Abstract
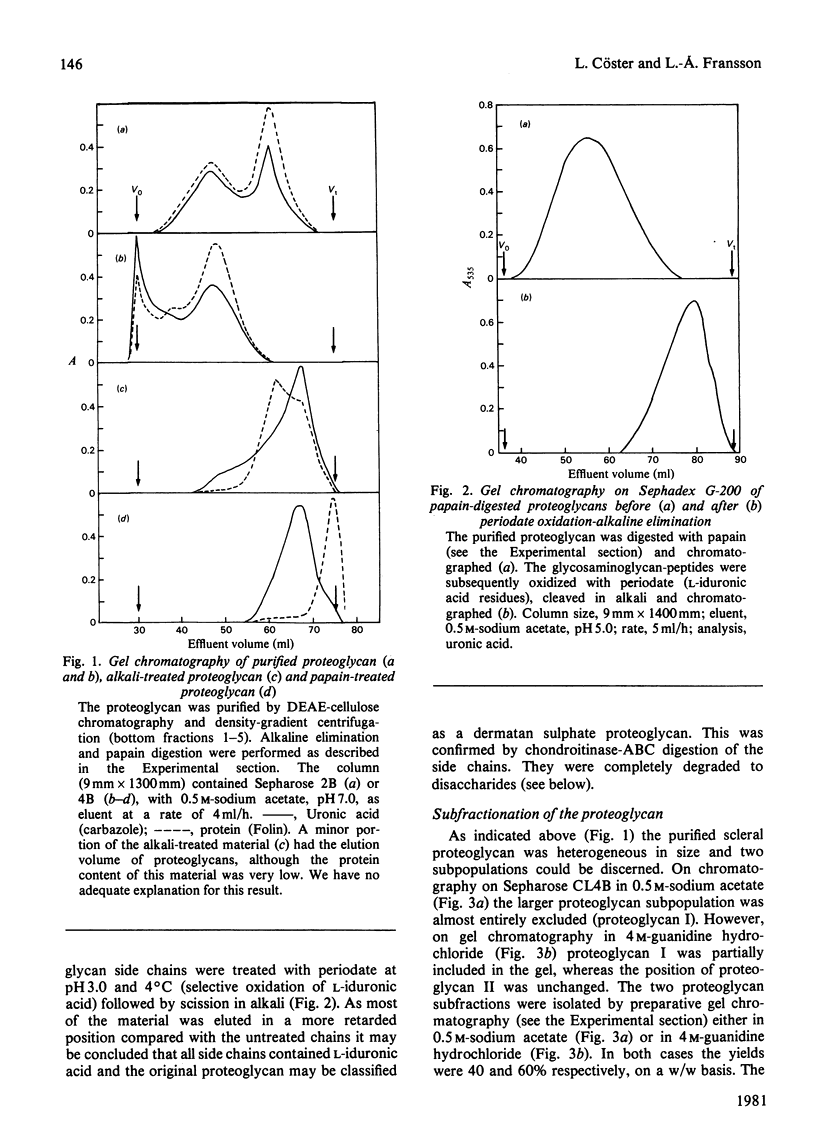
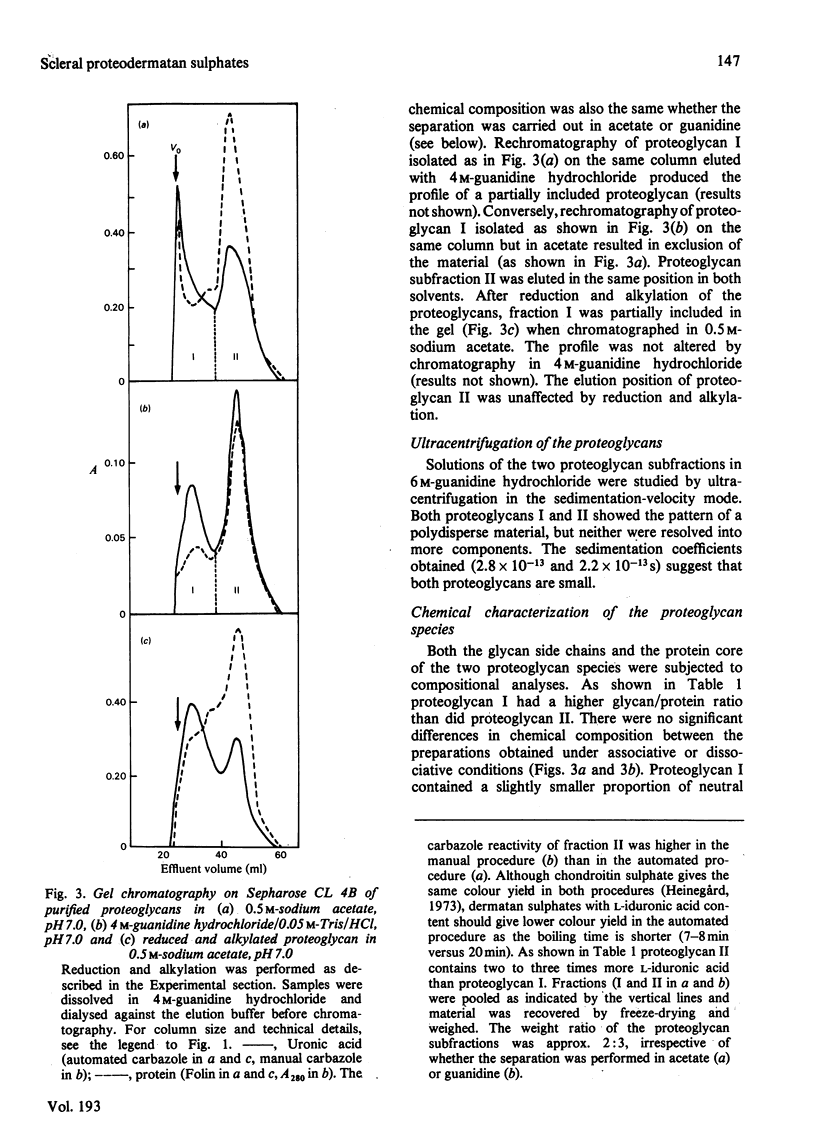
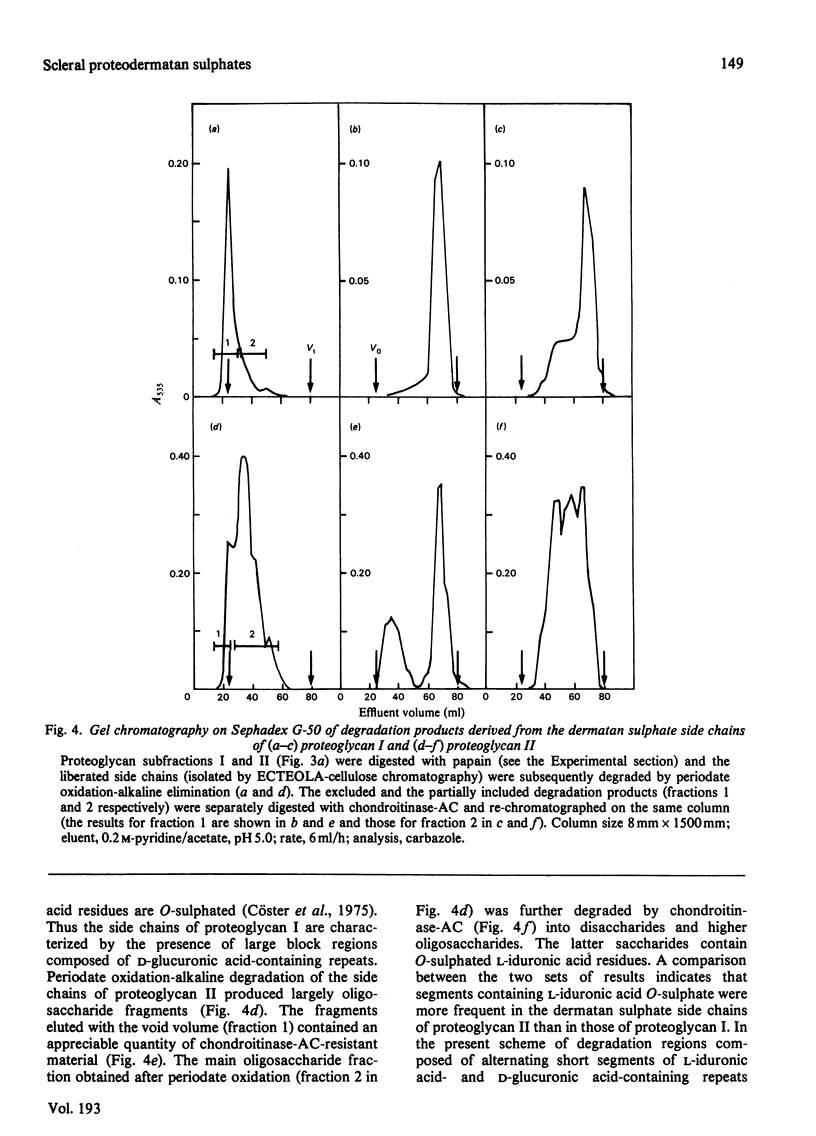
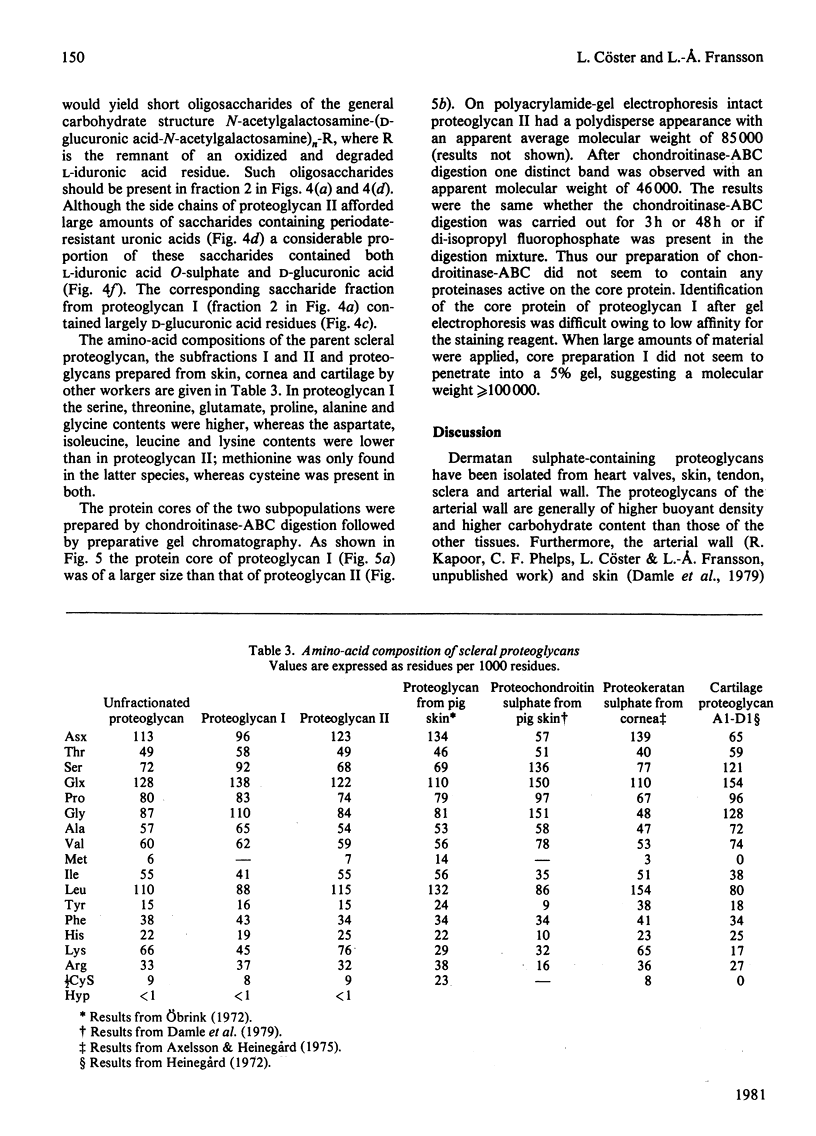
1. Proteoglycans were extracted from sclera with 4 M-guanidine hydrochloride in the presence of proteinase inhibitors and purified by ion-exchange chromatography and density-gradient centrifugation. 2. The entire proteoglycan pool was characterized by compositional analyses and by specific chemical (periodate oxidation) and enzymic (chondroitinases) degradations. The glycan moieties of the molecules were exclusively galactosaminoglycans (dermatan sulphate-chondroitin sulphate co-polymers). In addition, the preparations contained small amounts of oligosaccharides. 3. The scleral proteodermatan sulphates were fractionated into one larger (I) and one smaller (II) component by gel chromatography. Proteoglycan I was eluted in a more excluded position on gel chromatography in 0.5 M-sodium acetate than in 4.0 M-guanidine hydrochloride. Reduced and alkylated proteoglycan I was eluted in the same position (in 0.5 M-sodium acetate) as was the starting material (in 4.0 M-guanidine hydrochloride). The elution position of proteoglycan II was the same in both solvents. Proteoglycans I and II had s0 20,w values of 2.8 x 10(-13) and 2.2 x 10(-13) s respectively in 6.0 M-guanidine hydrochloride. 4. The two proteoglycans differed with respect to the nature of the protein core and the co-polymeric structure of their side chains. Also proteoglycan I contained more side chains than did proteoglycan II. The dermatan sulphate side chains of proteoglycan I were D-glucuronic acid-rich (80%), whereas those of proteoglycan II contained equal amounts of D-glucuronic acid and L-iduronic acid. Furthermore, the co-polymeric features of the side chains of proteoglycans I and II were different. The protein core of proteoglycan I was of larger size than that of proteoglycan II. The latter had an apparent molecular weight of 46 000 (estimated by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis), whereas the former was greater than 100 000. In addition, the amino-acid composition of the two core preparations was different. 5. As proteoglycan I altered its elution position on gel chromatography in 4 M-guanidine hydrochloride compared with 0.5 M-sodium acetate it is proposed that a change in conformation or a disaggregation took place. If the latter hypothesis is favoured, aggregation may be due to self-association or mediated by an extrinsic molecule, e.g. hyaluronic acid.
Full text
PDF



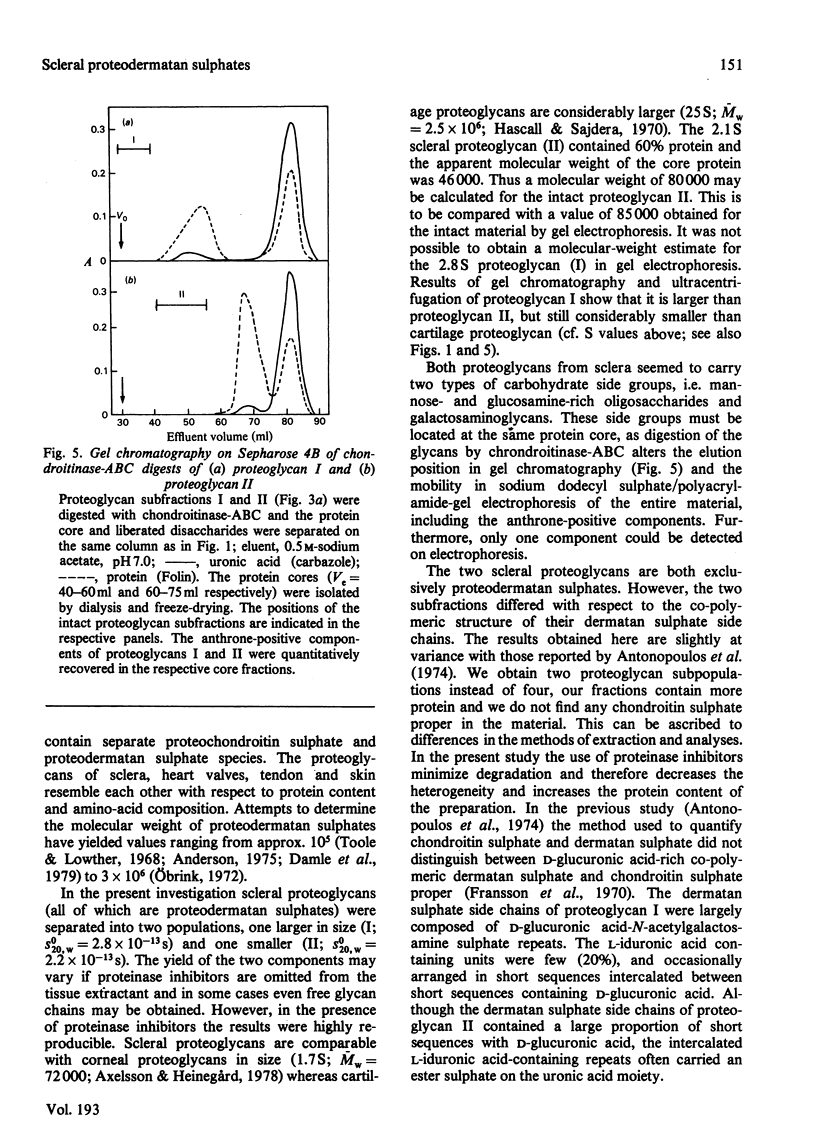


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANSETH A. Studies on corneal polysaccharides. III. Topographic and comparative biochemistry. Exp Eye Res. 1961 Dec;1:106–115. doi: 10.1016/s0014-4835(61)80015-8. [DOI] [PubMed] [Google Scholar]
- ANTONOPOULOS C. A., GARDELL S., SZIRMAI J. A., DETYSSONSK E. R. DETERMINATION OF GLYCOSAMINOGLYCANS (MUCOPOLYSACCHARIDES) FROM TISSUE ON THE MICROGRAM SCALE. Biochim Biophys Acta. 1964 Mar 2;83:1–19. doi: 10.1016/0926-6526(64)90045-x. [DOI] [PubMed] [Google Scholar]
- Anderson J. C. Isolation of a glycoprotein and proteodermatan sulphate from bovine achilles tendon by affinity chromatography on concanavalin A-Sepharose. Biochim Biophys Acta. 1975 Feb 27;379(2):444–455. doi: 10.1016/0005-2795(75)90151-8. [DOI] [PubMed] [Google Scholar]
- Axelsson I., Heinegård D. Characterization of the keratan sulphate proteoglycans from bovine corneal stroma. Biochem J. 1978 Mar 1;169(3):517–530. doi: 10.1042/bj1690517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson I., Heinegård D. Fractionation of proteoglycans from bovine corneal stroma. Biochem J. 1975 Mar;145(3):491–500. doi: 10.1042/bj1450491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Cöster L., Malmström A., Sjöberg I., Fransson L. The co-polymeric structure of pig skin dermatan sulphate. Distribution of L-iduronic acid sulphate residues in co-polymeric chains. Biochem J. 1975 Feb;145(2):379–389. doi: 10.1042/bj1450379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damle S. P., Kieras F. J., Tzeng W. K., Gregory J. D. Isolation and characterization of proteochondroitin sulfate from pig skin. J Biol Chem. 1979 Mar 10;254(5):1614–1620. [PubMed] [Google Scholar]
- Ehrlich K. C., Radhakrishnamurthy B., Berenson G. S. Isolation of a chondroitin sulfate--dermatan sulfate proteoglycan from bovine aorta. Arch Biochem Biophys. 1975 Nov;171(1):361–369. doi: 10.1016/0003-9861(75)90043-0. [DOI] [PubMed] [Google Scholar]
- Eisenstein R., Larsson S. E., Kuettner K. E., Sorgente N., Hascal V. C. The ground substance of the arterial wall. Part 1. Extractability of glycosaminoglycans and the isolation of a proteoglycan from bovine aorta. Atherosclerosis. 1975 Jul-Aug;22(1):1–17. doi: 10.1016/0021-9150(75)90064-7. [DOI] [PubMed] [Google Scholar]
- Fransson L. A., Carlstedt I. Alkaline and smith degradation of oxidized dermatan sulphate-chondroitin sulphate copolymers. Carbohydr Res. 1974 Sep;36(2):349–358. doi: 10.1016/s0008-6215(00)83056-6. [DOI] [PubMed] [Google Scholar]
- Fransson L. A., Cöster L. Interaction between dermatan sulphate chains. II. Structural studies on aggregating glycan chains and oligosaccharides with affinity for dermatan sulphate-substituted agarose. Biochim Biophys Acta. 1979 Jan 4;582(1):132–144. doi: 10.1016/0304-4165(79)90296-4. [DOI] [PubMed] [Google Scholar]
- Fransson L. A. Interaction between dermatan sulphate chains. I. Affinity chromatography of copolymeric galactosaminioglycans on dermatan sulphate-substituted agarose. Biochim Biophys Acta. 1976 Jun 23;437(1):106–115. doi: 10.1016/0304-4165(76)90351-2. [DOI] [PubMed] [Google Scholar]
- Fransson L. A. Periodate oxidation of L-iduronic acid residues in dermatan sulphate. Carbohydr Res. 1974 Sep;36(2):339–348. doi: 10.1016/s0008-6215(00)83055-4. [DOI] [PubMed] [Google Scholar]
- Fransson L. A. Structure of dermatan sulfate. IV. Glycopeptides from the carbohydrate-protein linkage region of pig skin dermatan sulfate. Biochim Biophys Acta. 1968 Mar 11;156(2):311–316. [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Hyaluronic acid in cartilage and proteoglycan aggregation. Biochem J. 1974 Jun;139(3):565–581. doi: 10.1042/bj1390565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Riolo R. L., Hayward J., Jr, Reynolds C. C. Treatment of bovine nasal cartilage proteoglycan with chondroitinases from Flavobacterium heparinum and Proteus vulgaris. J Biol Chem. 1972 Jul 25;247(14):4521–4528. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Physical properties and polydispersity of proteoglycan from bovine nasal cartilage. J Biol Chem. 1970 Oct 10;245(19):4920–4930. [PubMed] [Google Scholar]
- Heinegård D. Extraction, fractionation and characterization of proteoglycans from bovine tracheal cartilage. Biochim Biophys Acta. 1972 Nov 28;285(1):181–192. doi: 10.1016/0005-2795(72)90190-0. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Keiser H. D., Hatcher V. B. A comparison of bovine nasal cartilage proteoglycan core protein produced by chondroitinase and hyaluronidase: the possible role of protease contaminants. Connect Tissue Res. 1977;5(3):147–155. doi: 10.3109/03008207709152265. [DOI] [PubMed] [Google Scholar]
- Obrink B. Isolation and partial characterization of a dermatan sulphate proteoglycan from pig skin. Biochim Biophys Acta. 1972 Apr 21;264(2):354–361. [PubMed] [Google Scholar]
- Oegema T. R., Jr, Hascall V. C., Dziewiatkowski D. D. Isolation and characterization of proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1975 Aug 10;250(15):6151–6159. [PubMed] [Google Scholar]
- Radhakrishnamurthy B., Ruiz H. A., Jr, Berenson G. S. Isolation and characterization of proteoglycans from bovine aorta. J Biol Chem. 1977 Jul 25;252(14):4831–4841. [PubMed] [Google Scholar]
- Stegemann H., Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967 Nov;18(2):267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- Stern E. L., Lindahl B., Rodén L. The linkage of dermatan sulfate to protein. II. Monosaccharide sequence of the linkage region. J Biol Chem. 1971 Sep 25;246(18):5707–5715. [PubMed] [Google Scholar]
- Toole B. P., Lowther D. A. Dermatan sulfate-protein: isolation from and interaction with collagen. Arch Biochem Biophys. 1968 Dec;128(3):567–578. doi: 10.1016/0003-9861(68)90064-7. [DOI] [PubMed] [Google Scholar]
- Toole B. P., Lowther D. A. The isolation of a dermatan sulphate-protein complex from bovine heart valves. Biochim Biophys Acta. 1965 Nov 1;101(3):364–366. doi: 10.1016/0926-6534(65)90017-5. [DOI] [PubMed] [Google Scholar]


