to critical structures. For patients under this category, the treatment offered at the time of tumor progression will still be highly effective and is associated with excellent clinical outcomes when followed by an experienced management team; therefore, observation is recommended for patients under this category.
INAPPROPRIATE—For “inappropriate” patients, an observational approach is contraindicated because 1) immediate surgery has been shown to be beneficial and 2) tumor progression leads to significant morbidity and is associated with an increased risk of recurrence after surgery (55). Patients should be classified as inappropriate for AS when locoregional or distant metastasis or potential gross ETE is identified at the initial presentation, when the tumor presents with new features of potential gross ETE to the trachea or RLN, or locoregional or distant metastasis during US surveillance.
The US-based appropriateness category of a probable or proven PTMC for AS should be determined by the operator performing the real-time US scan and should be included in the US report.
With the increasing adoption of AS for pathologically proven low-risk PTMC, there has been a growing debate regarding the need for performing biopsies on sonographically suspicious subcentimeter thyroid nodules without ETE or LNM. Nevertheless, the majority of previous evidence had cytopathological confirmation before the initiation of AS, and the Japan Association of Endocrine Surgery (JAES) guideline continues to recommend fine-needle aspiration (FNA) for nodules measuring >0.5 cm and ≤1 cm that are strongly suspected of being malignant based on US findings (46). The malignancy rate of suspicious subcentimeter thyroid nodules ranges from 71.2% to 89.0% (59). Thus, up to 29.8% of suspicious thyroid nodules may be placed under unnecessary AS if a biopsy is not performed.
The 2015 American Thyroid Association (40) guideline recommends FNA only for thyroid nodules measuring ≥1 cm even if the thyroid nodules exhibit a highly suspicious US pattern. The 2023 European Thyroid Association guidelines also recommend FNA only for European Thyroid Imaging and Reporting Data System category 5 nodules measuring 5–10 mm that have suspicious lymph nodes (LNs), carry a risk of ETE, or are located in worrisome areas (e.g., close to the trachea and laryngeal nerve area) (52). Therefore, the number of pathologically proven low-risk PTMC should be low in countries that strictly follow thyroid nodule evaluation guidelines. Consequently, the role of AS in these settings may be less relevant (60). Most guidelines suggest AS without biopsy for subcentimeter thyroid nodules (52,61), and prospective studies have recently evaluated AS for highly suspicious subcentimeter thyroid nodules, even without prior biopsy confirmation (62). Hence, we recommend conducting AS for Bethesda V or VI nodules on FNA or core needle biopsy and suggest that AS can also be performed for highly suspicious (Korean Thyroid Imaging Reporting and Data System [K-TIRADS] 5) thyroid nodules on US without biopsy, taking patient preference into account.
IMAGING EVALUATION BEFORE THE INITIATION OF AS
KEY QUESTION 2: WHAT IS THE APPROPRIATE US EVALUATION FOR PATIENTS WITH PTMC WHEN CONSIDERING THE INITIATION OF AS?
[RECOMMENDATIONS]
2-1: Prior to the initiation of AS for thyroid cancer, high-quality US of the thyroid and neck should be performed by experts in thyroid and neck US imaging
2-2: CT of the neck may play a supplementary role in the detection of additional LNM in low-risk PTMC
2-3: Chest CT is not routinely indicated before AS of low-risk PTMC
US is regarded as the primary imaging modality for assessing thyroid nodules and determining the most appropriate management strategy (63,64). When AS is considered for management, meticulous and high-quality US is essential for patient selection and follow-up. High resolution US equipment with a high frequency linear transducer (10–15 MHz) is required to evaluate the thyroid gland, nodules, and cervical LN compartments (65). Additional low frequency scanning may be helpful for evaluating nodules in deep locations and those with large neck circumferences. The patient should lie down in the supine position with their neck extended in a neutral position (without head rotation). Head rotation to the contralateral side is helpful for evaluating level 6 lymph nodes located in the tracheoesophageal groove (TEG) and thyroid nodules close to the tracheal wall. Furthermore, head rotation facilitates the assessment of nodule echogenicity by minimizing artifactual changes (through a “sonic window” of the sternocleidomastoid muscle). However, head rotation may change the orientation (shape) of the thyroid nodules and the position of cervical LNs relative to the landmarks of the major vessels and sternocleidomastoid muscle. Therefore, the patient’s head should be held straight without rotation to achieve the natural orientation of the thyroid nodules (66) and enable LN imaging in an anatomically neutral position. During scanning, the US scan range should sufficiently cover the entire thyroid gland and caution should be exercised when scanning US-blind spots (67). Transverse and longitudinal grayscale and Doppler images of the thyroid gland and the target nodules should be acquired. Additionally, transverse scanning images of the neck compartments, including LNs in the central (levels 1A, 6, 7) and lateral (levels 1B, 2–5) compartments, should be obtained with a transducer sweeping from the submandibular region to the suprasternal notch, supraclavicular fossa, and posterior neck compartments.
Tumor size should be measured in three dimensions on transverse and longitudinal images to assess tumor growth in all possible directions and to evaluate volume changes (68). Furthermore, the risk of gross ETE in PTMC or evidence of LNM should be evaluated to determine whether the PTMC is appropriate for initiating or pursuing AS. When deciding whether AS should be initiated, experts should conduct US examinations because operator experience can considerably influence the detection of potential gross ETE or LNM (64).
The frequency of metastasis to cervical LNs in PTC has been reported to be as high as 30%–80% (69,70). Similarly, PTMC has an approximately 40% risk of pathological metastasis when LN dissection is performed (45,71,72,73,74,75). LNM is associated with a high-risk of locoregional recurrence after surgery (76). If cN1 disease is confirmed, immediate surgery (total thyroidectomy) is required, usually followed by radioactive iodine ablation therapy. While US is the established primary imaging modality for evaluating thyroid cancer and cervical LNs, it has a relatively low sensitivity for detecting metastatic LNs in the central neck (77,78,79,80). Current KSThR guideline recommends contrast-enhanced neck CT as an adjunct to US (81). The addition of CT to US has been reported to improve the detection of LNM in both the central and lateral neck compartments (77,78,79,80). Additionally, CT can identify metastasis in LNs that appear indeterminate or benign on US (82,83) and can detect LNM in compartments missed on US (e.g., the mediastinum or retropharyngeal area) (80). Therefore, CT findings may affect patient management decisions (84). However, data regarding the diagnostic performance of CT in patients with PTMC are limited. A recent study reported that CT provided the additional benefit of detecting LNM in patients with PTMC whose tumors had US characteristics suitable for AS (85).
The lung is the most common site of distant metastasis in differentiated thyroid cancer (40). However, in two studies conducted in Japan, none of the patients with T1aN0 PTMC showed distant metastasis or recurrence at follow-up (8,86). In another study conducted at the Memorial Sloan Kettering Cancer Center (MSKCC), none of 4927 patients with low-risk PTMCs exhibited distant metastases (87). In a report from Kuma Hospital, none of the 1000 consecutive patients with low-risk PTMC who underwent chest CT as a routine preoperative examination had metastatic lesions (88). In a more recent study reporting long-term data, two cases of lung metastasis (one after AS and the other 12 years after surgery) were identified. Both cases had no lung metastasis at the time of initial diagnosis and were discovered after at least one occurrence of neck recurrence (15). Considering these findings, the incidence of distant metastases in low-risk PTMC is extremely low. Therefore, routine chest CT is not recommended for evaluating AS candidacy in patients with PTMC. The rationale against the use of routine chest CT is that the frequency of distant metastasis in PTMC cases without ETE or LNM is exceedingly low, according to previous observations (46).
KEY QUESTION 3. HOW SHOULD US EVALUATION OF ETE AND LNM BE PERFORMED?
[RECOMMENDATIONS]
3-1: Paratracheal tumors abutting the trachea or posteromedial subcapsular tumors should be carefully evaluated for the risk of gross ETE, especially with respect to the trachea and RLN
3-2: Anterior subcapsular tumors with strap muscle replacement, paratracheal tumors abutting ≥90° to the trachea, posterior paratracheal tumors without normal intervening parenchyma, and posterior subcapsular tumors with protrusion pose a high risk of gross ETE and immediate surgery should be considered
3-3: FNA with thyroglobulin testing should be performed for any suspicious LNs
Clinically evident LNM and gross ETE are relevant prognostic risk factors for patients with PTMC and are currently considered contraindications for AS. ETE is defined as the involvement of perithyroidal structures through the direct extension of primary thyroid cancer. ETE ranges from minor ETE identified on histological examinations to gross ETE identified using preoperative or intraoperative evidence. The presence of ETE has been established as an important prognostic factor in the 6th edition of the American Joint Committee on Cancer (AJCC). In the latest version (8th edition), minor ETE has been eliminated from the staging system because not only does minimal ETE have little impact on patient prognosis but also pathological diagnosis is subjective and challenging. Meanwhile, the gross ETE of the strap muscles constitutes T3b, and the ETE of the major neck organs constitutes T4. Even sub-centimeter cancers with a high possibility of major neck structure involvement are considered high-risk thyroid cancers, and immediate surgery is recommended for these tumors. Accurate identification of gross ETE on US is important for AS. During AS, the new development of gross ETE is considered tumor progression, and conversion to surgery is recommended.
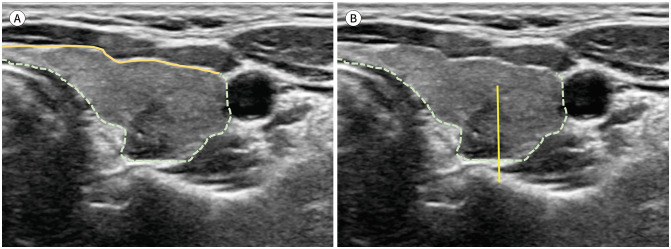
Tumors that need to be assessed for ETE can be classified into the following categories: two types of subcapsular tumors (anterior and posterior subcapsular) and paratracheal tumors (Fig. 1) (89,90). First, a subcapsular tumor describes tumors abutting the anterior or posterior capsule (margin) of the thyroid gland. The anterior thyroid capsule is defined as the anterior part of the thyroid capsule that is in contact with the strap muscles, and the posterior thyroid capsule is defined as the posterior part of the thyroid capsule that is not in contact with the strap muscles. Subcapsular tumors in contact with the anterior thyroid capsule are classified as anterior subcapsular tumors, whereas those in contact with the posterior thyroid capsule are classified as posterior subcapsular tumors. Among these, the medial half can be further categorized as posteromedial subcapsular tumors, and the lateral half as posterolateral subcapsular tumors. Finally, tumors in contact with the trachea were classified as paratracheal tumors. Tumors can be further divided into anterior and posterior paratracheal tumors based on the horizontal midline of the anteroposterior (AP) distance of the trachea.
Fig. 1. Classification of subcapsular tumors based on US imaging.
A. Anterior versus posterior subcapsular location. The thyroid capsule (echogenic line) contacting the anterior strap muscles can be regarded as the anterior capsule (solid line). Other areas can be classified as the posterior capsule (dotted line).
B. Posteromedial versus posterolateral thyroid capsule. The posterior thyroid capsule can be divided into halves, namely, the posteromedial and posterolateral capsule.
For anterior subcapsular tumors, the possibility of gross ETE in the strap muscles should be assessed. The JAES proposed that tumors on the ventral side of the thyroid and tumors with muscle invasion may not necessarily require immediate surgery, considering that extended resection of the neck muscles has little influence on patient quality of life (QOL) and prognosis (46). Recent studies have reported that T3b disease may be associated with an increased recurrence rate (91,92,93,94). For anterior subcapsular tumors, US features predictive of ETE include capsular abutment, disruption, protrusion, and replacement of the strap muscles (81,95). Immediate surgery is not necessarily indicated for tumors with capsular abutment, disruption, and protrusion. Given the potentially high recurrence rate of T3b tumors (91,92,93,94), AS would be inappropriate for rare microcarcinomas showing strap muscle replacement on US, which is a high-risk feature of gross ETE to the strap muscles.
Tumor invasion of the posterolateral capsule and adjacent soft tissue is not currently considered gross ETE according to the AJCC staging system. Tumors with a suspicious invasion of the posterolateral capsule can be described as those showing capsular contact, disruption, and protrusion (96). A recent study reported that a posterolateral capsular abutment was indicative of a posterior minor ETE to the thyroid capsule and associated with an increased risk of lymphovascular invasion and lateral LNM (96). However, its significance in the AS setting has not yet been validated. The KSThR Task Force reached a consensus that initiating AS would be inappropriate for posterolateral subcapsular tumors with tumor protrusion into the perithyroidal soft tissue, as this could be relevant to gross ETE of perithyroidal structures. However, further studies are required to validate the significance of posterior ETE in AS.
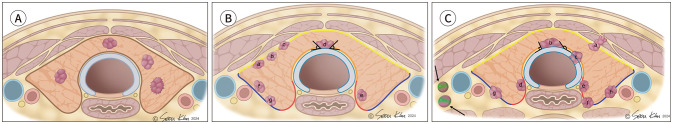
For paratracheal and posteromedial subcapsular tumors, the relationship between thyroid cancer and the trachea or RLN is one of the most important factors in enrolling patients with AS. Immediate surgery is recommended if imaging features suggest ETE to the trachea and RLN, as invasion into these structures is associated with a poorer prognosis, and extended surgery is associated with increased morbidity and poor QOL (40). The risk of tracheal invasion is assessed by the angle formed by the PTMC and tracheal cartilage (95,97). An obtuse angle between the cancer and trachea has been suggested as the most sensitive and accurate criterion for diagnosing tracheal invasion (81,95,97). As appropriate discrimination between right- and wide-angle abutments may be difficult and subject to reader variability, we recommend that paratracheal tumors with right- or wide-angle abutments are inappropriate for initiating or pursuing AS. The risk of RLN invasion can be assessed based on the presence of a normal rim of the thyroid between the TEG and the cancer (46,95,97). Table 2 and Figure 2 show the ideal, appropriate, and inappropriate US features of AS.
Fig. 2. Imaging-based appropriateness criteria for AS in thyroid cancer (≤1 cm).
A. Ideal tumor characteristics include tumors confined to the thyroid gland with no contact with the thyroid capsule and with no metastasis.
B. Appropriate tumor characteristics. Among anterior subcapsular tumors (near the yellow line), tumors showing capsular contact (a), disruption (b), and protrusion (c) are appropriate for AS. Paratracheal tumors (near the orange line) abutting the trachea with acute angle (d) are appropriate for AS. Among posteromedial subcapsular tumors (near the red line), tumors showing preserved thyroid parenchyma between the tumor and tracheoesophageal groove are appropriate (e). Posterolateral subcapsular tumors with capsular abutment (f, g) are appropriate for AS.
C. Inappropriate tumor characteristics. Anterior subcapsular tumors (near the yellow line) demonstrating strap muscle replacement (a), paratracheal tumors (near the orange line) showing right- or wide-angle abutment to trachea (b) (including tumors showing obvious tracheal cartilage invasion; c), posteromedial subcapsular tumors (near the red line) with loss of normal thyroid parenchyma (d, e) or obvious protrusion (f), and posterolateral subcapsular tumors (near the blue line) with obvious protrusion (g, h), biopsy-proven metastasis (arrows) are inappropriate candidates for AS.
AS = active surveillance
Ito et al. (97) reported that significant tracheal invasion requiring tracheal cartilage resection occurred only in PTMCs ≥0.7 cm with an obtuse angle between the tumor and the trachea. Similarly, significant invasions requiring dissection of the RLN only occurred in PTMCs ≥0.7 cm without a normal rim between the tumor and the course of the RLN. Newman et al. (98) reported that PTMCs larger than 0.9 cm were unsuitable for AS, even if US or CT did not show signs of RLN invasion, as subcapsular tumors located in the paratracheal and right lateral posterior lobe areas may have gross RLN invasion. In this study, RLN invasion of the right posterolateral subcapsular tumor was observed in only one case, which was >1 cm, indicating a remarkably low incidence. Excluding such tumors would unnecessarily exclude many tumors with a low risk of gross ETE from AS. Moreover, to enhance the simplicity of the guideline application, the current guidelines include posterolateral subcapsular tumors as an appropriate group for AS, irrespective of laterality. US-based diagnostic criteria for ETE remain controversial. Well-organized prospective studies are required to validate the diagnostic performance of US criteria for ETE.
PTMC has an approximately 10%–30% risk of pathological metastases when LN dissection is performed in patients without clinical LNM (45,71,72,73,74,75). Inadequate assessment of cervical LNs before AS can lead to extended neck surgery, increased morbidity, and impaired QOL. Clinically evident LNM is a relevant risk factor for patients with PTMC and should be carefully assessed at the beginning and during AS (46,68).
According to US features, LNs can be classified as suspicious, indeterminate, or probably benign based on their malignancy risk (81). The four suspicious US features of cortical hyperechogenicity, cystic changes, echogenic foci, and abnormal vascularity have high specificity and are highly predictive of metastasis (malignancy risk, 73%–88%). Probably benign LNs are defined as those with either an echogenic hilum or radiating hilar vascularity (malignancy risk <3%). Indeterminate LNs indicate LNs with loss of the hilum and hilar vascularity and have low specificity for differentiating between benign and malignant LNs (malignancy risk, 20%–29%) (83,99,100,101,102). Therefore, FNA is recommended in patients with suspicious LNs. A recent study showed that suspicious LNs exhibit a high malignancy risk, regardless of their size (82,100). The FNA criteria covering suspicious LNs of all sizes resulted in the highest specificity, minimized unnecessary biopsy rate, and maintained sensitivity (82). Additionally, no associations were identified between malignancy risk and the size or L/S ratio of the indeterminate LNs (102). Primary tumor characteristics such as nonparallel orientation and multiplicity of tumors were associated with metastasis in indeterminate LNs (100,101). Therefore, indeterminate LNs can be selectively biopsied by considering primary tumor characteristics and nodal size (>5 mm in short diameter) (81,83,100,102).
A previous study reported that FNA with thyroglobulin (Tg) generally yields conclusive results after cervical LNs (103). However, some LNs could demonstrate imaging-cytology (Tg) discordance, and repeat biopsy should be performed according to the imaging pattern to definitely exclude LNM, especially for sonographically suspicious but cytologically benign LNs because of their relatively high malignancy rates (22.9%) (104).
Table 2 describes a checklist that shows AS candidacy, including the risk of tumors and the appropriateness of AS based on US features.
US EVALUATION OF THYROID TUMORS DURING AS
KEY QUESTION 4. WHAT IS THE APPROPRIATE US IMAGING TECHNIQUE FOR EVALUATING THYROID NODULES UNDER AS?
[RECOMMENDATIONS]
4-1: We recommend using consistent measurement methods throughout the initial and follow-up examinations during AS
4-2: Measurement for three axes should be performed during AS using the same imaging plane and slice as previously measured
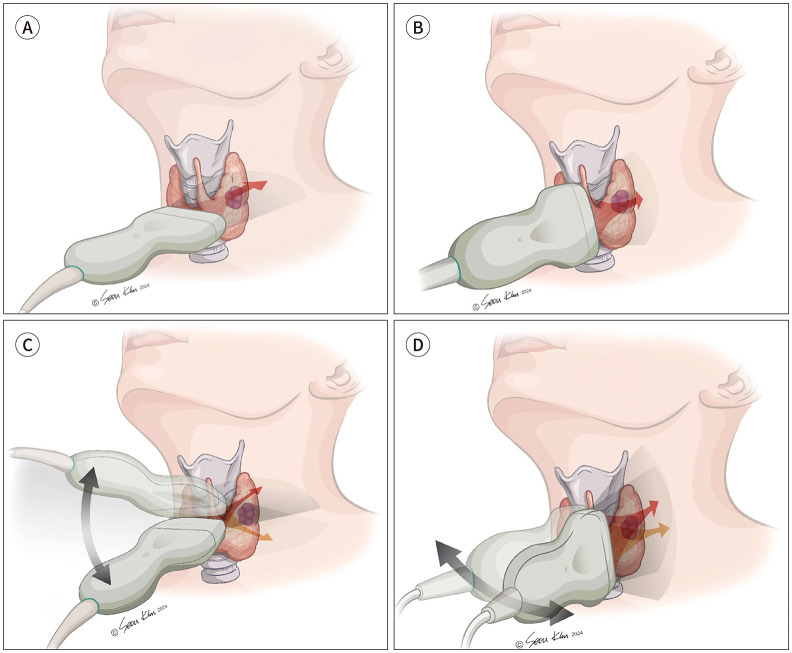
Although the definition of tumor enlargement differs across guidelines and studies, tumor growth is considered a surrogate marker of tumor progression in AS. Thus, accurate, consistent, and reproducible measurements of tumor size are essential for AS (68). US size measurements of thyroid nodules are known to have high intra- and inter-observer variability (105,106). Although tumor diameter and volume can be used to evaluate tumor growth, tumor volume, which multiplies the measurement values of the three axes, may be more prone to higher measurement variability (46). We recommend the use of the three-axis measurement technique proposed by the ACR Thyroid Imaging, Reporting, and Data System (TI-RADS) to ensure consistent and reproducible measurements (68,107). Measurements should be performed using transverse and longitudinal scans of the thyroid nodules (Fig. 3). The transverse plane for nodule size measurement should be set in a slice that shows the maximum dimension (transverse diameter) on transverse scans, and the second measurement (AP diameter) should be the maximum dimension measured perpendicular to the transverse diameter on the same image (Fig. 4). The longitudinal plane should be parallel to the central longitudinal plane of the thyroid gland, and not necessarily the true anatomical sagittal plane. Angulation of the probe to <45° can be used to measure the maximal tumor dimension in the transverse (transverse diameter) or longitudinal planes (craniocaudal [CC] diameter). This approach provides reproducible measurements of obliquely oriented nodules. When measuring the size of irregularly shaped or obliquely oriented nodules, CC angulation of the AP axis of the nodule is allowed during transverse scanning. Similarly, transverse angulation of the CC axis of the nodule is permitted during longitudinal scanning to measure the maximum diameter. Measurements should also include the nodule’s halo if present (105).
Fig. 3. Basic planes for nodule size measurement on ultrasound.
A, B. Transverse (A) and longitudinal planes (B). The transverse plane for size measurement of nodules should be set in the slice that shows the maximum dimension (transverse diameter) on transverse scans, and the longitudinal plane should be determined parallel to the central longitudinal plane of the thyroid gland, not necessarily the true anatomical sagittal plane.
C, D. Angulation of the probe to <45° can be used to measure the maximal tumor dimension on the transverse (transverse diameter) (C) or longitudinal plane (craniocaudal diameter) (D).
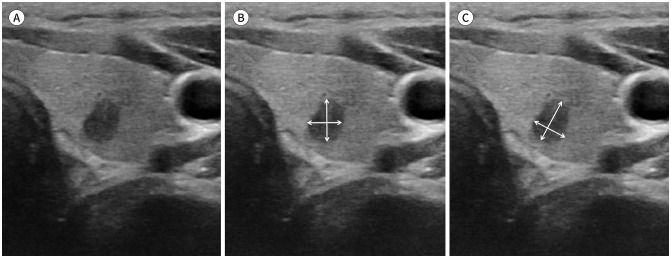
Fig. 4. Recommended reproducible size measurement methods for obliquely oriented thyroid nodules under active surveillance.
A. A 48-year-old female patient with papillary thyroid microcarcinoma in the left thyroid lobe.
B. Incorrect axis measurement method. The transverse and anteroposterior diameters are measured in the plane of the thyroid gland.
C. Correct caliper placement for size measurement should be placed along the orientation of the nodule (i.e., axes of maximum dimension of the nodule in the transverse image and maximum dimension perpendicular to the first measurement). Inaccurate caliper placement at baseline or follow-up may potentially lead to inadvertent decision of progression and surgery conversion.
Operators may multiply the measured linear dimensions to determine the nodule volume using the volume formula function available in US units or by manual calculation (nodule volume = transverse diameter × AP diameter × CC diameter × 0.524). Operators should consistently measure and report nodules to facilitate serial comparisons, and we recommend using the same image plane or slice as those previously measured during follow-up examinations. Because size measurement is performed at the slice and location where the maximum diameter is measured, the location of measurement can vary according to changes in nodule growth and shape.
KEY QUESTION 5. HOW SHOULD TUMOR PROGRESSION BE EVALUATED BY US IMAGING?
[RECOMMENDATIONS]
5-1: Tumor progression can be assessed by evaluating tumor size enlargement and new US features of potential gross ETE or LNM
5-2: Tumor size enlargement can be assessed by measuring the maximal tumor diameter increase of ≥3 mm or increase of ≥2 mm in at least two dimensions
The definition of tumor progression during AS remains controversial. However, tumor progression is typically defined as follows: tumor growth, gross ETE, LNM, or distant metastasis (45,46,48,49,51). New gross ETE, LNM, or distant metastasis is generally accepted as an indicator of tumor progression and an indication for surgical conversion across all prospective studies and guidelines. However, the definitions of tumor growth and enlargement vary. The most commonly used criterion is an increase of ≥3 mm in tumor diameter (45,46). The MSKCC study defined tumor growth as a tumor size increase of ≥3 mm in the greatest dimension and a tumor volume increase of ≥50% compared with baseline (30). In a Korean multicenter study, tumor growth was defined as a size increase of ≥3 mm in at least one dimension or ≥2 mm in at least two dimensions (21,24). The US Cedars-Sinai Medical Center study defined tumor growth as a diameter increase of ≥5 mm or a volume increase of ≥100% (31). We adopted the criteria for tumor size enlargement from a recent multicenter prospective PTMC cohort study (24), defining tumor size enlargement as an increase of ≥3 mm in one dimension or ≥2 mm in two dimensions. In this study, of the 57 patients who showed tumor size enlargement, 22 met both the 3 mm and 2 × 2 mm criteria, 19 met only the 3 mm one-dimension criteria, and 16 met only the 2 × 2 mm criterion. The time to progression in those meeting the 3 mm criterion was earlier than that in those meeting the 2 × 2 increments. The 2 × 2 mm criterion may be less sensitive than the 3 mm criterion. However, considering that the 2 × 2 mm criterion can detect roundly enlarging tumors, these two criteria can be used complementarily.
While some guidelines and studies require meeting the growth criteria only once, the Japan Kuma Hospital (15), Canadian prospective studies (32,68), and SFE/AFCE/SFMN (47) recommend surgery only when the criteria are met twice during consecutive US examinations. The requirement for two consecutive confirmations is due to inter- and intra-observer variability in measuring tumor size using US (105,106,108) and the possibility that a tumor meeting the growth criterion may subsequently decrease in size (109).
During the follow-up, new thyroid nodules may be detected and confirmed as additional PTMC via biopsy. Such newly detected PTMC can be considered either intrathyroidal metastases (110,111) or a new separate cancer (112,113). Currently, there is no consensus in the guidelines or prospective studies on whether newly developed PTMC within the thyroid gland indicate tumor progression or warrant surgery. If a new nodule is found during AS, its management should be evaluated according to the US risk stratification system. For highly suspicious (K-TIRADS 5) thyroid nodules ≤1 cm, AS may be performed without biopsy, and biopsy may be considered if a new, highly suspicious nodule exhibits US features that are inappropriate for AS. We recommend performing a biopsy for new nodules when management may be changed.
KEY QUESTION 6. WHAT IS THE APPROPRIATE INTERVAL BETWEEN US EXAMINATIONS DURING AS?
[RECOMMENDATIONS]
6: US evaluations of changes in tumor size and the appearance of novel potential gross ETE and LNM are recommended every 6 months for the first 1–2 years after the initiation of AS and once a year thereafter if no tumor progression is detected
No comparative studies have been published regarding the appropriate imaging intervals. In a prospective study conducted at the Kuma Hospital, patients visited the hospital once or twice a year for blood tests and neck US examinations (16). In most other prospective studies, patients were scheduled for hospital visits every 6 months during the first 2 years, followed by annual visits thereafter (21,30,32). A recent study conducted in Korea reported that progressive cases were sporadically observed throughout the follow-up period (24). In addition, a study conducted at the MSKCC demonstrated that the identification of LNM was not related to tumor size kinetic pattern/volume changes and was variable at different time points (57). To date, no evidence is available regarding when the interval between examinations can be further extended, such as every 2–3 years, even for primary tumor stability. Based on these protocols, we recommend a 6-month follow-up in the first 1 or 2 years, followed by regular annual follow-ups thereafter. However, the follow-up intervals can be adjusted to the short term, depending on the growth pattern observed on the last US examination, to confirm a significant size increase and other imaging features (impending gross ETE and suspicion of LNM) at the discretion of the physicians, if necessary.
KEY QUESTION 7. WHEN SHOULD A CONVERSION TO SURGERY BE CONSIDERED DURING FOLLOW-UP?
[RECOMMENDATIONS]
7: Surgical conversion can be considered when the PTMC grows to 13 mm (or 12 mm in two dimensions) or when new features inappropriate for AS appear
As for the timing of surgery, the JAES recommends continued AS until the tumor reaches 13 mm while considering patient preference (46) because a 3-mm increase in the 10-mm PTMC creates a 13-mm tumor. Considering the 2-mm two-dimension criteria, a 2 × 2 mm increase in 10-mm PTMC results in a tumor measuring 12 mm in two dimensions. We recommend surgery when this criterion is met at least twice on consecutive observations within at least 6 months, considering the inter- and intra-observer variability in measuring tumor size using US.
A recent study demonstrated that the 5- and 10-year progression rates of T1aN0M0 and T1bN0M0 PTC were not significantly different (56). Another study showed that many tumors shrank after enlargement (109). These findings suggest that surgery for PTMCs immediately after they exceed 10 mm is unnecessary. In such cases, the decision to proceed with conversion to surgery should be made after careful consultation while considering patient preferences. Regarding tumor progression other than size enlargement, conversion to surgery should be considered when new features that are inappropriate for AS appear (high-risk features for gross ETE or LNM). When a highly suspicious nodule shows enlargement during AS initiated without biopsy, biopsy should be considered to exclude the possibility of a rare high-grade malignant tumor that is inappropriate for AS.
SUMMARY
Table 1 summarizes the key questions and recommendations. AS is a feasible and reliable option for the management of low-risk PTMC. However, it carries the risk of cancer progression. Optimal decision-making is highly dependent on accurate staging of the extent of the disease at presentation. We present a standardized approach to imaging evaluations that incorporates the newly updated 2021 K-TIRADS and 2024 Korean Thyroid Association recommendations and addresses pertinent questions for thyroid physicians. By ensuring comprehensive imaging assessment and improving clarity in the thyroid US methodology, we seek to improve patient access to optimized care.
Acknowledgments
We would like to express our deepest gratitude to Dr. Young Joo Park and Dr. Eun Kyung Lee for their invaluable advisory role in the development of this guideline. Their expertise and guidance were instrumental in shaping the content of this work. We also extend our sincere thanks to Ms. Seou Kim for her outstanding efforts in creating the illustrations that significantly enhance the clarity and visual impact of this article.
Footnotes
This article is being published jointly in Journal of the Korean Society of Radiology and Korean Journal of Radiology.
Conflicts of Interest: Jung Hee Shin has been a Editorial Board Member of the Journal of the Korean Society of Radiology since 2013; however, she was not involved in the peer reviewer selection, evaluation, or decision process of this article. The other authors have declared no conflicts of interest.
- Conceptualization, L.J.Y., N.D.G.
- Data curation, L.J.Y., L.M.K., N.D.G.
- Formal analysis, L.J.Y., L.M.K., N.D.G.
- Funding acquisition, N.D.G.
- Investigation, all authors.
- Methodology, L.J.Y., L.M.K. N.D.G.
- Project administration, N.D.G.
- Resources, L.J.Y., L.M.K., N.D.G.
- Software, L.J.Y.
- Supervision, L.J.Y., L.M.K., K.J., J.S.L., B.J.H., N.D.G.
- Validation, L.J.Y., L.M.K., N.D.G.
- Visualization, L.J.Y.
- Writing—original draft, L.J.Y., N.D.G.
- Writing—review & editing, all authors.
Funding: This study was supported by a grant from the 2024 Clinical Practice Guideline Research Fund by the Korean Society of Radiology & Korean Society of Thyroid Radiology, and by a grant of the Korea Health Technology R&D Project through the Patient-Doctor Shared Decision Marking Research Center (PDSDM), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: RS-2023-KH142322).
Supplementary Materials
The Supplement is available with this article at http://doi.org/10.3348/jksr.2024.0132.
Scope of guideline
Methodology
Identifying information and availability
References
- 1.Pizzato M, Li M, Vignat J, Laversanne M, Singh D, La Vecchia C, et al. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol. 2022;10:264–272. doi: 10.1016/S2213-8587(22)00035-3. [DOI] [PubMed] [Google Scholar]
- 2.Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. 2017;317:1338–1348. doi: 10.1001/jama.2017.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. 2016;375:614–617. doi: 10.1056/NEJMp1604412. [DOI] [PubMed] [Google Scholar]
- 4.Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic”--screening and overdiagnosis. N Engl J Med. 2014;371:1765–1767. doi: 10.1056/NEJMp1409841. [DOI] [PubMed] [Google Scholar]
- 5.Mazzaferri EL. Management of low-risk differentiated thyroid cancer. Endocr Pract. 2007;13:498–512. doi: 10.4158/EP.13.5.498. [DOI] [PubMed] [Google Scholar]
- 6.Hay ID. Management of patients with low-risk papillary thyroid carcinoma. Endocr Pract. 2007;13:521–533. doi: 10.4158/EP.13.5.521. [DOI] [PubMed] [Google Scholar]
- 7.Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg. 2010;34:28–35. doi: 10.1007/s00268-009-0303-0. [DOI] [PubMed] [Google Scholar]
- 8.Ito Y, Uruno T, Nakano K, Takamura Y, Miya A, Kobayashi K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid. 2003;13:381–387. doi: 10.1089/105072503321669875. [DOI] [PubMed] [Google Scholar]
- 9.Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Papillary microcarcinoma of the thyroid: how should it be treated? World J Surg. 2004;28:1115–1121. doi: 10.1007/s00268-004-7644-5. [DOI] [PubMed] [Google Scholar]
- 10.Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014;24:27–34. doi: 10.1089/thy.2013.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oda H, Miyauchi A, Ito Y, Yoshioka K, Nakayama A, Sasai H, et al. Incidences of unfavorable events in the management of low-risk papillary microcarcinoma of the thyroid by active surveillance versus immediate surgery. Thyroid. 2016;26:150–155. doi: 10.1089/thy.2015.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oda H, Miyauchi A, Ito Y, Sasai H, Masuoka H, Yabuta T, et al. Comparison of the costs of active surveillance and immediate surgery in the management of low-risk papillary microcarcinoma of the thyroid. Endocr J. 2017;64:59–64. doi: 10.1507/endocrj.EJ16-0381. [DOI] [PubMed] [Google Scholar]
- 13.Miyauchi A, Kudo T, Ito Y, Oda H, Sasai H, Higashiyama T, et al. Estimation of the lifetime probability of disease progression of papillary microcarcinoma of the thyroid during active surveillance. Surgery. 2018;163:48–52. doi: 10.1016/j.surg.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 14.Miyauchi A, Kudo T, Ito Y, Oda H, Yamamoto M, Sasai H, et al. Natural history of papillary thyroid microcarcinoma: kinetic analyses on tumor volume during active surveillance and before presentation. Surgery. 2019;165:25–30. doi: 10.1016/j.surg.2018.07.045. [DOI] [PubMed] [Google Scholar]
- 15.Miyauchi A, Ito Y, Fujishima M, Miya A, Onoda N, Kihara M, et al. Long-term outcomes of active surveillance and immediate surgery for adult patients with low-risk papillary thyroid microcarcinoma: 30-year experience. Thyroid. 2023;33:817–825. doi: 10.1089/thy.2023.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito Y, Miyauchi A, Fujishima M, Noda T, Sano T, Sasaki T, et al. Thyroid-stimulating hormone, age, and tumor size are risk factors for progression during active surveillance of low-risk papillary thyroid microcarcinoma in adults. World J Surg. 2023;47:392–401. doi: 10.1007/s00268-022-06770-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki T, Miyauchi A, Fujishima M, Ito Y, Kudo T, Noda T, et al. Comparison of postoperative unfavorable events in patients with low-risk papillary thyroid carcinoma: immediate surgery versus conversion surgery following active surveillance. Thyroid. 2023;33:186–191. doi: 10.1089/thy.2022.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto M, Miyauchi A, Ito Y, Fujishima M, Sasaki T, Kudo T. Active surveillance outcomes of patients with low-risk papillary thyroid microcarcinoma according to levothyroxine treatment status. Thyroid. 2023;33:1182–1189. doi: 10.1089/thy.2023.0046. [DOI] [PubMed] [Google Scholar]
- 19.Fujishima M, Miyauchi A, Ito Y, Kudo T, Noda T, Sano T, et al. Active surveillance is an excellent management technique for identifying patients with progressive low-risk papillary thyroid microcarcinoma requiring surgical treatment. Endocr J. 2023;70:411–418. doi: 10.1507/endocrj.EJ22-0559. [DOI] [PubMed] [Google Scholar]
- 20.Fukuoka O, Sugitani I, Ebina A, Toda K, Kawabata K, Yamada K. Natural history of asymptomatic papillary thyroid microcarcinoma: time-dependent changes in calcification and vascularity during active surveillance. World J Surg. 2016;40:529–537. doi: 10.1007/s00268-015-3349-1. [DOI] [PubMed] [Google Scholar]
- 21.Moon JH, Kim JH, Lee EK, Lee KE, Kong SH, Kim YK, et al. Study protocol of multicenter prospective cohort study of active surveillance on papillary thyroid microcarcinoma (MAeSTro) Endocrinol Metab (Seoul) 2018;33:278–286. doi: 10.3803/EnM.2018.33.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong SH, Ryu J, Kim MJ, Cho SW, Song YS, Yi KH, et al. Longitudinal assessment of quality of life according to treatment options in low-risk papillary thyroid microcarcinoma patients: active surveillance or immediate surgery (interim analysis of MAeSTro) Thyroid. 2019;29:1089–1096. doi: 10.1089/thy.2018.0624. [DOI] [PubMed] [Google Scholar]
- 23.Moon JH, Ryu CH, Cho SW, Choi JY, Chung EJ, Hah JH, et al. Effect of initial treatment choice on 2-year quality of life in patients with low-risk papillary thyroid microcarcinoma. J Clin Endocrinol Metab. 2021;106:724–735. doi: 10.1210/clinem/dgaa889. [DOI] [PubMed] [Google Scholar]
- 24.Lee EK, Moon JH, Hwangbo Y, Ryu CH, Cho SW, Choi JY, et al. Progression of low-risk papillary thyroid microcarcinoma during active surveillance: interim analysis of a multicenter prospective cohort study of active surveillance on papillary thyroid microcarcinoma in Korea. Thyroid. 2022;32:1328–1336. doi: 10.1089/thy.2021.0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim K, Choi JY, Kim SJ, Lee EK, Lee YK, Ryu JS, et al. Active surveillance versus immediate surgery for low-risk papillary thyroid microcarcinoma patients in South Korea: a cost-minimization analysis from the MAeSTro study. Thyroid. 2022;32:648–656. doi: 10.1089/thy.2021.0679. [DOI] [PubMed] [Google Scholar]
- 26.Hwangbo Y, Choi JY, Lee EK, Ryu CH, Cho SW, Chung EJ, et al. A cross-sectional survey of patient treatment choice in a multicenter prospective cohort study on active surveillance of papillary thyroid microcarcinoma (MAeSTro) Thyroid. 2022;32:772–780. doi: 10.1089/thy.2021.0619. [DOI] [PubMed] [Google Scholar]
- 27.Hwang H, Choi JY, Yu HW, Moon JH, Kim JH, Lee EK, et al. Surgical outcomes in patients with low-risk papillary thyroid microcarcinoma from MAeSTro study: immediate operation versus delayed operation after active surveillancea multicenter prospective cohort study. Ann Surg. 2023;278:e1087–e1095. doi: 10.1097/SLA.0000000000005841. [DOI] [PubMed] [Google Scholar]
- 28.Lee JY, Kim JH, Kim YK, Lee CY, Lee EK, Moon JH, et al. US predictors of papillary thyroid microcarcinoma progression at active surveillance. Radiology. 2023;309:e230006. doi: 10.1148/radiol.230006. [DOI] [PubMed] [Google Scholar]
- 29.Jeon MJ, Kang YE, Moon JH, Lim DJ, Lee CY, Lee YS, et al. Protocol for a Korean multicenter prospective cohort study of active surveillance or surgery (KoMPASS) in papillary thyroid microcarcinoma. Endocrinol Metab (Seoul) 2021;36:359–364. doi: 10.3803/EnM.2020.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuttle RM, Fagin JA, Minkowitz G, Wong RJ, Roman B, Patel S, et al. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg. 2017;143:1015–1020. doi: 10.1001/jamaoto.2017.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho AS, Kim S, Zalt C, Melany ML, Chen IE, Vasquez J, et al. Expanded parameters in active surveillance for low-risk papillary thyroid carcinoma: a nonrandomized controlled trial. JAMA Oncol. 2022;8:1588–1596. doi: 10.1001/jamaoncol.2022.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sawka AM, Ghai S, Tomlinson G, Rotstein L, Gilbert R, Gullane P, et al. A protocol for a Canadian prospective observational study of decision-making on active surveillance or surgery for low-risk papillary thyroid cancer. BMJ Open. 2018;8:e020298. doi: 10.1136/bmjopen-2017-020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sawka AM, Ghai S, Tomlinson G, Baxter NN, Corsten M, Imran SA, et al. A protocol for a pan-Canadian prospective observational study on active surveillance or surgery for very low risk papillary thyroid cancer. Front Endocrinol (Lausanne) 2021;12:686996. doi: 10.3389/fendo.2021.686996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sawka AM, Ghai S, Rotstein L, Irish JC, Pasternak JD, Gullane PJ, et al. A quantitative analysis examining patients’ choice of active surveillance or surgery for managing low-risk papillary thyroid cancer. Thyroid. 2022;32:255–262. doi: 10.1089/thy.2021.0485. [DOI] [PubMed] [Google Scholar]
- 35.Sanabria A. Experience with active surveillance of thyroid low-risk carcinoma in a developing country. Thyroid. 2020;30:985–991. doi: 10.1089/thy.2019.0522. [DOI] [PubMed] [Google Scholar]
- 36.Rosario PW, Mourão GF, Calsolari MR. Active surveillance in adults with low-risk papillary thyroid microcarcinomas: a prospective study. Horm Metab Res. 2019;51:703–708. doi: 10.1055/a-1015-6684. [DOI] [PubMed] [Google Scholar]
- 37.Smulever A, Pitoia F. Active surveillance in papillary thyroid carcinoma: not easily accepted but possible in Latin America. Arch Endocrinol Metab. 2019;63:462–469. doi: 10.20945/2359-3997000000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molinaro E, Campopiano MC, Pieruzzi L, Matrone A, Agate L, Bottici V, et al. Active surveillance in papillary thyroid microcarcinomas is feasible and safe: experience at a single Italian center. J Clin Endocrinol Metab. 2020;105:e172–e180. doi: 10.1210/clinem/dgz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campopiano MC, Matrone A, Rago T, Scutari M, Prete A, Agate L, et al. Assessing mPTC progression during active surveillance: volume or diameter increase? J Clin Med. 2021;10:4068. doi: 10.3390/jcm10184068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takami H, Ito Y, Okamoto T, Yoshida A. Therapeutic strategy for differentiated thyroid carcinoma in Japan based on a newly established guideline managed by Japanese Society of Thyroid Surgeons and Japanese Association of Endocrine Surgeons. World J Surg. 2011;35:111–121. doi: 10.1007/s00268-010-0832-6. [DOI] [PubMed] [Google Scholar]
- 42.Pacini F, Basolo F, Bellantone R, Boni G, Cannizzaro MA, De Palma M, et al. Italian consensus on diagnosis and treatment of differentiated thyroid cancer: joint statements of six Italian societies. J Endocrinol Invest. 2018;41:849–876. doi: 10.1007/s40618-018-0884-2. [DOI] [PubMed] [Google Scholar]
- 43.Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, et al. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1856–1883. doi: 10.1093/annonc/mdz400. [DOI] [PubMed] [Google Scholar]
- 44.Ito Y, Onoda N, Okamoto T. The revised clinical practice guidelines on the management of thyroid tumors by the Japan Associations of Endocrine Surgeons: core questions and recommendations for treatments of thyroid cancer. Endocr J. 2020;67:669–717. doi: 10.1507/endocrj.EJ20-0025. [DOI] [PubMed] [Google Scholar]
- 45.Horiguchi K, Yoshida Y, Iwaku K, Emoto N, Kasahara T, Sato J, et al. Position paper from the Japan Thyroid Association task force on the management of low-risk papillary thyroid microcarcinoma (T1aN0M0) in adults. Endocr J. 2021;68:763–780. doi: 10.1507/endocrj.EJ20-0692. [DOI] [PubMed] [Google Scholar]
- 46.Sugitani I, Ito Y, Takeuchi D, Nakayama H, Masaki C, Shindo H, et al. Indications and strategy for active surveillance of adult low-risk papillary thyroid microcarcinoma: consensus statements from the Japan Association of Endocrine Surgery task force on management for papillary thyroid microcarcinoma. Thyroid. 2021;31:183–192. doi: 10.1089/thy.2020.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leboulleux S, Lamartina L, Lecornet Sokol E, Menegaux F, Leenhardt L, Russ G. SFE-AFCE-SFMN 2022 consensus on the management of thyroid nodules : follow-up: how and how long? Ann Endocrinol (Paris) 2022;83:407–414. doi: 10.1016/j.ando.2022.10.010. [DOI] [PubMed] [Google Scholar]
- 48.Jarząb B, Dedecjus M, Słowinska-Klencka D, Lewinski A, Adamczewski Z, Anielski R, et al. Guidelines of Polish National Societies diagnostics and treatment of thyroid carcinoma. 2018 update. Endokrynol Pol. 2018;69:34–74. doi: 10.5603/EP.2018.0014. [DOI] [PubMed] [Google Scholar]
- 49.Ward LS, Scheffel RS, Hoff AO, Ferraz C, Vaisman F. Treatment strategies for low-risk papillary thyroid carcinoma: a position statement from the Thyroid Department of the Brazilian Society of Endocrinology and Metabolism (SBEM) Arch Endocrinol Metab. 2022;66:522–532. doi: 10.20945/2359-3997000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koot A, Soares P, Robenshtok E, Locati LD, de la Fouchardiere C, Luster M, et al. Position paper from the Endocrine Task Force of the European Organisation for Research and Treatment of Cancer (EORTC) on the management and shared decision making in patients with low-risk micro papillary thyroid carcinoma. Eur J Cancer. 2023;179:98–112. doi: 10.1016/j.ejca.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 51.Park YJ, Lee EK, Song YS, Kang SH, Koo BS, Kim SW, et al. [2023 Korean Thyroid Association management guidelines for patients with thyroid nodules] Int J Thyroidol. 2023;16:1–31. Korean. [Google Scholar]
- 52.Durante C, Hegedüs L, Czarniecka A, Paschke R, Russ G, Schmitt F, et al. 2023 European Thyroid Association clinical practice guidelines for thyroid nodule management. Eur Thyroid J. 2023;12:e230067. doi: 10.1530/ETJ-23-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghai S, Goldstein DP, Sawka AM. Ultrasound imaging in active surveillance of small, low-risk papillary thyroid cancer. Korean J Radiol. 2024;25:749–755. doi: 10.3348/kjr.2024.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee EK, Park YJ, Jung CK, Na DG. A narrative review of the 2023 Korean Thyroid Association Management guideline for patients with thyroid nodules. Endocrinol Metab (Seoul) 2024;39:61–72. doi: 10.3803/EnM.2024.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chou R, Dana T, Haymart M, Leung AM, Tufano RP, Sosa JA, et al. Active surveillance versus thyroid surgery for differentiated thyroid cancer: a systematic review. Thyroid. 2022;32:351–367. doi: 10.1089/thy.2021.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakai T, Sugitani I, Ebina A, Fukuoka O, Toda K, Mitani H, et al. Active surveillance for T1bN0M0 papillary thyroid carcinoma. Thyroid. 2019;29:59–63. doi: 10.1089/thy.2018.0462. [DOI] [PubMed] [Google Scholar]
- 57.Tuttle RM, Fagin J, Minkowitz G, Wong R, Roman B, Patel S, et al. Active surveillance of papillary thyroid cancer: frequency and time course of the six most common tumor volume kinetic patterns. Thyroid. 2022;32:1337–1345. doi: 10.1089/thy.2022.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brito JP, Ito Y, Miyauchi A, Tuttle RM. A clinical framework to facilitate risk stratification when considering an active surveillance alternative to immediate biopsy and surgery in papillary microcarcinoma. Thyroid. 2016;26:144–149. doi: 10.1089/thy.2015.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ha SM, Baek JH, Na DG, Suh CH, Chung SR, Choi YJ, et al. Diagnostic performance of practice guidelines for thyroid nodules: thyroid nodule size versus biopsy rates. Radiology. 2019;291:92–99. doi: 10.1148/radiol.2019181723. [DOI] [PubMed] [Google Scholar]
- 60.Lončar I, van Dijk SPJ, Metman MJH, Lin JF, Kruijff S, Peeters RP, et al. Active surveillance for papillary thyroid microcarcinoma in a population with restrictive diagnostic workup strategies. Thyroid. 2021;31:1219–1225. doi: 10.1089/thy.2020.0845. [DOI] [PubMed] [Google Scholar]
- 61.Do Cao C, Haissaguerre M, Lussey-Lepoutre C, Donatini G, Raverot V, Russ G. SFE-AFCE-SFMN 2022 consensus on the management of thyroid nodules: initial work-up for thyroid nodules. Ann Endocrinol (Paris) 2022;83:380–388. doi: 10.1016/j.ando.2022.10.009. [DOI] [PubMed] [Google Scholar]
- 62.Zhuge L, Huang Z, Cai H, Wang S, Niu L, Li Z. The optimal age threshold for stratifying the risks of disease progression in patients with highly suspicious sub-centimeter thyroid nodules. Ann Surg Oncol. 2023;30:5463–5469. doi: 10.1245/s10434-023-13497-1. [DOI] [PubMed] [Google Scholar]
- 63.Ha EJ, Lim HK, Yoon JH, Baek JH, Do KH, Choi M, et al. Primary imaging test and appropriate biopsy methods for thyroid nodules: guidelines by Korean Society of Radiology and National Evidence-Based Healthcare Collaborating Agency. Korean J Radiol. 2018;19:623–631. doi: 10.3348/kjr.2018.19.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee JY, Baek JH, Ha EJ, Sung JY, Shin JH, Kim JH, et al. 2020 imaging guidelines for thyroid nodules and differentiated thyroid cancer: Korean Society of Thyroid Radiology. Korean J Radiol. 2021;22:840–860. doi: 10.3348/kjr.2020.0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee MK, Na DG, Joo L, Lee JY, Ha EJ, Kim JH, et al. Standardized imaging and reporting for thyroid ultrasound: Korean Society of Thyroid Radiology consensus statement and recommendation. Korean J Radiol. 2023;24:22–30. doi: 10.3348/kjr.2022.0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim SY, Na DG, Paik W. Which ultrasound image plane is appropriate for evaluating the taller-than-wide sign in the risk stratification of thyroid nodules? Eur Radiol. 2021;31:7605–7613. doi: 10.1007/s00330-021-07936-4. [DOI] [PubMed] [Google Scholar]
- 67.Choi SH, Kim EK, Kim SJ, Kwak JY. Thyroid ultrasonography: pitfalls and techniques. Korean J Radiol. 2014;15:267–276. doi: 10.3348/kjr.2014.15.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghai S, O’Brien C, Goldstein DP, Sawka AM Canadian Thyroid Cancer Active Surveillance Study Group. Ultrasound in active surveillance for low-risk papillary thyroid cancer: imaging considerations in case selection and disease surveillance. Insights Imaging. 2021;12:130. doi: 10.1186/s13244-021-01072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mulla MG, Knoefel WT, Gilbert J, McGregor A, Schulte KM. Lateral cervical lymph node metastases in papillary thyroid cancer: a systematic review of imaging-guided and prophylactic removal of the lateral compartment. Clin Endocrinol (Oxf) 2012;77:126–131. doi: 10.1111/j.1365-2265.2012.04336.x. [DOI] [PubMed] [Google Scholar]
- 70.Stack BC, Jr, Ferris RL, Goldenberg D, Haymart M, Shaha A, Sheth S, et al. American Thyroid Association consensus review and statement regarding the anatomy, terminology, and rationale for lateral neck dissection in differentiated thyroid cancer. Thyroid. 2012;22:501–508. doi: 10.1089/thy.2011.0312. [DOI] [PubMed] [Google Scholar]
- 71.Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid. A “normal” finding in Finland. A systematic autopsy study. Cancer. 1985;56:531–538. doi: 10.1002/1097-0142(19850801)56:3<531::aid-cncr2820560321>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto Y, Maeda T, Izumi K, Otsuka H. Occult papillary carcinoma of the thyroid. A study of 408 autopsy cases. Cancer. 1990;65:1173–1179. doi: 10.1002/1097-0142(19900301)65:5<1173::aid-cncr2820650524>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 73.Hyun SM, Song HY, Kim SY, Nam SY, Roh JL, Han MW, et al. Impact of combined prophylactic unilateral central neck dissection and hemithyroidectomy in patients with papillary thyroid microcarcinoma. Ann Surg Oncol. 2012;19:591–596. doi: 10.1245/s10434-011-1995-6. [DOI] [PubMed] [Google Scholar]
- 74.Choi SJ, Kim TY, Lee JC, Shong YK, Cho KJ, Ryu JS, et al. Is routine central neck dissection necessary for the treatment of papillary thyroid microcarcinoma? Clin Exp Otorhinolaryngol. 2008;1:41–45. doi: 10.3342/ceo.2008.1.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oh HS, Park S, Kim M, Kwon H, Song E, Sung TY, et al. Young age and male sex are predictors of large-volume central neck lymph node metastasis in clinical N0 papillary thyroid microcarcinomas. Thyroid. 2017;27:1285–1290. doi: 10.1089/thy.2017.0250. [DOI] [PubMed] [Google Scholar]
- 76.Randolph GW, Duh QY, Heller KS, LiVolsi VA, Mandel SJ, Steward DL, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid. 2012;22:1144–1152. doi: 10.1089/thy.2012.0043. [DOI] [PubMed] [Google Scholar]
- 77.Suh CH, Baek JH, Choi YJ, Lee JH. Performance of CT in the preoperative diagnosis of cervical lymph node metastasis in patients with papillary thyroid cancer: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2017;38:154–161. doi: 10.3174/ajnr.A4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim E, Park JS, Son KR, Kim JH, Jeon SJ, Na DG. Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid. 2008;18:411–418. doi: 10.1089/thy.2007.0269. [DOI] [PubMed] [Google Scholar]
- 79.Ahn JE, Lee JH, Yi JS, Shong YK, Hong SJ, Lee DH, et al. Diagnostic accuracy of CT and ultrasonography for evaluating metastatic cervical lymph nodes in patients with thyroid cancer. World J Surg. 2008;32:1552–1558. doi: 10.1007/s00268-008-9588-7. [DOI] [PubMed] [Google Scholar]
- 80.Lee Y, Kim JH, Baek JH, Jung SL, Park SW, Kim J, et al. Value of CT added to ultrasonography for the diagnosis of lymph node metastasis in patients with thyroid cancer. Head Neck. 2018;40:2137–2148. doi: 10.1002/hed.25202. [DOI] [PubMed] [Google Scholar]
- 81.Ha EJ, Chung SR, Na DG, Ahn HS, Chung J, Lee JY, et al. 2021 Korean thyroid imaging reporting and data system and imaging-based management of thyroid nodules: Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2021;22:2094–2123. doi: 10.3348/kjr.2021.0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jeon YH, Lee JY, Yoo RE, Rhim JH, Lee KH, Choi KS, et al. Validation of ultrasound and computed tomography-based risk stratification system and biopsy criteria for cervical lymph nodes in preoperative patients with thyroid cancer. Korean J Radiol. 2023;24:912–923. doi: 10.3348/kjr.2023.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yoo RE, Kim JH, Hwang I, Kang KM, Yun TJ, Choi SH, et al. Added value of computed tomography to ultrasonography for assessing LN metastasis in preoperative patients with thyroid cancer: node-by-node correlation. Cancers (Basel) 2020;12:1190. doi: 10.3390/cancers12051190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jeong SY, Chung SR, Baek JH, Choi YJ, Kim S, Sung TY, et al. Impact of additional preoperative computed tomography imaging on staging, surgery, and postsurgical survival in patients with papillary thyroid carcinoma. Korean J Radiol. 2023;24:1284–1292. doi: 10.3348/kjr.2023.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee DH, Kim YK, Yu HW, Choi JY, Park SY, Moon JH. Computed tomography for detecting cervical lymph node metastasis in patients who have papillary thyroid microcarcinoma with tumor characteristics appropriate for active surveillance. Thyroid. 2019;29:1653–1659. doi: 10.1089/thy.2019.0100. [DOI] [PubMed] [Google Scholar]
- 86.Sugitani I, Fujimoto Y. Symptomatic versus asymptomatic papillary thyroid microcarcinoma: a retrospective analysis of surgical outcome and prognostic factors. Endocr J. 1999;46:209–216. doi: 10.1507/endocrj.46.209. [DOI] [PubMed] [Google Scholar]
- 87.Choi JB, Lee WK, Lee SG, Ryu H, Lee CR, Kang SW, et al. Long-term oncologic outcomes of papillary thyroid microcarcinoma according to the presence of clinically apparent lymph node metastasis: a large retrospective analysis of 5,348 patients. Cancer Manag Res. 2018;10:2883–2891. doi: 10.2147/CMAR.S173853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kawano S, Miyauchi A, Ito Y. Routine chest computed tomography at presentation does not identify distant metastasis in cT1aN0 papillary thyroid carcinoma. Thyroid. 2020;30:1620–1624. doi: 10.1089/thy.2020.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jiang J, Lu H. Immediate surgery might be a better option for subcapsular thyroid microcarcinomas. Int J Endocrinol. 2019;2019:3619864. doi: 10.1155/2019/3619864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin M, Su Y, Wei W, Gong Y, Huang Y, Zeng J, et al. Extra-thyroid extension prediction by ultrasound quantitative method based on thyroid capsule response evaluation. Med Sci Monit. 2021;27:e929408. doi: 10.12659/MSM.929408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park SY, Kim HI, Kim JH, Kim JS, Oh YL, Kim SW, et al. Prognostic significance of gross extrathyroidal extension invading only strap muscles in differentiated thyroid carcinoma. Br J Surg. 2018;105:1155–1162. doi: 10.1002/bjs.10830. [DOI] [PubMed] [Google Scholar]
- 92.Amit M, Boonsripitayanon M, Goepfert RP, Tam S, Busaidy NL, Cabanillas ME, et al. Extrathyroidal extension: does strap muscle invasion alone influence recurrence and survival in patients with differentiated thyroid cancer? Ann Surg Oncol. 2018;25:3380–3388. doi: 10.1245/s10434-018-6563-x. [DOI] [PubMed] [Google Scholar]
- 93.Danilovic DLS, Castroneves LA, Suemoto CK, Elias LO, Soares IC, Camargo RY, et al. Is there a difference between minimal and gross extension into the strap muscles for the risk of recurrence in papillary thyroid carcinomas? Thyroid. 2020;30:1008–1016. doi: 10.1089/thy.2019.0753. [DOI] [PubMed] [Google Scholar]
- 94.Kang IK, Kim K, Bae JS, Kim JS. [Is completion thyroidectomy necessary in patients with papillary thyroid carcinoma who underwent lobectomy?] Korean J Head Neck Oncol. 2021;37:25–31. Korean. [Google Scholar]
- 95.Chung SR, Baek JH, Choi YJ, Sung TY, Song DE, Kim TY, et al. Sonographic assessment of the extent of extrathyroidal extension in thyroid cancer. Korean J Radiol. 2020;21:1187–1195. doi: 10.3348/kjr.2019.0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jeong SY, Chung SR, Baek JH, Choi YJ, Sung TY, Song DE, et al. Sonographic assessment of minor extrathyroidal extension of papillary thyroid microcarcinoma involving the posterior thyroid capsule. Eur Radiol. 2022;32:6090–6096. doi: 10.1007/s00330-022-08765-9. [DOI] [PubMed] [Google Scholar]
- 97.Ito Y, Miyauchi A, Oda H, Kobayashi K, Kihara M, Miya A. Revisiting low-risk thyroid papillary microcarcinomas resected without observation: was immediate surgery necessary? World J Surg. 2016;40:523–528. doi: 10.1007/s00268-015-3184-4. [DOI] [PubMed] [Google Scholar]
- 98.Newman SK, Harries V, Wang L, McGill M, Ganly I, Girshman J, et al. Invasion of a recurrent laryngeal nerve from small well-differentiated papillary thyroid cancers: patient selection implications for active surveillance. Thyroid. 2022;32:164–169. doi: 10.1089/thy.2021.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chung SR, Baek JH, Rho YH, Choi YJ, Sung TY, Song DE, et al. Sonographic diagnosis of cervical lymph node metastasis in patients with thyroid cancer and comparison of European and Korean guidelines for stratifying the risk of malignant lymph node. Korean J Radiol. 2022;23:1102–1111. doi: 10.3348/kjr.2022.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee JY, Yoo RE, Rhim JH, Lee KH, Choi KS, Hwang I, et al. Validation of ultrasound risk stratification systems for cervical lymph node metastasis in patients with thyroid cancer. Cancers (Basel) 2022;14:2106. doi: 10.3390/cancers14092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chung SR, Baek JH, Choi YJ, Sung TY, Song DE, Kim TY, et al. Risk factors for metastasis in indeterminate lymph nodes in preoperative patients with thyroid cancer. Eur Radiol. 2022;32:3863–3868. doi: 10.1007/s00330-021-08478-5. [DOI] [PubMed] [Google Scholar]
- 102.Yoo RE, Kim JH, Bae JM, Hwang I, Kang KM, Yun TJ, et al. Ultrasonographic indeterminate lymph nodes in preoperative thyroid cancer patients: malignancy risk and ultrasonographic findings predictive of malignancy. Korean J Radiol. 2020;21:598–604. doi: 10.3348/kjr.2019.0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pacini F, Fugazzola L, Lippi F, Ceccarelli C, Centoni R, Miccoli P, et al. Detection of thyroglobulin in fine needle aspirates of nonthyroidal neck masses: a clue to the diagnosis of metastatic differentiated thyroid cancer. J Clin Endocrinol Metab. 1992;74:1401–1404. doi: 10.1210/jcem.74.6.1592886. [DOI] [PubMed] [Google Scholar]
- 104.Chung SR, Baek JH, Choi YJ, Sung TY, Song DE, Kim TY, et al. Diagnostic algorithm for metastatic lymph nodes of differentiated thyroid carcinoma. Cancers (Basel) 2021;13:1338. doi: 10.3390/cancers13061338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Choi YJ, Baek JH, Hong MJ, Lee JH. Inter-observer variation in ultrasound measurement of the volume and diameter of thyroid nodules. Korean J Radiol. 2015;16:560–565. doi: 10.3348/kjr.2015.16.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee HJ, Yoon DY, Seo YL, Kim JH, Baek S, Lim KJ, et al. Intraobserver and interobserver variability in ultrasound measurements of thyroid nodules. J Ultrasound Med. 2018;37:173–178. doi: 10.1002/jum.14316. [DOI] [PubMed] [Google Scholar]
- 107.Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR thyroid imaging, reporting and data system (TI-RADS): white paper of the ACR TI-RADS committee. J Am Coll Radiol. 2017;14:587–595. doi: 10.1016/j.jacr.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 108.Chung SR, Choi YJ, Lee SS, Kim SO, Lee SA, Jeon MJ, et al. Interobserver reproducibility in sonographic measurement of diameter and volume of papillary thyroid microcarcinoma. Thyroid. 2021;31:452–458. doi: 10.1089/thy.2020.0317. [DOI] [PubMed] [Google Scholar]
- 109.Ito Y, Miyauchi A, Kudo T, Higashiyama T, Masuoka H, Kihara M, et al. Kinetic analysis of growth activity in enlarging papillary thyroid microcarcinomas. Thyroid. 2019;29:1765–1773. doi: 10.1089/thy.2019.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lu Z, Sheng J, Zhang Y, Deng J, Li Y, Lu A, et al. Clonality analysis of multifocal papillary thyroid carcinoma by using genetic profiles. J Pathol. 2016;239:72–83. doi: 10.1002/path.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kuhn E, Teller L, Piana S, Rosai J, Merino MJ. Different clonal origin of bilateral papillary thyroid carcinoma, with a review of the literature. Endocr Pathol. 2012;23:101–107. doi: 10.1007/s12022-012-9202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moniz S, Catarino AL, Marques AR, Cavaco B, Sobrinho L, Leite V. Clonal origin of non-medullary thyroid tumours assessed by non-random X-chromosome inactivation. Eur J Endocrinol. 2002;146:27–33. doi: 10.1530/eje.0.1460027. [DOI] [PubMed] [Google Scholar]
- 113.Nakazawa T, Kondo T, Tahara I, Kasai K, Inoue T, Oishi N, et al. Multicentric occurrence of multiple papillary thyroid carcinomas--HUMARA and BRAF mutation analysis. Cancer Med. 2015;4:1272–1280. doi: 10.1002/cam4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scope of guideline
Methodology
Identifying information and availability