Abstract
Background and purpose
Limited options exist for migraine prevention after stopping anti‐calcitonin gene‐related peptide monoclonal antibodies. A systematic review examining the benefits of switching between different classes (ligand vs. receptor monoclonal antibody) is essential, alongside well‐designed real‐world studies.
Methods
In this cohort study 67 patients were included, who discontinued their first treatment with erenumab or fremanezumab. Patients (n = 31) switched to another monoclonal antibody class within 3 months, whilst those in the control group (n = 36) received standard care. Allocation to either group relied largely on the availability of alternative monoclonal antibody treatments, introducing pseudo‐random allocation. Changes in monthly migraine days were compared between groups 3 months post‐discontinuation of the first monoclonal antibody or initiation of a different monoclonal antibody class. A multivariate regression model was conducted that accounted for potential confounding factors.
Results
The groups were comparable at baseline and poor treatment response was the main reason for treatment discontinuation of the first monoclonal antibody. The switching cohort experienced a reduction of 3.9 monthly migraine days (95% confidence interval −6.4, −1.3, p = 0.004) compared with the control group.
Conclusion
Transitioning to a different anti‐calcitonin gene‐related peptide monoclonal class yields reduction in monthly migraine days compared to returning to standard care for patients with inadequate initial treatment response.
Keywords: calcitonin gene‐related peptide, migraine, monoclonal antibodies, narrative review, preventive treatment, refractory migraine, switch
INTRODUCTION
Monoclonal antibodies targeting calcitonin gene‐related peptide (CGRP‐mAbs) have demonstrated their effectiveness as prophylactic treatment for patients experiencing frequent migraine attacks [1, 2, 3]. CGRP‐mAbs are typically reserved as a final resort in preventive treatment options [4]. In the Netherlands and other European countries, CGRP‐mAbs are typically only considered after other preventive medications have been tried. These antibodies can be differentiated based on whether they target the CGRP ligand (fremanezumab, galcanezumab or eptinezumab) or its receptor (erenumab), effectively blocking the actions of CGRP [5, 6]. Despite these advancements in the treatment of migraine, uncertainty persists regarding the optimal course of action following the discontinuation of CGRP‐mAb treatment. Discontinuation frequently occurs due to factors such as insufficient response, adverse events or the emergence of new treatment contraindications [2, 7, 8, 9]. Most patients have tried various prophylactic treatment options, narrowing down the alternatives to a few: either returning to previous commonly used preventives which were the only option before the introduction of CGRP‐mAbs, or switching to an alternative CGRP‐mAb. Given the absence of supportive evidence for any of these treatment options, the current decision making is based on clinical expertise and experience or reimbursement possibilities [10].
Considering the high costs associated with CGRP‐mAb treatment, evaluating the potential benefits of switching to an alternative CGRP‐mAb is of great importance. Uncertainty regarding the additional benefits of switching prompts inquiries about the imperative to thoroughly evaluate all available CGRP‐mAb options for each patient. As all CGRP‐mAbs show consistent outcomes in trials and share similar pharmacokinetic and pharmacodynamic properties, it was suggested that switching might not provide an additional beneficial effect [1, 8, 10, 11, 12, 13]. Although there is a lack of robust scientific evidence, the European Headache Federation's guidelines consider switching to another CGRP‐mAb in certain cases to be a viable therapeutic option. This recommendation is supported by real‐world studies involving patients who experienced treatment failure with one CGRP‐mAb and subsequently switched to another [10, 14]. Despite their similarities, CGRP‐mAbs differ in key pharmacokinetic aspects, such as isoelectric points and administration routes (e.g., intravenous for eptinezumab). Additionally, there are slight differences in pharmacodynamic properties between CGRP‐mAbs targeting the receptor and those targeting the ligand, as well as variations in their affinity for other receptors, such as the amylin receptor (AMY1) [15]. This distinction implies a potential therapeutic benefit to switching to a different target for CGRP blockade [10]. Nevertheless, the evidence for switching remains constrained to a handful of case series and single‐arm cohort studies [16, 17, 18]. These studies suggested that both responders and non‐responders may experience improvements upon switching to an alternative CGRP‐mAb class. However, the inherent nature of these studies and the absence of a control group make it challenging to draw definite conclusions [10].
In this cohort study, the aim was to examine the effectiveness of switching to a second CGRP‐mAb following treatment discontinuation in comparison to standard care. Additionally, a systematic review of the existing literature on switching between CGRP‐mAb treatments was conducted.
METHOD
Literature search
An extensive literature search was performed (PubMed, Embase) up to August 2024 to find all evidence regarding the effectivity of switching CGRP‐mAbs. Papers were included that had a full text available, in English or Spanish, and reported effectivity in absolute change in monthly migraine days (MMDs) or monthly headache days (MHDs). The validity of the studies was estimated based on the following characteristics: clear description of study population and study design, inclusion of control group, definition of outcome measurement, a defined baseline measurement prior to switching, and comparison of follow‐up to baseline specified for a particular month versus an average across an entire treatment period [19].
Design
This observational cohort study was conducted at the Leiden Headache Centre, with data collected from November 2018 to April 2023. Patients who switched to a different CGRP‐mAb class—either from a receptor targeting or to a ligand targeting CGRP‐mAb, or vice versa—within 3 months of discontinuing their first CGRP‐mAb formed the intervention group. Patients who did not receive a second CGRP‐mAb but received standard care were the control group. Limited availability of CGRP‐mAbs at the time of treatment discontinuation created pseudo‐random allocation. Standard care involved either starting another preventive medication or no additional preventive treatment, based on shared decision making between the patient and physician. MMD changes between groups were compared after 3 months.
Standard protocol approvals, registrations and patient consents
The study is performed in accordance with the Declaration of Helsinki Ethical Principles and Good Clinical Practices and was approved by the local and national ethics committees (reference number 22‐3075).
Population of interest
All patients were diagnosed with migraine by a neurology resident or neurologist with headache expertise, based on the International Classification of Headache Disorders (ICHD) 3 criteria [20]. Patients treated with CGRP‐mAbs were monitored by a physician and headache nurse, who documented treatment initiation and discontinuation dates.
Eligibility required a minimum of two injections (at least 2 months of treatment) and a history of at least six MMDs and failure with four prophylactic migraine treatments, in line with CGRP‐mAb regulations in the Netherlands and compassionate use programmes. Patients could not have active medication overuse headache, as CGRP‐mAbs are currently not reimbursed for this condition in the Netherlands. Additional preventive migraine treatments were not allowed, as polypharmacy is not standard in the Netherlands. A baseline month of e‐diary data, covering the 28 days before discontinuation of the first CGRP‐mAbs, was required, with at least 60% compliance to ensure accurate imputation. Patients with <60% compliance at 3 months were classified as lost to follow‐up.
Study procedure
Eligible patients started treatment with erenumab (70 mg, increasing to 140 mg after 3 months), fremanezumab (225 mg monthly) or galcanezumab (240 mg initially, then 120 mg every 4 weeks). Eptinezumab was not available in the Netherlands during the study. The first injection was supervised by a physician or headache nurse, whilst subsequent doses were self‐administered. Patients at the Leiden Headache Centre used a validated e‐diary to monitor treatment, recording daily headache presence, characteristics, symptoms and medication use A validated algorithm classified each day as a migraine, headache or non‐headache day. A headache day was defined by a headache lasting ≥1 h or requiring acute medication, whilst migraine days met the ICHD‐3 criteria [21, 22].
All endpoints were defined as the mean change from baseline (days −28–0) in a 28‐day period at the 3‐month mark. A review of medical records established baseline characteristics, treatment dates, reasons for treatment discontinuation of the first CGRP‐mAb, and, for the control group, reasons for not starting a second CGRP‐mAb within 3 months. Reasons were categorized as (1) unavailability of other CGRP‐mAbs, (2) contraindications or adverse events, (3) logistic delays, (4) personal reasons, (5) adjusted treatment indications for CGRP‐mAbs. Other outcomes included days of acute medication (migraine‐specific and non‐migraine‐specific medication), pain coping ability (0–10 scale) and general well‐being (0–100 scale).
A responder was defined as having a ≥50% reduction in MMDs for episodic migraine or ≥30% for chronic migraine [23, 24]. The proportion of responders was reported. If a patient did not meet the responder criteria, the reason for treatment discontinuation was labelled ‘poor treatment response’. The decision to discontinue the first CGRP‐mAb treatment was based upon the reduction of MMDs and shared decision making between the patient and treating physician.
Statistical analysis
Baseline characteristics were summarized using means, standard deviations, medians and interquartile ranges. Comparisons between the intervention and control group at baseline were performed using an unpaired t test for continuous variables, a chi‐squared test for categorical data and a Mann–Whitney U test for non‐normally distributed data.
The primary outcome was the mean change in MMDs from baseline to the third month after discontinuing the first CGRP‐mAb (control group) or switching to a second CGRP‐mAb (intervention group). Prespecified secondary outcomes included change in MHDs, days of acute medication or triptan use, and changes in pain coping and general well‐being. Subgroup analysis assessed MMD changes in chronic and episodic migraine patients. A multivariable linear regression model compared changes in primary and secondary outcomes between groups. For left‐skewed general well‐being data, a squared root transformation was applied, with estimates back‐transformed to the original scale. Covariates were pre‐selected based upon background knowledge (Figure S1). Continuous covariates in the model were age and baseline MMDs, whilst categorical predictors included sex, insufficient response to the first CGRP‐mAb and the first administered CGRP‐mAb. The power calculation showed that, with 45 patients per group, assuming a true difference of 3.6 MMDs and a standard deviation of 6.02, the study would have 80% power (α = 0.05, two‐sided) [2, 25]. Missing e‐diary days were imputed using ratio imputation based on the follow‐up month. The proportion of responders was assessed in the switcher and control groups 3 months after the switch, compared with the period before initiating the first CGRP‐mAb. Additionally, the change in MMDs from baseline to follow‐up in both the switcher and control group was estimated.
Multiple sensitivity analyses were conducted to ensure data robustness. First, MMDs in the switcher group at 3 months were compared to baseline MMDs in the control group, excluding the effect of stopping the first CGRP‐mAb. Second, the effect direction was tested by excluding controls who did not switch due to external factors. Third, only controls who received preventive treatment after stopping the first CGRP‐mAb were included. Lastly, patients in the standard care group who did not switch within 3 months due to contraindications or adverse events were excluded.
RESULTS
Baseline characteristics
In total, 269 patients were initially treated with CGRP‐mAbs, of whom 168 (62%) continued and 101 (38%) patients discontinued CGRP‐mAb treatment. In total 89 patients were initially eligible, of whom 15 patients from the control group were lost to follow‐up. Additionally, six patients had more than 10 missing days during the follow‐up period, with five of these patients belonging to the switcher group. One patient did not switch CGRP‐mAb class and was therefore excluded. As a result, a final inclusion of 67 patients was achieved, with the switcher group accounting for n = 31 and the control group for n = 36 (Figure 1). Baseline characteristics of the included patients are presented in Table 1, which were similar to baseline characteristics in the initial sample including the patients lost to follow‐up (Table S1).
FIGURE 1.
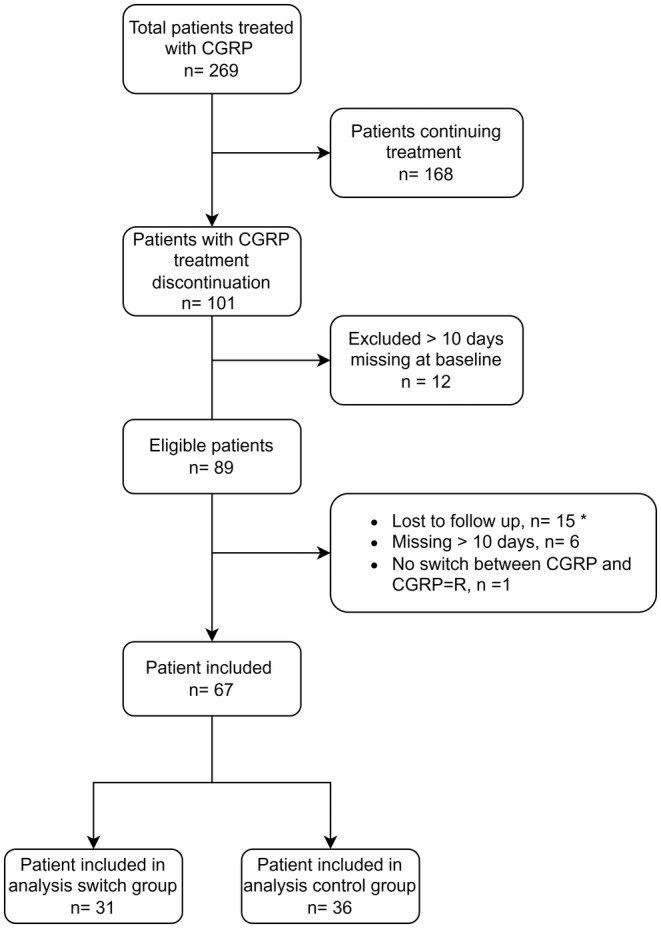
Flowchart screening eligible patients. *All patients completely lost to follow‐up belonged to the control group. Abbreviation: CGRP‐L, anti‐calcitonin gene‐related peptide against the ligand; CGRP‐R, anti‐calcitonin gene‐related peptide against the receptor.
TABLE 1.
Baseline characteristics of the intervention group, patients switching to another anti‐CGRP‐mAb treatment, and controls receiving standard care.
| Switchers | Controls | p value | |
|---|---|---|---|
| Number of patients, n | 31 | 36 | |
| Age (years), mean ± SD | 41 (14) | 43 (12) | 0.503 |
| Sex (female), mean ± SD | 23 (74) | 32 (89) | 0.213 |
| BMI (kg/m2), mean ± SD | 24.9 (5.5) | 25.0 (4.8) | 0.933 |
| Migraine with aura, n (%) | 12 (39) | 21 (58) | 0.175 |
| Chronic migraine, n (%) | 15 (48) | 18 (50) | 1.000 |
| History of medication overuse headache, n (%) | 15 (48) | 21 (58) | 0.570 |
| Tension headache, n (%) | 13 (42) | 12 (33) | 0.637 |
| First CGRP medication a | |||
| Fremanezumab, n (%) | 14 (45) | 12 (33) | 0.460 |
| Erenumab, n (%) | 17 (55) | 24 (66) | |
| Days first CGRP were given, median ± IQR | 236 [168, 392] | 229 [152, 354] | 0.734 |
| Days between first and second CGRP‐mAbs, median ± IQR | 33 (29, 55) | – | – |
| Reasons treatment discontinuation | |||
| Poor treatment response, n (%) | 30 (97) | 28 (78) | 0.050 |
| Adverse events, n (%) | 1 (3.2) | 6 (16.7) | |
| Desire for pregnancy, n (%) | 0 (0.0) | 2 (5.6) | |
| MMD baseline, mean ± SD | 13.3 (6.8) | 13.6 (7.0) | 0.848 |
| MHD baseline, mean ± SD | 17.6 (7.7) | 17.4 (7.9) | 0.920 |
| Acute medication days baseline, mean ± SD | 5.7 (3.4) | 4.8 (2.9) | 0.298 |
| Completed diaries at baseline, median ± IQR | 28 (27–28) | 28 (27–28) | 0.698 |
| MMD baseline (imputed) b | 13.7 (6.8) | 14.2 (7.30) | 0.802 |
| MHD baseline(imputed) b | 18.2 (7.8) | 18.1 (8.1) | 0.957 |
Note: Comparisons between intervention and control group at baseline were performed using an unpaired t test for continuous variables, a chi‐squared test for categorical data and a Mann–Whitney U test for non‐normally distributed data.
Abbreviations: BMI, body mass index; CGRP, anti‐calcitonin gene‐related peptide; CGRP‐mAb, anti‐calcitonin gene‐related peptide monoclonal antibody; IQR, interquartile range; MHD, monthly headache day; MMD, monthly migraine day.
In this sample patients started with either fremanezumab or erenumab.
A ratio imputation was performed for patients missing ≤10 days at baseline (in total 3.6%).
Both groups were comparable at baseline, with 74% of the switcher group and 89% of the control group being female. Chronic migraine diagnoses were similar: 48% in the switcher group and 50% in the control group. Median treatment duration for the first CGRP‐mAb was 236 days (interquartile range 168–392) for switchers and 229 days (interquartile range 152–354) for controls. Poor treatment response was the reason for discontinuation in 97% of switchers and 78% of controls. In total 65 patients received a minimum of three injections of the first CGRP‐mAb treatment. Two patients received only two injections of the first CGRP‐mAb, as the treatment was discontinued due to side effects. Overall, there were no significant baseline differences between the groups.
Table 2 presents an overview, outlining the reasons from control patients for not switching to a second CGRP‐mAb within the 3‐month period. In the control group, 33% received additional preventive treatment as part of the standard of care (Table 2). All switchers received both CGRP‐L (ligand) and CGRP‐R (receptor) as preventive medication (Table S2). In total, 96% (n = 55) of the patients treated with erenumab received a dose of 140 mg. However, two patients discontinued erenumab treatment before the dose increment due to the occurrence of side effects.
TABLE 2.
Overview of reasons for control group (n = 36) refraining from transitioning to a second CGRP‐mAb within a 3‐month period.
| Control group | |
|---|---|
| Number of patients, n | 36 |
| Reasons for not switching to another CGRP‐mAb | |
| Other CGRP‐mAb was not available, n (%) | 11 (30.6) |
| Contraindication/adverse events, n (%) | 10 (27.8) |
| Treatment delay due to logistics, n (%) | 7 (19.4) |
| Treatment delay due to personal reasons, n (%) | 7 (19.4) |
| Change in treatment indication, n (%) | 1 (2.8) |
| Standard of care without medication, n (%) | 24 (67) |
| Standard of care with medication, n (%) a | 12 (33) |
| Candesartan, n | 3 |
| Amitriptyline, n | 2 |
| Flunarizine, n | 1 |
| Topiramate, n | 2 |
| Pizotifen, n | 1 |
| Botulinum toxin, n | 2 |
| Lamotrigine, n | 1 |
Abbreviation: CGRP‐mAb, anti‐calcitonin gene‐related peptide monoclonal antibody.
Preventive medication treatment given after withdrawal of the initial CGRP‐mAb treatment.
Primary analysis
At baseline patients in the switcher group reported mean (SD) MMDs of 13.7 (6.8) and the control group 14.2 (7.3). At follow‐up the switchers had a mean (SD) of 11.9 (6.6) MMDs and the control group 16.1 (7.5). The switcher group had a greater reduction in MMDs, −3.87 MMDs (95% confidence interval [CI] −6.41, −1.33; p = 0.004), from baseline to 3 months follow‐up compared with the control group (Table 3, Figure 2 and Table S3).
TABLE 3.
Primary and secondary outcomes.
| Control | Switcher | Difference (95% CI) a | p value* | |
|---|---|---|---|---|
| Primary endpoint | (n = 36) | (n = 31) | ||
| ∆ Monthly migraine days mean ± SD | 2.0 (3.6) | −1.8 (6.4) | −3.9 (−6.4, −1.3) | 0.004 |
| Secondary endpoint | ||||
| ∆ Monthly headache days mean ± SD | 1.4 (3.2) | −0.5 (4.9) | −1.9 (−4.0, 0.13) | 0.07 |
| Chronic migraine | (n = 18) | (n = 16) | ||
| ∆ Monthly migraine days mean ± SD | 1.3 (4.3) | −3.7 (7.5) | −4.56 (−9.1, −0.03) | 0.06 |
| Episodic migraine | (n = 18) | (n = 15) | ||
| ∆ Monthly migraine days mean ± SD | 2.6 (2.9) | 0.2 (4.3) | −2.66 (−5.5, 0.20) | 0.20 |
Note: Data are mean (SD).
Abbreviation: CI, confidence interval.
Difference in least‐squares mean from control group.
p values are for the control group versus switcher group, p‐value < 0.05 was considered statistically significant.
FIGURE 2.
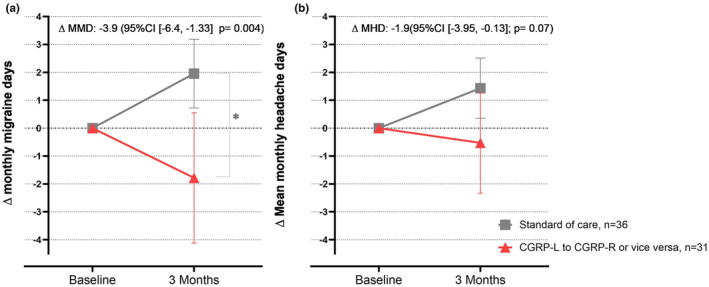
(a) ∆ Mean monthly migraine days and (b) ∆ mean monthly headache days with 95% CI from baseline and 3 months’ follow‐up in the switcher (n = 31) and control group (n = 36).
Secondary analysis
At baseline, the switcher group reported mean (SD) MHDs of 18.2 (7.8) and the control group 18.1 (8.1). At follow‐up, the switcher group had a mean (SD) of 17.7 (7.8) MHDs and the control group 19.5 (7.5). There was no difference in MHDs between groups from baseline to 3 months, with −1.91 MHDs in the switcher group compared to the control group (95% CI −3.95, −0.13; p = 0.072) (Tables 3 and S5). In the switcher group, 32.2% were responders to the second CGRP‐mAbs: 25.0% of chronic migraine patients (≥30% reduction in MMDs) and 13.3% of episodic migraine patients (≥50% reduction in MMDs). In the control group, 13.9% were responders, including 11.1% of chronic and 5.6% of episodic migraine patients.
There was no difference in acute medication days between the switcher and control groups. The mean change in pain coping increased by 0.5 points in the switcher group (95% CI 0.05, 0.85; p = 0.03) compared to the control group. The switcher group experienced an increase in general well‐being, with a mean change of 2.3 points (95% CI 0.5, 4.9; p = 0.01) compared to the control group (Table 4).
TABLE 4.
Linear regression with change in acute medication, triptans, pain coping and general well‐being at 3 months in patients switching to a second CGRP‐mAb (n = 31) and controls (n = 36).
| Switchers (n = 31) | Controls (n = 36) | Difference (95% CI) a | p value* | |
|---|---|---|---|---|
| ∆ Acute medication days, mean ± SD | −1.2 (2.7) | −0.03 (2.6) | −0.4 (−1.6, 2.8) | 0.48 |
| ∆ Triptan days, mean ± SD | −0.94 (2.05) | 0.06 (2.06) | −0.4 (−1.3, 0.6) | 0.46 |
| ∆ Pain coping b , mean ± SD | 0.24 (0.74) | −0.22 (0.75) | 0.5 (0.05, 0.85) | 0.03 |
| ∆ General well‐being c , median ± IQR | 0.5 (4.5) | −1.7 (3.5) | 2.3 (0.5, 4.9) | 0.01 |
Abbreviations: CGRP‐mAb, anti‐calcitonin gene‐related peptide monoclonal antibody; CI, confidence interval; IQR, interquartile range.
Difference in least‐squares mean from control group.
Scored on a 0–10 visual analogue scale, with 0,very bad; 10, very good.
Scored on a 0–100 visual analogue scale, with 0, very bad; 100, very good.
p values are for the control group versus switcher group. p‐value < 0.05 was considered statistically significant.
Sensitivity analyses
First, compared with the control baseline, the direction and magnitude of the effect were similar to the primary analysis (−3.34 MMDs, 95% CI −6.96, 0.25; p = 0.07; Table S8). There was no difference from baseline to 3 months in the switcher group (−1.95 MMDs, 95% CI −4.19, 0.30) or the control group (1.95 MMDs, 95% CI 0.72, 3.18).
Second, excluding controls who did not switch for random (external) reasons showed a similar direction and effect magnitude as the primary analysis (−4.3 MMD reduction, 95% CI −7.0, −1.5; p = 0.003) (Table S4). Third, including only controls receiving additional preventive treatment yielded a similar direction and effect magnitude (−3.3 MMDs, 95% CI −7.3, 0.7; p = 0.12) (Figure S2 and Table S6). Last, excluding controls who did not switch due to adverse events or contraindications showed a similar direction and effect magnitude of the effect (−3.96 MMDs, 95% CI −6.79, −1.12; p = 0.009) (Table S7).
Literature search
A total of 14 papers were identified and summarized (Table 5), 13 single‐arm retrospective cohort studies and one case report. None of the studies included a control group. Six cohort studies investigated MMDs, of which five reported a reduction of 5.0–6.9 MMDs at 3 or 6 months for patients who switched CGRP‐mAbs, with two involving class switchers and four involving both between‐class and within‐class switches. One small study (n = 20) reported a slight increase in MMDs following a within‐class switch; in contrast one study reported a reduction of 3.7 days in subgroup (n = 18) analysis of within‐class switches. Three cohort studies investigated MHDs and reported a reduction of 2.5–4.1 MHDs at 3 months for patients switching between CGRP‐mAb classes. Three studies classified 28.5%–50% of the switchers as responders, but it is unknown what kind of switching was included.
TABLE 5.
Narrative review: summary table.
| Reference | Design | Control group | N (total) | N* | Population | Intervention (n) | Baseline | Outcome (switch analyses) | Measurement of outcome | Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Suliman et al. (2024) [32] | Cohort | No | 53 | 53 | CM, EM a , b , c | ere‐gal ere‐epti gal‐epti | Before switch | Response rate at 3 and 6 months in MMDs (EM ≥ 50%, CM ≥ 30% reduction) | Headache diaries and electronic medical records (not specified) | Decrease of 5.0 MMDs IQR 5 at 6 months |
| Hong et al. (2024) [33] | Cohort | No | 655 | 135 | CM, EM a , b , c | ere‐fre ere‐gal gal‐fre | Before initiating first CGRP‐mAb | Response rate ≥ 50% MMDs at 3 and 6 months. MIDAS and HIT‐6 at 6 months | Clinical visits with self‐reported migraine days |
At 3 months ≥ 50% response was 33% and 41% at 6 months No statistical analysis was performed for the MIDAS and HIT‐6 |
| Month of 28 days | ||||||||||
| Talbot et al. (2024) [34] | Cohort | No | 54 | 54 | CM | Not specified | Before switch | Response at 3 months (CM ≥ 30% reduction) | Headache diary (not specified) | At 3 months n = 22 responders (33%) |
| Ihara et al. (2023) [35] | Cohort | No | 20 | 15 | CM, EM a | gal‐fre | Before switch | Change MMDs at 4 months | Headache diary (not specified) | Increase of 0.7 MMDs (95% CI −4.1, –5.5) |
| Number of days in month not given | ||||||||||
| Suzuki et al. (2023) [36] | Cohort | No | 110 | 35 | CM, EM a , b | gal‐fre (23) | Before switch | Change MMDs at 3 and 6 months | Headache diary (not specified) | At 3 months, reduction ~6 MMDs |
|
ere‐fre (4) |
Number of days in month not given | At 6 months, reduction ~7 MMDs | ||||||||
| ere + gal‐fre (8) | (95% CI not given, deduced from figure) | |||||||||
| Lambru et al. (2023) [12] | Cohort | No | 39 | 39 | CM c | ere‐fre | Before switch | Change MMDs at 3 and 6 months | Headache diary (not specified) |
At 3 months, reduction of 6.5 MMDs At 6 months, reduction 12.9 MMDs |
| Month of 30 days | (95% CI not given) | |||||||||
| Straube et al. (2023) [14] | Cohort | No | 153 | 138 | CM, EM b | gal‐fre (16) | Before switch | Change MMDs at 3 months | Headache diary (not specified) | At 3 months, reduction of 6.4 MMDs |
| ere‐gal (145) | Month of 28 days | (95% CI not given) | ||||||||
| ere + gal‐fre (8) | ||||||||||
| Kaltseis et al. (2023) [26] | Cohort | No | 196 | 34 | CM, EM a , b | Not specified | Not specified | Response rate at 3 months (EM ≥ 50%, CM ≥ 30% reduction) | Headache diary (not specified) | At 3 months n = 17 responders (50%) |
| Number of days in month not given | (Absolute reduction not given) | |||||||||
| Overeem et al. (2023) [27] | Cohort | No | 29 | 20 (at 6 months 14) | CM, EM c |
gal‐ere (14) fre‐ere (6) |
Before switch | Change MHDs at 3 and 6 months | Headache diary (not specified) |
At 3 months, reduction of 4.1 MHDs At 6 months, reduction of 7.0 MHDs |
| Month of 28 days | (95% CI not given) | |||||||||
| Iannone et al. (2023) [28] | Cohort | No | 31 | 22 | CM, EM |
Ligand–receptor (110) Receptor– ligand (11) |
Before switch | Change in MHDs at 3 months | Paper headache diary | At 3 months, reduction of 2.5 MHDs (95% CI not given) |
| Months of 30 days | ||||||||||
| López‐Moreno et al. (2022) [37] | Cohort | No | 14 | 14 | CM, EM c |
ere‐gal (9) gal‐ere (4) ere‐fre (1) |
Not specified | Response rate d | Not specified | At 3 months 28.5% were responders |
| Overeem et al. (2022) [16] | Cohort | No | 78 | 25 | CM c | ere‐gal (12) | Before switch | Change in MHDs at 3 months | Unstandardized headache diary | At 3 months, reduction of 3.0 MHDs (95% CI not given) |
| ere‐fre (13) | Month of 28 days | |||||||||
| Ziegeler and May (2020) [18] | Casereport | No | 3 | 3 | CM, EM c | ere‐gal | Before switch | Change in MHDs at 3 months | Not specified | At 3 months, reduction of 12.7 MHDs (95% CI not given) |
| Ruiz et al. (2022) [17] | Cohort | No | 30 | 15 | CM, EM c | ere‐gal | Not specified | Mean change over 3 months | Not specified | At 3 months, reduction of 6.9 MMDs (95% CI not given) |
Note: The study designs were classified based upon epidemiology guidelines, to distinguish between case series and retrospective cohort studies [17]. N* is the number of patients included in switch analysis.
Abbreviations: CI, confidence interval; CM, chronic migraine; EM, episodic migraine, epti , eptinezumab; ere, erenumab; fre, fremanezumab; gal, galcanezumab; HIT‐6, Headache Impact Test; IQR, interquartile range; MHDs, monthly headache days; MIDAS, Migraine Disability Assessment Test; MMDs, monthly migraine days.
Medication overuse headache not excluded.
Psychiatric disorders not excluded.
Inclusion and exclusion criteria comorbidities not specified.
Defined as reduction in MMDs or MHDs >50% or 5‐point reduction on HIT‐6 or, if baseline was 11–20, a 5‐point reduction on MIDAS or a reduction of >30% if baseline score is >20.
DISCUSSION
The findings of this controlled cohort study demonstrated a higher effectiveness of switching to another CGRP‐mAb class after treatment discontinuation of the first CGRP‐mAbs, in comparison with standard care without CGRP‐mAbs at 3 months. A reduction of −3.9 (95% CI [‐6.41, ‐1.33]) MMDs from baseline to 3 months’ follow‐up was found in the switcher group compared with the control group. An ineffective treatment response to the first CGRP‐mAbs was the most frequent reason for treatment discontinuation, 97% in the switcher group and 78% in the control group. Notably, the switcher group demonstrated an increase in pain coping and an improvement in general well‐being compared to the control group. Switching to another CGRP‐mAb class can be effective for patients who have failed their first anti‐CGRP‐mAb treatment [10, 26].
A cohort study with pseudo‐randomization is presented, where treatment allocation is primarily determined by time, as shown in Table 2. This approach minimizes confounding by indication and allows exploration of the potential benefits of switching to an alternative CGRP‐mAb class. Our findings align with previous research, supporting the benefit of switching to an alternative CGRP‐mAb class after treatment discontinuation due to inadequate response [12, 13, 16, 17, 18, 26, 27, 28]. Notably, earlier studies lacked a control group, making our study a stronger basis for substantiating the effect of switching. The response rate after switching in our study was 32.3%, consistent with previous research (Table S1).
A strength of this study is its observational design, which allows for examination of the effect of switching to an alternative CGRP‐mAb class compared with a control group in a real‐world setting, enhancing generalizability of the results. Additionally, the inclusion of a control group enabled a more accurate estimation of the true effect of switching, as it accounts for natural variation and helps mitigate the regression to the mean phenomenon. Participants were restricted from using concurrent preventive medication, enabling an assessment of the isolated effect of switching CGRP‐mAb classes. Additional strengths include sensitivity analyses that (1) considered only controls with independent reasons for not switching, (2) included only controls receiving preventive medication as standard care and (3) excluded controls who did not switch due to adverse events or contraindications. These analyses demonstrated that the direction and magnitude of the effect remained unchanged, further supporting the findings. Lastly, prospective daily measurements recorded in a validated e‐diary, along with an algorithm for monitoring MMDs and MHDs, minimized potential sources of measurement error, misclassification and recall bias [21, 22].
Whilst this study provides insights into switching to an alternative CGRP‐mAb class, it has limitations. First, some patients, mainly in the control group, were lost to follow‐up due to their treatment allocation, as they no longer received care in our outpatient clinic. Consequently, missing e‐diary data are probably unrelated to study outcomes. Although baseline characteristics were comparable between included participants and those lost to follow‐up, this loss reduced the sample size and may have widened the 95% CIs. Second, the required sample size was 45 patients per group, which was not achieved, suggesting the study may have been underpowered; however, a statistically significant difference was still found. Third, the heterogeneous control group, which included all non‐CGRP‐mAb interventions, is a limitation to this study. Fourth, the varied timing of follow‐up measurements between the switcher and control groups may have underestimated the benefits of switching to an alternative CGRP‐mAb class. Fifth, treatment with CGRP‐mAbs is only reimbursed in the Netherlands if patients have no active medication overuse headache, which limits the generalizability of the results of this study to patients with migraine diagnosis without active medication overuse headache. Sixth, the reasons for discontinuation of the first CGRP‐mAbs for poor treatment response were unbalanced; therefore this has been added to the model as a covariate. However, as depicted in Figure S1, the occurrence of adverse events is unlikely to have influenced the primary outcome. Finally, the lack of blinding in treatment allocation may have biased the results towards the CGRP‐mAb group.
In our study, the control group experienced an MMD increase post‐discontinuation of the first CGRP‐mAbs, whilst the switcher group experienced a decrease. To assess switching's effect independently of MMD changes post‐discontinuation, a sensitivity analysis was conducted. Comparing the switcher group to the control group's baseline, corresponding to the last 28 days of the initial CGRP‐mAb treatment, showed a −3.34 MMD reduction (95% CI −6.96, 0.25), not statistically significant but with an effect estimate that is considerable (3.34 MMDs). The response rate after switching (32.2%) suggested positive outcomes for many patients.
The interpretation of our study extends beyond the outcomes to explore potential mechanisms behind these effects. All switcher group patients alternated between the CGRP receptor (erenumab) and CGRP ligand monoclonal antibodies (fremanezumab or galcanezumab), in both orders. The observed effects may arise from differences between the interchanged CGRP‐mAb classes as, despite targeting the same pathway, their mechanisms of action vary due to differences in signalling and intracellular pathways associated with receptor and ligand binding [29].
A distinction between receptor and ligand monoclonal antibodies is erenumab' s ability to antagonize AMY1, possibly through its interaction with receptor activity modifying proteins (RAMP1), a component of the amylin receptor [29, 30, 31]. Erenumab, unlike fremanezumab, exhibits internalization at both the RAMP1 and AMY1 receptors [29]. The role of amylin receptor in migraine pathophysiology is speculative, but the AMY1 receptor may contribute to erenumab's mechanism of action, and our findings provide some support for this hypothesis [12]. Since CGRP is part of the calcitonin family of peptides, the effect of CGRP probably extends beyond the CGRP receptor, suggesting that binding ligands may have additional effects [30]. The differences in the modes of action of the two CGRP‐mAb classes might explain the differences in efficacy and tolerability in patients with migraine [8, 10, 13, 18, 29]. Another explanation for the efficacy differences in switching might be the sustained antagonism of the CGRP pathway. However, as both groups were first treated with CGRP‐mAbs for nearly a year, this may not fully explain the differences found between the two groups.
Based on our findings and the different mechanisms of action at the cellular level, switching to an alternative CGRP‐mAb class, receptor or ligand, may benefit patients with limited responses to their first CGRP‐mAb treatment. Two meta‐analyses have suggested similar effectiveness across classes, suggesting that the sequence of CGRP‐mAb treatment may not impact the effect of switching [6, 11].
Considering our results and prior published research, switching to an alternative CGRP‐mAb class can be considered for patients who demonstrated insufficient response to their first CGRP‐mAb treatment and continue to experience frequent MMDs. Clinicians are recommended to consider this option for these patients.
AUTHOR CONTRIBUTIONS
Nancy van Veelen: Conceptualization; methodology; formal analysis; project administration; writing – original draft; data curation. Britt W. H. van der Arend: Writing – review and editing; conceptualization; formal analysis. E. Hiele: Methodology; data curation; project administration; writing – review and editing. E. W. van Zwet: Methodology; conceptualization; writing – review and editing. Gisela M. Terwindt: Supervision; writing – review and editing; conceptualization.
FUNDING INFORMATION
No funding to report.
CONFLICT OF INTEREST STATEMENT
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: B.W.H. van der Arend reports independent support from the Dutch Brain Foundation and the Dutch Brain Council. G.M. Terwindt reports consultancy or industry support from Novartis, Lilly and Teva, Allergan/Abbvie, Lundbeck, Pfizer, Interactive Studios and independent supportfromtheEuropeanCommunity,DutchHeart Foundation, Dutch Research Council, Dutch Brain Foundation, and Dioraphte. N. van Veelen, E. Hiele, B.W.H. van der Arend and E.W. van Zwet have no COI to report.
Supporting information
Figure S1. Directed acyclic graph. The red dots represent confounding factors and the red lines a biased path. The green dots represent exposures and the green line a causal path. The blue dots represent the outcome. All the red dots will be included in the data analysis as covariates, as they represent potential confounders.
Figure S2. ∆Mean monthly migraine days with 95% CI from baseline and 3 months’ follow‐up in the switcher (n = 31), control group (n = 12) with initiation of another preventive treatment and the control group (n = 24) without preventive treatment in the follow‐up.
Figure S3. Violin plot depicting the distribution of the total number of days the first CGRP‐mAbs were administered. The dashed line within the violin depicts the median. The solid lines above and below the dashed line represent the 25th and 75th quartiles, respectively.
Table S1–S12.
van Veelen N, van der Arend BWH, Hiele E, van Zwet EW, Terwindt GM. Switching from ligand to receptor anti‐calcitonin gene‐related peptide (CGRP) antibodies or vice versa in non‐responders: A controlled cohort study. Eur J Neurol. 2025;32:e16542. doi: 10.1111/ene.16542
Nancy van Veelen and Britt W.H. van der Arend are shared first authors.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on reasonable request from the corresponding author. Results will be communicated to participants, healthcare professionals, the public and other relevant groups through publication.
REFERENCES
- 1. Huang IH, Wu PC, Lin EY, Chen CY, Kang YN. Effects of anti‐calcitonin gene‐related peptide for migraines: a systematic review with meta‐analysis of randomized clinical trials. Int J Mol Sci. 2019;20(14): 3527. doi: 10.3390/ijms20143527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Vries Lentsch S, Verhagen IE, van den Hoek TC, MaassenVanDen Brink A, Terwindt GM. Treatment with the monoclonal calcitonin gene‐related peptide receptor antibody erenumab: a real‐life study. Eur J Neurol. 2021;28(12):4194‐4203. doi: 10.1111/ene.15075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verhagen IE, de Vries LS, van der Arend BWH, le Cessie S, MaassenVanDen Brink A, Terwindt GM. Both perimenstrual and nonperimenstrual migraine days respond to anti‐calcitonin gene‐related peptide (receptor) antibodies. Eur J Neurol. 2023;30(7):2117‐2121. doi: 10.1111/ene.15794 [DOI] [PubMed] [Google Scholar]
- 4. Eigenbrodt AK, Ashina H, Khan S, et al. Diagnosis and management of migraine in ten steps. Nat Rev Neurol. 2021;17(8):501‐514. doi: 10.1038/s41582-021-00509-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ashina M, Terwindt GM, Al‐Karagholi MA, et al. Migraine: disease characterisation, biomarkers, and precision medicine. Lancet. 2021;397(10283):1496‐1504. doi: 10.1016/s0140-6736(20)32162-0 [DOI] [PubMed] [Google Scholar]
- 6. Haghdoost F, Puledda F, Garcia‐Azorin D, Huessler EM, Messina R, Pozo‐Rosich P. Evaluating the efficacy of CGRP mAbs and gepants for the preventive treatment of migraine: a systematic review and network meta‐analysis of phase 3 randomised controlled trials. Cephalalgia. 2023;43(4):3331024231159366. doi: 10.1177/03331024231159366 [DOI] [PubMed] [Google Scholar]
- 7. Ornello R, Baraldi C, Guerzoni S, et al. Comparing the relative and absolute effect of erenumab: is a 50% response enough? Results from the ESTEEMen study. J Headache Pain. 2022;23(1):38. doi: 10.1186/s10194-022-01408-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kanaan S, Hettie G, Loder E, Burch R. Real‐world effectiveness and tolerability of erenumab: a retrospective cohort study. Cephalalgia. 2020;40(13):1511‐1522. doi: 10.1177/0333102420946725 [DOI] [PubMed] [Google Scholar]
- 9. de Vries LS, van der Arend BWH, de Boer I, van Zwet EW, MaassenVanDenBrink A, Terwindt GM. Depression and treatment with anti‐calcitonin gene related peptide (CGRP) (ligand or receptor) antibodies for migraine. Eur J Neurol. 2024;31(2):e16106. doi: 10.1111/ene.16106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sacco S, Amin FM, Ashina M, et al. European Headache Federation guideline on the use of monoclonal antibodies targeting the calcitonin gene related peptide pathway for migraine prevention—2022 update. J Headache Pain. 2022;23(1):67. doi: 10.1186/s10194-022-01431-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Messina R, Huessler EM, Puledda F, Haghdoost F, Lebedeva ER, Diener HC. Safety and tolerability of monoclonal antibodies targeting the CGRP pathway and gepants in migraine prevention: a systematic review and network meta‐analysis. Cephalalgia. 2023;43(3):3331024231152169. doi: 10.1177/03331024231152169 [DOI] [PubMed] [Google Scholar]
- 12. Lambru G, Caponnetto V, Hill B, et al. Long‐term effect of switching from an anti‐CGRP receptor to an anti‐CGRP ligand antibody in treatment‐refractory chronic migraine: a prospective real‐world analysis. Neurotherapeutics. 2023;20:1284‐1293. doi: 10.1007/s13311-023-01394-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Straube A, Broessner G, Gaul C, et al. Real‐world effectiveness of fremanezumab in patients with migraine switching from another mAb targeting the CGRP pathway: a subgroup analysis of the finesse study. J Headache Pain. 2023;24(1):59. doi: 10.1186/s10194-023-01593-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wells‐Gatnik WD, Martelletti P. Switching CGRP(r) MoAbs in migraine: what evidence? Expert Opin Biol Ther. 2024;24(5):327‐333. doi: 10.1080/14712598.2024.2354386 [DOI] [PubMed] [Google Scholar]
- 15. Al‐Hassany L, Goadsby PJ, Danser AHJ, MaassenVanDenBrink A. Calcitonin gene‐related peptide‐targeting drugs for migraine: how pharmacology might inform treatment decisions. Lancet Neurol. 2022;21(3):284‐294. doi: 10.1016/s1474-4422(21)00409-9 [DOI] [PubMed] [Google Scholar]
- 16. Overeem LH, Peikert A, Hofacker MD, et al. Effect of antibody switch in non‐responders to a CGRP receptor antibody treatment in migraine: a multi‐center retrospective cohort study. Cephalalgia. 2022;42(4–5):291‐301. doi: 10.1177/03331024211048765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patier Ruiz I, Sánchez‐Rubio Ferrández J, Cárcamo Fonfría A, Molina GT. Early experiences in switching between monoclonal antibodies in patients with nonresponsive migraine in Spain: a case series. Eur Neurol. 2022;85(2):132‐135. doi: 10.1159/000518899 [DOI] [PubMed] [Google Scholar]
- 18. Ziegeler C, May A. Non‐responders to treatment with antibodies to the CGRP‐receptor may profit from a switch of antibody class. Headache. 2020;60(2):469‐470. doi: 10.1111/head.13729 [DOI] [PubMed] [Google Scholar]
- 19. Dekkers OM, Egger M, Altman DG, Vandenbroucke JP. Distinguishing case series from cohort studies. Ann Intern Med. 2012;156(1 Pt 1):37‐40. doi: 10.7326/0003-4819-156-1-201,201,030-00006 [DOI] [PubMed] [Google Scholar]
- 20. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders 3rd edition. Cephalalgia. 2018;38(1):1‐211. doi: 10.1177/0333102417738202 [DOI] [PubMed] [Google Scholar]
- 21. van der Arend BWH, Verhagen IE, van Leeuwen M, van der Arend M, van Casteren DS, Terwindt GM. Defining migraine days, based on longitudinal E‐diary data. Cephalalgia. 2023;43(5):3331024231166625. doi: 10.1177/03331024231166625 [DOI] [PubMed] [Google Scholar]
- 22. van Casteren DS, Verhagen IE, de Boer I, et al. E‐diary use in clinical headache practice: a prospective observational study. Cephalalgia. 2021;41(11–12):1161‐1171. doi: 10.1177/03331024211010306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tassorelli C, Diener HC, Dodick DW, et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia. 2018;38(5):815‐832. doi: 10.1177/0333102418758283 [DOI] [PubMed] [Google Scholar]
- 24. Diener HC, Tassorelli C, Dodick DW, et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of migraine attacks in episodic migraine in adults. Cephalalgia. 2020;40(10):1026‐1044. doi: 10.1177/0333102420941839 [DOI] [PubMed] [Google Scholar]
- 25. de Vries LS, van der Arend BWH, Maassen VanDenBrink A, Terwindt GM. Blood pressure in patients with migraine treated with monoclonal anti‐CGRP (receptor) antibodies: a prospective follow‐up study. Neurology. 2022;99(17):e1897‐e1904. doi: 10.1212/wnl.0000000000201008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaltseis K, Filippi V, Frank F, Eckhardt C, Schiefecker A, Broessner G. Monoclonal antibodies against CGRP (R): non‐responders and switchers: real world data from an Austrian case series. BMC Neurol. 2023;23(1):174. doi: 10.1186/s12883-023-03203-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Overeem LH, Lange KS, Fitzek MP, et al. Effect of switching to erenumab in non‐responders to a CGRP ligand antibody treatment in migraine: a real‐world cohort study. Front Neurol. 2023;14:1154420. doi: 10.3389/fneur.2023.1154420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iannone LF, Burgalassi A, Vigani G, et al. Switching anti‐CGRP(R) monoclonal antibodies in multi‐assessed non‐responder patients and implications for ineffectiveness criteria: a retrospective cohort study. Cephalalgia. 2023;43(4):3331024231160519. doi: 10.1177/03331024231160519 [DOI] [PubMed] [Google Scholar]
- 29. Bhakta M, Vuong T, Taura T, Wilson DS, Stratton JR, Mackenzie KD. Migraine therapeutics differentially modulate the CGRP pathway. Cephalalgia. 2021;41(5):499‐514. doi: 10.1177/0333102420983282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Russo AF, Hay DL. CGRP physiology, pharmacology, and therapeutic targets: migraine and beyond. Physiol Rev. 2023;103(2):1565‐1644. doi: 10.1152/physrev.00059.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun H, Dodick DW, Silberstein S, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet Neurol. 2016;15(4):382‐390. doi: 10.1016/s1474-4422(16)00019-3 [DOI] [PubMed] [Google Scholar]
- 32. Suliman R, Santos V, Al Qaisi I, et al. Effectiveness of switching CGRP monoclonal antibodies in non‐responder patients in the UAE: a retrospective study. Neurol Int. 2024;16(1):274‐288. doi: 10.3390/neurolint16010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hong JB, Israel‐Willner H, Peikert A, et al. Therapeutic patterns and migraine disease burden in switchers of CGRP‐targeted monoclonal antibodies – insights from the German NeuroTransData registry. J Headache Pain. 2024;25(1):90. doi: 10.1186/s10194-024-01790-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Talbot J, Stuckey R, Wood N, Gordon A, Crossingham G, Weatherby S. Switching anti‐CGRP monoclonal antibodies in chronic migraine: real‐world observations of erenumab, fremanezumab and galcanezumab. Eur J Hosp Pharm. 2024. doi: 10.1136/ejhpharm-2023-003779 [DOI] [PubMed] [Google Scholar]
- 35. Ihara K, Ohtani S, Watanabe N, et al. Switching between anti‐calcitonin gene‐related peptide monoclonal antibodies: a comparison of monthly and quarterly dosing. J Neurol Sci. 2023;453:120811. doi: 10.1016/j.jns.2023.120811 [DOI] [PubMed] [Google Scholar]
- 36. Suzuki S, Suzuki K, Shiina T, Haruyama Y, Hirata K. Real‐world experience with monthly and quarterly dosing of fremanezumab for the treatment of patients with migraine in Japan. Front Neurol. 2023;14:1220285. doi: 10.3389/fneur.2023.1220285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. López‐Moreno Y, Castro‐Sánchez MV, García‐Trujillo L, Serrano‐Castro P. [Failure of an anti‐CGRP monoclonal antibody in the treatment of migraine. Is it worthwhile trying another one?]. Rev Neurol. 2022;75(4):87‐91. doi: 10.33588/rn.7504.2021526 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Directed acyclic graph. The red dots represent confounding factors and the red lines a biased path. The green dots represent exposures and the green line a causal path. The blue dots represent the outcome. All the red dots will be included in the data analysis as covariates, as they represent potential confounders.
Figure S2. ∆Mean monthly migraine days with 95% CI from baseline and 3 months’ follow‐up in the switcher (n = 31), control group (n = 12) with initiation of another preventive treatment and the control group (n = 24) without preventive treatment in the follow‐up.
Figure S3. Violin plot depicting the distribution of the total number of days the first CGRP‐mAbs were administered. The dashed line within the violin depicts the median. The solid lines above and below the dashed line represent the 25th and 75th quartiles, respectively.
Table S1–S12.
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author. Results will be communicated to participants, healthcare professionals, the public and other relevant groups through publication.


