Abstract
Background and purpose
Autoimmune screening panels (ASPs) are often ordered as a part of the diagnostic workup in people with suspected multiple sclerosis (MS). However, data on the significance of ASP seropositivity in MS are scarce. This study aimed to investigate whether routine implementation of ASPs is viable in MS diagnostic workup.
Methods
In this retrospective study, we included patients from the Vienna Multiple Sclerosis Database who were diagnosed with MS according to current McDonald criteria between 2014 and 2021 and had an ASP performed.
Results
We analyzed 212 patients (mean age at serology = 30.4 [SD = 8.5] years, 67% female). Red flag symptoms for presence of systemic autoimmune disease were reported by 5.6% of patients during initial evaluation (sicca syndrome [n = 5], joint pain [n = 4], dermatitis [n = 4]). Complement levels (C3c and C4) were below the lower reference level in 26 of 134 (19.4%) and three of 134 (2.2%), respectively. Antinuclear antibodies (ANAs) were positive in 24 of 210 (11.4%), with 18 (8.6%), five (2.4%), and one (0.5%) having mildly, moderately, and strongly positive ANA titers. Extractable nuclear antibody subsets were positive in 10 of 211 (4.7%) patients. ASPs led to the diagnosis of mixed connective tissue disease (n = 1), psoriatic arthritis (n = 1), and Sjögren syndrome (n = 2; positive predictive value [PPV] = 4.9%, negative predictive value [NPV] = 99.3%). Among patients presenting with red flag symptoms, ASPs had better overall test performance (PPV = 100%, NPV = 88.9%).
Conclusions
The rate of ASP seropositivity in MS is low and within the range of the general population. Performance of ASPs without clinical suspicion of systemic autoimmune disease seems unwarranted.
Keywords: autoimmune diseases, diagnostic techniques and procedures, differential diagnosis, multiple sclerosis, serology
INTRODUCTION
Although some studies suggest an overall increased risk of autoimmune comorbidities in patients with multiple sclerosis (pwMS), current evidence is generally conflicting, as these associations have not been consistently demonstrated in population‐based studies [1, 2, 3]. Although the specific predisposition of MS to autoimmune comorbidities remains debatable, there is general consensus that certain autoimmune diseases (AIDs), such as rheumatologic conditions (e.g., systemic lupus erythematosus [SLE], Sjögren syndrome [SS]), vasculitis (e.g., Behçet disease), or antiphospholipid antibody syndrome (APS), can involve the central nervous system (CNS) and might mimic symptoms or paraclinical findings of MS [4]. This is at least partly reflected by several reports implying a relatively high proportion of MS misdiagnosis, especially if consideration of differential diagnosis is not applied carefully [5, 6]. Therefore, it is essential to definitively exclude these and other disorders to accurately diagnose MS, as specifically emphasized by the McDonald 2017 criteria. This, at least in principle, would support the concept of implementing autoimmune screening panels (ASPs) as part of the initial diagnostic evaluation in patients suspected of having MS [7]. However, studies have challenged the concept of performing ASPs to exclude alternative diagnoses, as emerging data suggest that performing neither routine antinuclear antibody (ANA) screening nor broad antibody screening is advisable, evidenced by a limited diagnostic yield for autoimmune conditions despite frequent elevation of such parameters [8, 9]. These findings are somewhat reflected in recent revisions of the MS guideline by the German Society for Neurology, which now suggest that ASPs should primarily be performed when there is clinical suspicion of an AID [10]. Nonetheless, there remains a substantial lack of evidence concerning the effectiveness of routine ASPs, as well as the overall seroprevalence of positive antibody screening findings in pwMS, especially in relation to AID follow‐up diagnosis. Therefore, this study aimed to expand on and confirm recent trends regarding ASPs in a broad population of pwMS and to contextualize these results in relation to diagnosis of AID [8].
METHODS
Data collection
This retrospective study analyzed patients diagnosed with relapsing MS (RMS) based on concurrent McDonald criteria at the Department of Neurology, Medical University of Vienna from April 2013 to October 2021 [7, 11]. This data analysis was restricted to patients with RMS who had ASPs performed as part of their initial diagnostic evaluation. Data were utilized from the Vienna Multiple Sclerosis Database (VMSD), which is a comprehensive repository of clinical and paraclinical data, with the primary objective to gather detailed information on MS by adhering to a minimal core dataset as defined by institutional expert recommendations [12].
Patients were excluded from our analysis if they had a diagnosis of primary progressive or pediatric MS, if follow‐up data were incomplete (i.e., absence of a follow‐up visit after ASPs or insufficient documentation on ASP results by the treating neurologist), or if no ASP was conducted at diagnosis. In addition to data extracted from the VMSD, medical reports were screened to identify any red flag symptoms indicative of a systemic AID (e.g., rheumatologic disease) present at the time of initial evaluation (such as joint pain, sicca syndrome, and/or recurring fever). No standardized assessment was implemented, and as such symptoms had to be reported by patients spontaneously during evaluation. Diagnoses of autoimmune conditions other than MS were extracted from follow‐up medical records.
Autoimmune screening panels
ASP parameter selection was standardized based on institutional expert opinion and previous suggestions by guidelines; however, selection of parameters could differ between patients depending on the clinician's judgment to include or exclude specific laboratory values based on the patients' clinical symptoms or reported family history [10]. The following parameters were collected: complement factor C3c and C4, rheumatoid factor (RF), ANA, extractable nuclear antibody (ENA) subsets (anti‐SSA/Ro, anti‐SSB/La, Centromere B, SCL70, SM, u1RNP, and Jo‐1), smooth muscle cell antibody (SMA), cytoplasmic or perinuclear antineutrophil cytoplasmic antibodies (cANCA/pANCA), IgG and IgM against cardiolipin, antibodies against double‐stranded DNA (dsDNA), IgG and IgM against beta‐2 glycoprotein (ß2GP), and lastly antimitochondrial antibodies. ANA, cANCA/pANCA, and SMA titers were defined as either negative, or mildly (1:80–1:160), moderately (1:320–1:640), or strongly (≥1:1280) positive. Positivity for ENA and dsDNA was classified as either borderline positive if present within concentrations of 5 U/mL to 10 U/mL for most ENA and 10 U/mL to 15 U/mL for dsDNA or definitively positive if greater than 10 U/mL for most ENA and 15 U/mL for dsDNA. Complete reference ranges for each parameter are shown in Table S1.
ASPs were defined as positive if at least one autoantibody, including borderline positive cases, was detectable, when complement C3c or C4 levels were abnormal, and/or when RF was elevated. In this study, systemic AIDs were defined as conditions characterized by simultaneous, successive, or variable involvement of multiple organs or systems (e.g., skin, muscle, joints, kidney, CNS), distinguished by the presence of autoantibodies [13].
Laboratory analysis of ASP parameters
ASP measurements were performed in the local Department of Laboratory Medicine at the Medical University of Vienna. Methods of measurement for each parameter are shown in Table S1.
Statistical analysis
The statistical analysis was performed using IBM SPSS Statistics (version 29.0.2; SPSS, Chicago, IL, USA). Categorical variables were expressed as frequency and percentage; continuous variables were displayed either as mean and SD, or median and range, as appropriate.
To evaluate diagnostic accuracy of ASPs in correctly identifying systematic AID, sensitivity and specificity along with positive and negative predictive values (PPV, NPV) were calculated with 95% confidence intervals.
Ethics
The study was approved by the ethics committee of the Medical University of Vienna (ethical approval no. 1668/2023). As datasets were exported pseudonymously from the local VMSD including data obtained in routine practice, the need for written informed consent from study participants was waived by the ethics committee. This study adheres to the reporting guidelines outlined within the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement.
RESULTS
Patient characteristics
After screening of the VMSD, 212 patients were included (Figure 1). Of those, 142 patients (66.9%) were female and mean age at time of ASP was 30.4 (SD = 8.5) years. Characteristics of study cohorts are shown in Table 1 and did not significantly differ from the whole VMSD cohort (Table S2).
FIGURE 1.
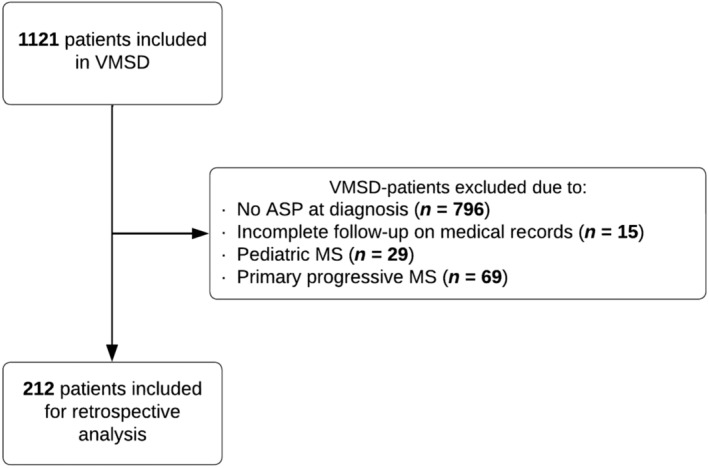
Inclusion flowchart of the patient cohort. ASP, autoimmune screening panel; MS, multiple sclerosis; VMSD, Vienna Multiple Sclerosis Database.
TABLE 1.
Patient demographics.
| Patient characteristic | Results |
|---|---|
| Age at disease onset, years a | 29.8 (8.4) |
| Age at diagnosis, years a | 30.4 (8.5) |
| Age at serology, years a | 30.4 (8.5) |
| Disease onset to serology, days b | 28.5 (9–139.5) |
| Female, n (%) | 142 (67) |
| Positive ASP results, n (%) | 61 (28.7) |
| Patients with autoantibodies present, n (%) | 42 (19.8) |
Abbreviation: ASP, autoimmune screening panel.
Mean (SD).
Median (interquartile range).
Apart from two patients with known and managed autoimmune thyroiditis, none of the analyzed patients had a definitive diagnosis of any systemic AID prior to evaluation for MS. The total number of each parameter ordered is presented in Table 2.
TABLE 2.
Results of autoimmune screening panel.
| Screening parameter | n total | n positive | % positive |
|---|---|---|---|
| ASL | 162 | 32 | 19.75 |
| C3c and C4 abnormal a | 134 | 31 a | 23.13 |
| C3c decreased | 134 | 26 | 19.40 |
| C3c increased | 134 | 1 | 0.75 |
| C4 decreased | 134 | 3 | 2.24 |
| C4 increased | 134 | 3 | 2.24 |
| RF | 180 | 0 | 0.00 |
| ANA total | 210 | 24 | 11.43 |
| ANA mild | 210 | 18 | 8.57 |
| ANA moderate | 210 | 5 | 2.38 |
| ANA strong | 210 | 1 | 0.48 |
| Anti‐dsDNA total | 208 | 2 | 0.96 |
| dsDNA borderline | 208 | 1 | 0.48 |
| dsDNA definite | 208 | 1 | 0.48 |
| ENA total b | 211 | 10 b | 4.74 |
| SSA/antiRo total | 211 | 6 | 2.84 |
| SSA/antiRo borderline | 211 | 1 | 0.47 |
| SSA/antiRo definite | 211 | 5 | 2.37 |
| SSB/antiLa total | 211 | 2 | 0.95 |
| SSB/antiLa borderline | 211 | 1 | 0.47 |
| SSB/antiLa definite | 211 | 1 | 0.47 |
| SM total/borderline | 211 | 3 | 1.42 |
| Centromere B | 211 | 2 | 0.95 |
| u1RNP | 211 | 0 | 0.00 |
| SCL‐70 | 211 | 0 | 0.00 |
| Jo‐1 | 211 | 0 | 0.00 |
| Cardiolipin IgM | 205 | 4 | 1.95 |
| Cardiolipin IgG | 204 | 0 | 0.00 |
| SMA | 167 | 11 | 6.59 |
| ß2GP IgM, total/borderline | 203 | 5 | 2.46 |
| ß2GP IgG | 204 | 0 | 0.00 |
| cANCA | 205 | 0 | 0.00 |
| pANCA | 205 | 0 | 0.00 |
| Antimitochondrial | 173 | 0 | 0.00 |
Abbreviations: ANA, antinuclear antibody; anti‐dsDNA, anti‐double‐stranded DNA antibody; ASL, antistreptolysin; cANCA, cytoplasmic antineutrophil cytoplasmic antibody, ENA, extractable nuclear antigen; Jo‐1, myositis specific antibody; pANCA, perinuclear antineutrophil cytoplasmic antibody; RF, rheumatoid factor; SCL‐70, anti‐topoisomerase I; SM, Smith antibody; SMA, smooth muscle cell antibody; ß2GP, beta‐2 glycoprotein; SSA/antiRo and SSB/antiLa, anti‐Sjögren syndrome‐related antigen A/B; u1RNP, u1‐ribonucleoprotein.
Two patients had decreased levels of both C3c and C4.
Three patients were positive for multiple ENAs.
ASP findings
Abnormal screening results were found in 28.7% of all patients (Table 1). Among those with a positive ASP, most patients exhibited abnormal levels of complement C3c or C4 (31/134, 23.1%), with the majority having C3c levels below the respective reference range (26/134, 19.4%; Tables S1 and S2). In 2.2% (3/134) of patients, complement C4 concentrations were decreased, and in 1.5% (2/134) they were increased (Table 2).
Among the panel results that were positive for autoantibodies, the majority had ANA (24/211, 11.4%). Specifically, 8.6% (18/211) of patients demonstrated mildly, 2.4% (5/211) moderately, and 0.5% (1/210) strongly positive ANA titers. ENA titers were positive in 4.7% (10/211) of patients, with positivity for SSA/antiRo and SSB/antiLa autoantibodies being most frequent (7/211, 3.3%), whereas none of the patients tested positive for ENA subclasses u1RNP, SCL‐70, or Jo‐1. Other positive antibody results included SMA (11/167, 6.6%), ß2GP (borderline positive in 5/203, 2.5%), and IgM against cardiolipin (4/204, 1.9%). Complete results of ASPs are shown in Table 2. Overall, in 42 (19.8%) patients at least one antibody parameter was measured to be positive.
Diagnostic accuracy of ASPs
In 12 patients (5.6%), symptoms compatible with AID were observed; five exhibited sicca symptoms, four experienced arthralgia, and four presented with dermatitis (one patient presented with both sicca symptoms and dermatitis). Follow‐up examinations led to a diagnosis of systemic AID in four patients, all of whom displayed red flag symptoms. Two patients were diagnosed with SS, one with mixed connective tissue disease and another with psoriatic arthritis. All AIDs were identified as comorbid conditions in presence of MS with none involving the CNS, were diagnosed after formal diagnosis of MS, and did not alter the established diagnosis of MS. Whereas in three patients positive panel results were observed, the patient with psoriatic arthritis did not exhibit elevated RF or any other positive screening result (Table 3). Based on the findings in this study, ASPs were calculated to have an overall NPV of 99.3% and PPV of 4.9% for systemic AIDs that have been diagnosed as a direct consequence of ASP in this study cohort. In a subgroup analysis of all patients who presented with red flag symptoms, ASPs were calculated to have a PPV of 100% and an NPV of 88.9%. Test metrics are shown in Table 4.
TABLE 3.
AID diagnosis with ASP results.
| Follow‐up AID | Results |
|---|---|
| Total AID diagnosed, n | 4 |
| Psoriatic arthritis, n | 1 |
| ASP results | All negative (including RF) |
| Mixed connective tissue disease, n | 1 |
| ASP results | ASL (265), ANA (1:320), antinucleosome (29.2) |
| Sjögren syndrome, n | 2 |
| ASP results, Patient 1 | C3c (88.1), ASL (292), SSA (242), SSB (320), SM (7.9) |
| ASP results, Patient 2 | ANA (1:160) |
Abbreviations: AID, autoimmune disorder; ANA, antinuclear antibody; ASL, antistreptolysin; ASP, autoimmune screening panel; RF, rheumatoid factor; SM, Smith antibody; SSA/SSB, anti‐Sjögren syndrome‐related antigen A/B.
TABLE 4.
Overall test metrics for autoimmune screening panel.
| Statistics | Results | |
|---|---|---|
| All patients | Red flag symptoms | |
| Sensitivity, % (95% CI) | 75.0 (19.4–99.4) | 75 (19.4–99.4) |
| Specificity, % (95% CI) | 72.1 (65.5–78.1) | 100.0 (63.1–100.0) |
| Positive likelihood ratio (95% CI) | 2.7 (1.4–4.9) | NA |
| Negative likelihood ratio (95% CI) | 0.4 (0.1–1.9) | 0.25 (0.05–1.36) |
| AID prevalence, % | 1.9 | 33.3 |
| Positive predictive value, % (95% CI) | 4.9 (2.8–8.7) | 100.0 (29.2–100.0) |
| Negative predictive value, % (95% CI) | 99.3 (96.5–99.9) | 88.9 (59.4–97.8) |
| Accuracy, % | 72.2 (65.6–78.1) | 91.7 (61.5–99.8) |
Abbreviations: AID, autoimmune disease; CI, confidence interval; NA, not applicable.
DISCUSSION
Given the questionable diagnostic benefits of routine ASPs along with high direct and potential indirect economic costs associated with performing these [8, 9], the aim of this study was to provide a more comprehensive report on the general seroprevalence of positive antibody screening findings as well as to evaluate the test performance of ASPs in MS. The current lack of evidence regarding routine ASP testing in pwMS contributes to the tendency for ASPs to still be frequently performed in tertiary and quaternary care clinics. Based on our results, however, we assert that, although positive ASPs are not infrequent, occurring in nearly 30% positive of our study population, routine ASPs provide little added diagnostic value. This is evidenced by a particularly poor PPV across all pwMS, and as such, ASPs should not be performed in all patients suspected of having MS.
The most common findings were abnormal levels of complement C3c or C4. This, however, is not particularly surprising, as previous studies have already established a link between increased complement factor activity and MS pathology as well as disease activity and/or progression [14, 15, 16]. As such, a derangement of complement levels could be interpreted as a mechanism compatible with MS pathophysiology and would therefore not constitute a viable screening parameter to allow excluding or differentiating other autoimmune disorders. This is particularly evident in this study's findings, because among 31 patients with abnormal complement concentrations, only one was diagnosed with an AID (SS).
Among antibody screening results in our cohort, ANA was most commonly positive. Again, these results were to be expected, as the seroprevalence of ANA varies significantly, but is consistently high in both healthy individuals as well as in patients with nonrheumatic disorders, with positive results reported in 26.7%–63.5% of pwMS [8, 17, 18]. In contrast, in our current study, only 11.4% of pwMS tested positive for ANA, a rate that rather approximates the seroprevalence observed in the general “healthy” population [19, 20]. Due to a lack of specificity and sensitivity, the Canadian Rheumatology Association specifically advised against using ANA testing as a general screening tool for patients who do not exhibit specific signs or symptoms indicative of SLE or another connective tissue disease (CTD) [19, 21, 22]. Additionally, Becker et al. reported that although ANA titers were frequently elevated among various screening panel parameters, only four of 197 patients were ultimately diagnosed with rheumatologic conditions, with one of these patients being ANA‐negative [8]. The authors therefore concluded that routine ANA screening may not be advisable for patients with MS due to its limited sensitivity and specificity [8]. These observations are consistent with the results of our study, which also demonstrated a notably high rate of false positive and false negative results, as only half of the patients with a follow‐up diagnosis of systemic AID tested positive for ANA.
Nonetheless, in case of positive ANA results, ENA and dsDNA are typically part of a follow‐up serology (stepwise approach); however, simultaneous testing for ANA, dsDNA, and ENA is still used as a screening measure [23, 24]. Independent of the presence of MS or other disorders, data on the use of ENA and dsDNA as screening measures suggest that their diagnostic yield is low in “healthy” individuals without clinical suspicion of AID and those who test negative for ANA. In these patient populations, ENA and dsDNA demonstrate very limited PPV and sensitivity [25, 26]. In our study, 4.7% of patients tested positive for ENA, yet only one patient was diagnosed with SS upon follow‐up, having shown clinical symptoms of sicca syndrome consistent with SS prior to ASP testing. dsDNA was positive in two patients, but only one was subsequently diagnosed with mixed CTD, who also presented with symptoms consistent with CTD (joint pain and skin involvement). Similarly, another study involving 85 pwMS reported ENA positivity in 13 patients and dsDNA positivity in one patient, with none of these individuals exhibiting clinical manifestations of CTD or being diagnosed with CTD upon follow‐up [18]. Therefore, our observations along with previously reported data (inside and outside the realm of MS) suggest that there is no necessity to routinely screen for either ENA or dsDNA in patients suspected of having MS without clinical manifestations of systemic AID.
Regarding other antibody findings, ß2GP (only borderline) and IgM cardiolipin were elevated in few pwMS (5/203, 2.5% and 4/205, 2.0%), and none of the assessed patients was diagnosed with anti‐phospholipid syndrome (APS) systemic during follow‐up. Studies suggest that the general seroprevalence of APS antibodies in “healthy” individuals is somewhere between 1% and 14% and can be associated with other processes, such as infections, vaccination, and malignancies [27, 28]. Although APS can mimic symptomatology and paraclinical findings of MS, careful history taking (e.g., venous thrombosis) could most likely exclude APS without the need for ASPs [29]. SMA was within the range of healthy individuals and was not associated with any follow‐up AID. Although studies that assessed antigen‐specific autoimmune liver disease‐related autoantibodies found that, contrary to our results, SMA concentration in pwMS seemed to be surprisingly high, no clear increase in presence of autoimmune hepatitis (AIH) could be observed [30, 31]. Additionally, AIH is not thought to typically mimic MS symptomatology and therefore is not regarded a relevant differential diagnosis [4]. Finally, in our study, all patients were found to be negative for RF, cANCA, and pANCA, and although especially ANCA positivity can precede the onset symptoms in AID, it is not advisable to generally screen for these without clearly indicative symptoms or to distinguish between MS and non‐MS [8, 22, 32, 33].
Consistent with previous data, our study confirms that the prevalence of comorbid AID is low in pwMS and that the rate of seropositive autoantibodies approximates those observed within the general population [3, 20]. ASPs in the current study demonstrated a high rate of false positives along with correctly identifying only three of four with follow‐up AID, resulting in an overall poor test performance and a particularly low PPV of 4.9%. Notably, all patients diagnosed with an AID exhibited symptoms consistent with systemic AID, implying that routine ASPs are highly unlikely to provide any additional diagnostic benefits in effectively excluding autoimmune differential diagnoses or comorbidity in patients who do not present with highly atypical MS symptoms. This is further supported by our subgroup analysis, where every patient with clinical symptoms of systemic APS and a positive ASP was eventually diagnosed with an AID. Although our sample size in this subgroup was small, test accuracy appears exceptional in these patients, as indicated by both high PPV and NPV. Additionally, cost‐effectiveness should be considered as well when performing an ASP, because antibody screenings tend to have high direct costs (€932 for a complete ASP at our institution), along with indirect costs involving potentially unnecessary referrals to other specialists such as rheumatologists.
Some limitations of this study must be acknowledged. First, the sample size of 212 patients may be insufficient to adequately capture rare autoimmune disorders. However, if a significantly larger number of patients would be required to identify these disorders, routine application of ASPs may not be useful either way, but only in a selected population. It needs to be acknowledged that patients were not systematically interviewed regarding systemic symptoms, potentially introducing a risk of underestimating red flag symptoms, particularly for sicca syndrome. Additionally, our study did not include a control cohort, limiting the ability to make definitive conclusions about the general seroprevalence of positive ASP results in relation to follow‐up AID diagnosis. This limitation is compounded by the retrospective nature of our study stemming from a real‐world population, which included only patients with a confirmed MS diagnosis who received an ASP. Thus, there could be both selection and indication bias and our findings may not truly reflect the effectiveness of ASPs in patients evaluated for MS. However, the study cohort characteristics are similar to the whole cohort at our center, rendering the potential for a relevant selection/indication bias for ASPs unlikely. Also, we could not account for ethnicity in our analyses, because our study population consisted of >95% Caucasians, an issue which should be addressed by future research.
CONLUSIONS
The rate of seropositivity for ASPs in pwMS is low and aligns with the prevalence expected in the general population. Performing ASPs should only be considered in the presence of atypical MS symptoms or symptoms compatible with systemic AID.
AUTHOR CONTRIBUTIONS
Fabian Föttinger: Data curation; formal analysis; project administration; validation; writing – original draft. Nik Krajnc: Data curation; writing – review and editing; formal analysis; project administration. Katharina Riedl: Data curation; writing – review and editing; project administration. Fritz Leutmezer: Data curation; writing – review and editing. Markus Ponleitner: Data curation; writing – review and editing. Paulus Rommer: Data curation; writing – review and editing. Barbara Kornek: Data curation; writing – review and editing. Stefan Macher: Data curation; writing – review and editing. Christiane Schmied: Data curation; writing – review and editing. Karin Zebenholzer: Data curation; writing – review and editing. Gudrun Zulehner: Data curation; writing – review and editing. Tobias Zrzavy: Data curation; writing – review and editing. Thomas Berger: Data curation; writing – review and editing. Gabriel Bsteh: Data curation; conceptualization; writing – review and editing; validation; supervision; project administration; methodology.
FUNDING INFORMATION
This study was partly funded by the Austrian MS Research Society.
CONFLICT OF INTEREST STATEMENT
Fabian Föttinger: Has participated in meetings sponsored by, received speaker honoraria or travel funding from Novartis. Nik Krajnc: Has participated in meetings sponsored by, received speaker honoraria or travel funding from Alexion, BMS/Celgene, Janssen, Merck, Novartis, Roche and Sanofi‐Genzyme and held a grant for a Multiple Sclerosis Clinical Training Fellowship Programme from the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). Katharina Riedl: Nothing to disclose. Fritz Leutmezer: Has participated in meetings sponsored by, received speaker honoraria or travel funding from Actelion, Almirall, Biogen, Celgene, Johnson&Johnson, MedDay, Merck, Novartis, Roche, Sanofi‐Genzyme and Teva, and received honoraria for consulting Biogen, Celgene, Merck, Novartis, Roche, Sanofi‐Genzyme and Teva. Markus Ponleitner: Has participated in meetings sponsored by, received speaker or consulting honoraria from Amicus and travel funding from Amicus, Merck, Novartis and Sanofi‐Genzyme. Paulus Rommer: Has received honoraria for consultancy/speaking from Alexion/Astra Zeneca, Allmiral, Amgen/Horizon, Amicus, Biogen, Merck, Novartis, Roche, Sandoz, and Sanofi. He has received research grants from Amicus, Biogen, Merck, Roche. Barbara Kornek: Has received honoraria for speaking and for consulting from Biogen, BMS‐Celgene, Johnson&Johnson, Merck, Novartis, Roche, Teva and Sanofi‐Genzyme outside of the submitted work. No conflict of interest with respect to the present study. Stefan Macher: Declares no conflict of interest relevant to this study Christiane Schmied: Declares no conflict of interest relevant to this study. Karin Zebenholzer: Received speaking honoraria or travel grants from Biogen, Celgene/BMS, Novartis and Sanofi‐Genzyme. Gudrun Zulehner: Has participated in meetings sponsored by or received travel funding and speaking honoraria from Biogen, Merck, Novartis, Roche, Sanofi‐Genzyme and Teva. Tobias Zrzavy: Has participated in meetings sponsored by or received travel funding from Biogen, Merck, Novartis, Roche, Sanofi‐Genzyme and Teva. Thomas Berger: Has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for MS: Allergan, Bayer, Biogen, Bionorica, BMS/Celgene, Genesis, GSK, GW/Jazz Pharma, Horizon, Janssen‐Cilag, MedDay, Merck, Novartis, Octapharma, Roche, Sandoz, Sanofi‐Genzyme, Teva and UCB. His institution has received financial support in the past 12 months by unrestricted research grants (Biogen, Bayer, BMS/Celgene, Merck, Novartis, Roche, Sanofi‐Genzyme, Teva and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Merck, Novartis, Octapharma, Roche, Sanofi‐Genzyme, Teva. Gabriel Bsteh: Has participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, Celgene/BMS, Lilly, MedWhizz, Merck, Novartis, Roche, Sanofi‐Genzyme and Teva, and received honoraria for consulting Biogen, Celgene/BMS, Merck, Novartis, Roche, Sanofi‐Genzyme and Teva. He has received unrestricted research grants from Celgene/BMS and Novartis. He serves as a Member of the Executive Committee of the European Committee for Treatment and Research in Multiple Sclerosis.
Supporting information
APPENDIX S1. Supporting information.
APPENDIX S2. Supporting information.
Föttinger F, Krajnc N, Riedl K, et al. Autoimmune screening panel in patients with multiple sclerosis: A Vienna multiple sclerosis database study. Eur J Neurol. 2025;32:e16558. doi: 10.1111/ene.16558
DATA AVAILABILITY STATEMENT
Anonymized data supporting the findings of this study are available from the corresponding author upon reasonable request by a qualified researcher and upon approval by the data‐clearing committee of the Medical University of Vienna.
REFERENCES
- 1. Marrie RA, Reider N, Cohen J, et al. A systematic review of the incidence and prevalence of autoimmune disease in multiple sclerosis. Mult Scler. 2015;21(3):282‐293. doi: 10.1177/1352458514564490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leal Rato M, Santos M, de Sá J, Ferreira J. Comorbid autoimmune disorders in people with multiple sclerosis: a retrospective cohort study. J Neuroimmunol. 2023;385:578226. doi: 10.1016/j.jneuroim.2023.578226 [DOI] [PubMed] [Google Scholar]
- 3. Conrad N, Misra S, Verbakel JY, et al. Incidence, prevalence, and co‐occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population‐based cohort study of 22 million individuals in the UK. Lancet. 2023;401(10391):1878‐1890. doi: 10.1016/s0140-6736(23)00457-9 [DOI] [PubMed] [Google Scholar]
- 4. Solomon AJ, Arrambide G, Brownlee WJ, et al. Differential diagnosis of suspected multiple sclerosis: an updated consensus approach. Lancet Neurol. 2023;22(8):750‐768. doi: 10.1016/s1474-4422(23)00148-5 [DOI] [PubMed] [Google Scholar]
- 5. Solomon AJ, Klein EP, Bourdette D. “Undiagnosing” multiple sclerosis: the challenge of misdiagnosis in MS. Neurology. 2012;78(24):1986‐1991. doi: 10.1212/WNL.0b013e318259e1b2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: a multicenter study. Neurology. 2016;87(13):1393‐1399. doi: 10.1212/wnl.0000000000003152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162‐173. doi: 10.1016/s1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 8. Becker J, Geffken M, Diehl RR, Berlit P, Krämer M. Choosing wisely? Multiple sclerosis and laboratory screening for autoimmune differential diagnoses. Neurol Int Open: Thieme Verlag. 2017;1(4):E256‐E263. doi: 10.1055/s-0043-115429 [DOI] [Google Scholar]
- 9. Dal‐Bianco A, Wenhoda F, Rommer PS, et al. Do elevated autoantibodies in patients with multiple sclerosis matter? Acta Neurol Scand. 2019;139(3):238‐246. doi: 10.1111/ane.13054 [DOI] [PubMed] [Google Scholar]
- 10. Hemmer B, Bayas A, Berthele A, Christe K, Domurath B, Ebert J. Diagnose und Therapie der Multiplen Sklerose, Neuromyelitis‐optica‐Spektrum‐Erkrankungen und MOG‐IgG‐assoziierten Erkrankungen, S2k‐Leitlinie. Deutsche Gesellschaft für Neurologie; 2023; [Google Scholar]
- 11. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292‐302. doi: 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bsteh G, Hegen H, Riedl K, et al. Quantifying the risk of disease reactivation after interferon and glatiramer acetate discontinuation in multiple sclerosis: the VIAADISC score. Eur J Neurol. 2021;28(5):1609‐1616. doi: 10.1111/ene.14705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doria A, Sarzi‐Puttini P, Shoenfeld Y. 2nd conference on heart, rheumatism and autoimmunity, Pescara, Italy, May 19–20, 2005. Autoimmun Rev. 2006;5(1):55‐63. [DOI] [PubMed] [Google Scholar]
- 14. Oechtering J, Stein K, Schaedelin SA, et al. Complement activation is associated with disease severity in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2024;11(2):e200212. doi: 10.1212/nxi.0000000000200212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ingram G, Loveless S, Howell OW, et al. Complement activation in multiple sclerosis plaques: an immunohistochemical analysis. Acta Neuropathol Commun. 2014;2:53. doi: 10.1186/2051-5960-2-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Storch MK, Piddlesden S, Haltia M, Iivanainen M, Morgan P, Lassmann H. Multiple sclerosis: in situ evidence for antibody‐ and complement‐mediated demyelination. Ann Neurol. 1998;43(4):465‐471. doi: 10.1002/ana.410430409 [DOI] [PubMed] [Google Scholar]
- 17. Grygiel‐Górniak B, Rogacka N, Puszczewicz M. Antinuclear antibodies in healthy people and non‐rheumatic diseases—diagnostic and clinical implications. Reumatologia. 2018;56(4):243‐248. doi: 10.5114/reum.2018.77976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Szmyrka‐Kaczmarek M, Pokryszko‐Dragan A, Pawlik B, et al. Antinuclear and antiphospholipid antibodies in patients with multiple sclerosis. Lupus. 2012;21(4):412‐420. doi: 10.1177/0961203311427550 [DOI] [PubMed] [Google Scholar]
- 19. Tan EM, Feltkamp TE, Smolen JS, et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum. 1997;40(9):1601‐1611. doi: 10.1002/art.1780400909 [DOI] [PubMed] [Google Scholar]
- 20. Deshpande P, Lucas M, Brunt S, Lucas A, Hollingsworth P, Bundell C. Low level autoantibodies can be frequently detected in the general Australian population. Pathology. 2016;48(5):483‐490. doi: 10.1016/j.pathol.2016.03.014 [DOI] [PubMed] [Google Scholar]
- 21. Chow SL, Carter Thorne J, Bell MJ, et al. Choosing wisely: the Canadian rheumatology Association's list of 5 items physicians and patients should question. J Rheumatol. 2015;42(4):682‐689. doi: 10.3899/jrheum.141140 [DOI] [PubMed] [Google Scholar]
- 22. Ferrari R. Evaluation of the Canadian rheumatology association choosing wisely recommendation concerning anti‐nuclear antibody (ANA) testing. Clin Rheumatol. 2015;34(9):1551‐1556. doi: 10.1007/s10067-015-2985-z [DOI] [PubMed] [Google Scholar]
- 23. Man A, Shojania K, Phoon C, et al. An evaluation of autoimmune antibody testing patterns in a Canadian health region and an evaluation of a laboratory algorithm aimed at reducing unnecessary testing. Clin Rheumatol. 2013;32(5):601‐608. doi: 10.1007/s10067-012-2141-y [DOI] [PubMed] [Google Scholar]
- 24. Verstegen G, Duyck MC, Meeus P, Ravelingien I, De Vlam K. Detection and identification of antinuclear antibodies (ANA) in a large community hospital. Acta Clin Belg. 2009;64(4):317‐323. doi: 10.1179/acb.2009.049 [DOI] [PubMed] [Google Scholar]
- 25. Yeo AL, Ojaimi S, Le S, Leech M, Morand E. Frequency and clinical utility of antibodies to extractable nuclear antigen in the setting of a negative antinuclear antibody test. Arthritis Care Res. 2023;75(7):1595‐1601. doi: 10.1002/acr.24990 [DOI] [PubMed] [Google Scholar]
- 26. Ruffatti A, Calligaro A, Del Ross T, et al. Anti‐double‐stranded DNA antibodies in the healthy elderly: prevalence and characteristics. J Clin Immunol. 1990;10(6):300‐303. doi: 10.1007/bf00917474 [DOI] [PubMed] [Google Scholar]
- 27. Edwards CJ, Syddall H, Jameson K, et al. The presence of anticardiolipin antibodies in adults may be influenced by infections in infancy. QJM. 2008;101(1):41‐47. doi: 10.1093/qjmed/hcm119 [DOI] [PubMed] [Google Scholar]
- 28. Biggioggero M, Meroni PL. The geoepidemiology of the antiphospholipid antibody syndrome. Autoimmun Rev. 2010;9(5):A299‐A304. doi: 10.1016/j.autrev.2009.11.013 [DOI] [PubMed] [Google Scholar]
- 29. Knight JS, Branch DW, Ortel TL. Antiphospholipid syndrome: advances in diagnosis, pathogenesis, and management. BMJ. 2023;380:e069717. doi: 10.1136/bmj-2021-069717 [DOI] [PubMed] [Google Scholar]
- 30. Tsouris Z, Liaskos C, Dardiotis E, et al. A comprehensive analysis of antigen‐specific autoimmune liver disease related autoantibodies in patients with multiple sclerosis. Auto Immun Highlights. 2020;11(1):7. doi: 10.1186/s13317-020-00130-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nordal GJ, Vandvik B. Evidence of local synthesis of smooth‐muscle antibodies in the central nervous system in isolated cases of multiple sclerosis and chronic lymphocytic meningoencephalitis. Scand J Immunol. 1977;6(4):327‐334. doi: 10.1111/j.1365-3083.1977.tb00401.x [DOI] [PubMed] [Google Scholar]
- 32. Nielsen SF, Bojesen SE, Schnohr P, Nordestgaard BG. Elevated rheumatoid factor and long term risk of rheumatoid arthritis: a prospective cohort study. BMJ. 2012;345:e5244. doi: 10.1136/bmj.e5244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berglin E, Mohammad AJ, Dahlqvist J, et al. Anti‐neutrophil cytoplasmic antibodies predate symptom onset of ANCA‐associated vasculitis. A case‐control study. J Autoimmun. 2021;117:102579. doi: 10.1016/j.jaut.2020.102579 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1. Supporting information.
APPENDIX S2. Supporting information.
Data Availability Statement
Anonymized data supporting the findings of this study are available from the corresponding author upon reasonable request by a qualified researcher and upon approval by the data‐clearing committee of the Medical University of Vienna.


