Abstract
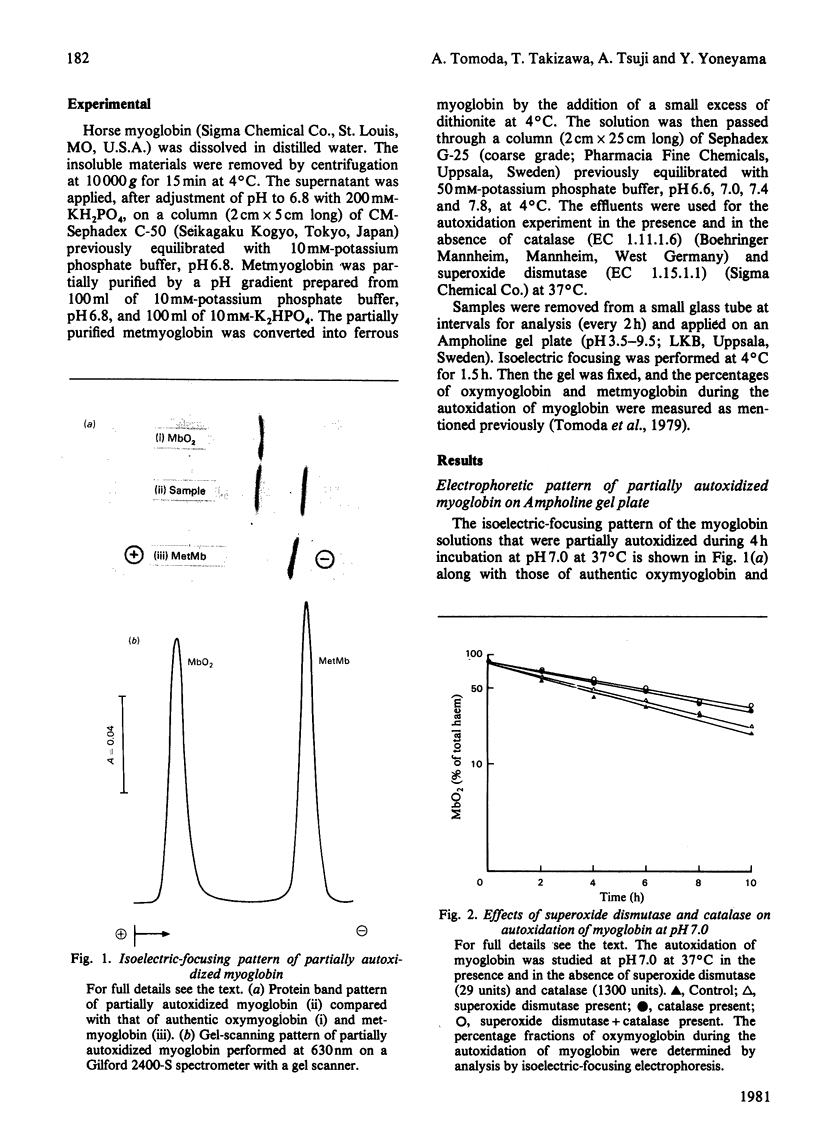
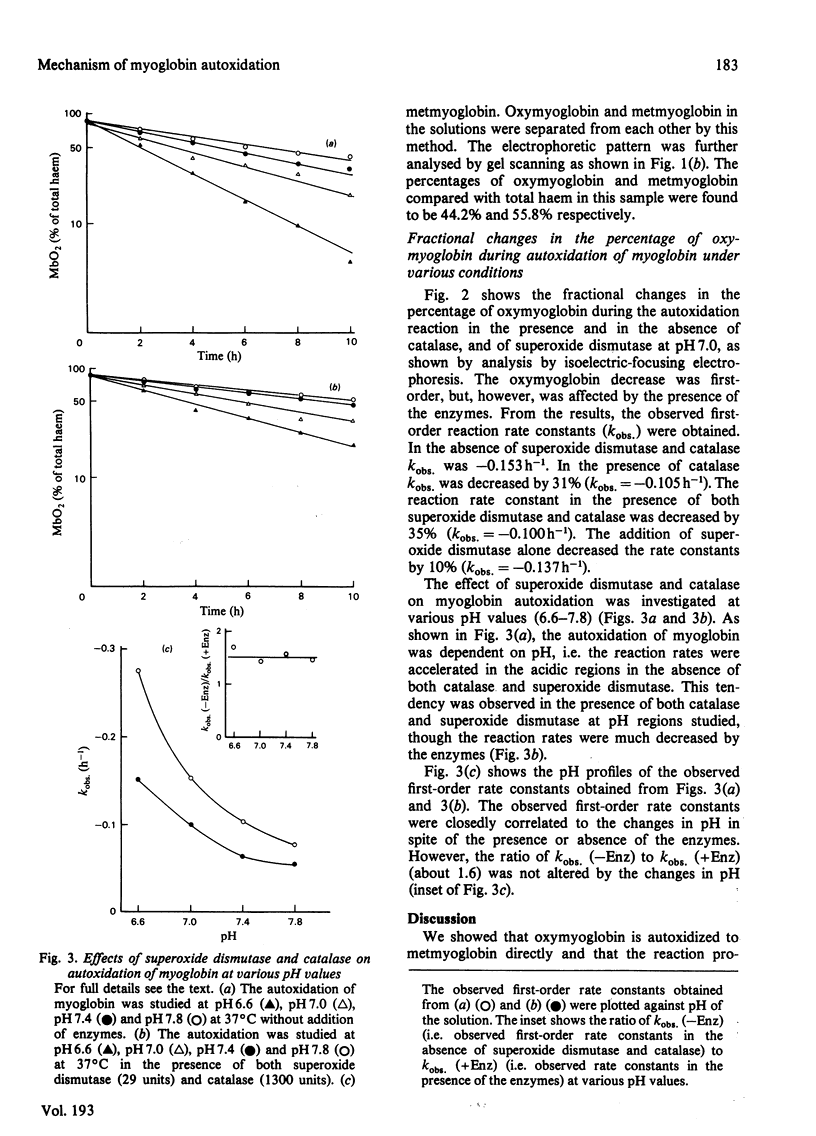
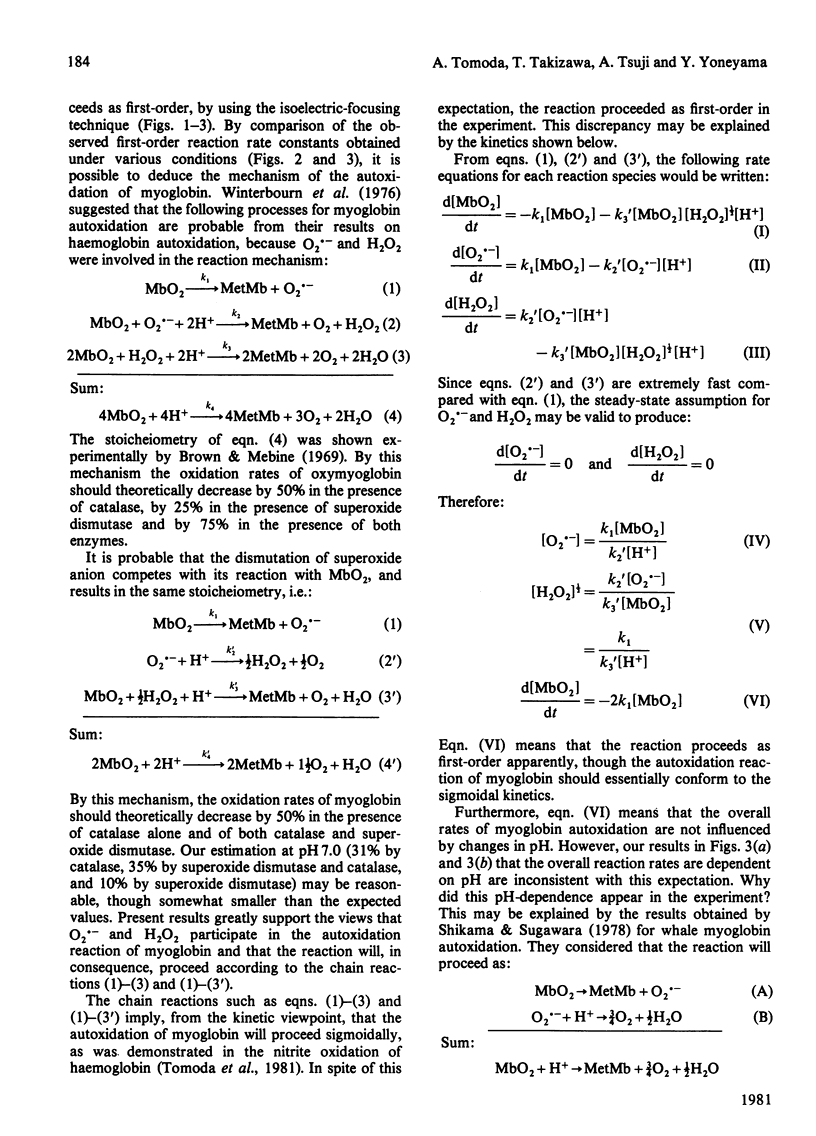
The autoxidation of horse myoglobin was studied in the presence or absence of catalase (EC 1.11.1.6) and/or superoxide dismutase (EC 1.15.1.1) at various pH values (6.6-7.8). Changes in the percentages of oxymyoglobin and metmyoglobin during the reaction were analysed by means of isoelectric focusing on Ampholine gel plates. Oxymyoglobin was decreased in a first-order manner, with an accompanying increase in metmyoglobin, under the various conditions studied. The observed reaction rate constants obtained under these conditions were pH-dependent; however, they were also greatly affected by the presence of the enzymes. The pH-dependence of the overall reaction was explained by the acid-base three-state model of myoglobin proposed by Shikama & Sugawara [(1978) Eur. J. Biochem. 91, 407-413]. The reaction process of myoglobin autoxidation was explained by the model suggested by Winterbourn, McGrath & Carrell [(1976) Biochem. J. 155, 493-502], indicating that superoxide anion and hydrogen peroxide are involved in the reaction mechanism.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown W. D., Mebine L. B. Autoxidation of oxymyoglobins. J Biol Chem. 1969 Dec 25;244(24):6696–6701. [PubMed] [Google Scholar]
- GEORGE P., STRATMANN C. J. The oxidation of myoglobin to metmyoglobin by oxygen. III. Kinetic studies in the presence of carbon monoxide, and at different hydrogen-ion concentrations with considerations regarding the stability of oxymyoglobin. Biochem J. 1954 Aug;57(4):568–573. doi: 10.1042/bj0570568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T., Shikama K. Autoxidation of native oxymyoglobin from bovine heart muscle. Arch Biochem Biophys. 1974 Aug;163(2):476–481. doi: 10.1016/0003-9861(74)90504-9. [DOI] [PubMed] [Google Scholar]
- Gotoh T., Shikama K. Generation of the superoxide radical during autoxidation of oxymyoglobin. J Biochem. 1976 Aug;80(2):397–399. doi: 10.1093/oxfordjournals.jbchem.a131289. [DOI] [PubMed] [Google Scholar]
- Shikama K., Sugawara Y. Autoxidation of native oxymyoglobin. Kinetic analysis of the pH profile. Eur J Biochem. 1978 Nov 15;91(2):407–413. doi: 10.1111/j.1432-1033.1978.tb12693.x. [DOI] [PubMed] [Google Scholar]
- Tomoda A., Imoto M., Hirano M., Yoneyama Y. Analyis of met-form haemoglobins in human erythrocytes of normal adults and of a patient with hereditary methaemoglobinaemia due to deficiency of NADH-cytochrome b5 reductase. Biochem J. 1979 Aug 1;181(2):505–507. doi: 10.1042/bj1810505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda A., Tsuji A., Yoneyama Y. Involvement of superoxide anion in the reaction mechanism of haemoglobin oxidation by nitrite. Biochem J. 1981 Jan 1;193(1):169–179. doi: 10.1042/bj1930169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C., McGrath B. M., Carrell R. W. Reactions involving superoxide and normal and unstable haemoglobins. Biochem J. 1976 Jun 1;155(3):493–502. doi: 10.1042/bj1550493. [DOI] [PMC free article] [PubMed] [Google Scholar]



