Abstract
The prevalence of chronic polyneuropathy will increase due to the aging population, and therefore, it becomes ever so important to optimize the diagnostic process. However, it is uncertain which blood tests are required and when nerve conduction studies (NCS) should be done in the workup of chronic polyneuropathy. We aimed to investigate the methodology used to develop national polyneuropathy guidelines and to provide an overview and strength of evidence of the recommendations. We searched PubMed and websites of national neurological associations as listed on the website of the World Federation of Neurology to identify national guidelines pertaining to the workup of chronic polyneuropathy by neurologists in an outpatient clinic setting. We identified three national guidelines in the United States and seven national guidelines in Denmark, France, Germany, the Netherlands, Norway, Spain, and Turkey. The methodology used to develop the guidelines differed greatly. All guidelines recommend a series of blood tests. Some guidelines advise to conduct NCS in all patients, while other guidelines advise to conduct NCS when certain symptoms are present. There is variation in recommendations about the extensiveness of NCS, but all mention measuring the sural nerve and the motor peroneal nerve. The evidence for the recommendations is graded as low. Despite some overlap, there are disparities between guidelines regarding the workup that is advised to do in patients with chronic polyneuropathy. It remains unclear which combination of blood tests are to be strongly recommended. Furthermore, it is undetermined whether NCS are always necessary.
Keywords: diagnostics, guidelines, polyneuropathy
1. INTRODUCTION
The overall prevalence of patients with polyneuropathy has been estimated at 4% in the general population, and in persons aged 80 years or older, this increases up to 13%. 1 , 2 The number of patients with polyneuropathy is expected to increase due to the aging population and rising prevalence rates of known risk factors for polyneuropathy such as diabetes, obesity, and cardiovascular disease. 1 , 2 , 3 , 4 , 5 , 6 , 7
History taking and neurological examination are essential and the first and most important steps when evaluating patients with suspected polyneuropathy. To identify causes and risk factors for polyneuropathy, neurologists commonly perform additional blood tests, and nerve conduction studies (NCS) are done to confirm the clinical diagnosis or to classify the polyneuropathy as axonal or demyelinating. 8 , 9 In view of present‐day limitations in healthcare resources and expenditures as opposed to the growing number of patients with polyneuropathy, it becomes ever so important to optimize this diagnostic process.
A routine extensive workup to uncover any potential cause of chronic polyneuropathy results in redundant and costly tests, and in many patients, a reliable and expedient diagnosis could probably still be made with a limited or protocolled workup. 10 , 11 , 12 , 13 , 14 , 15 Uncertainty remains about which investigations are necessary to perform in daily practice, which prompts the question which national polyneuropathy guidelines exist, how they have been developed, and what differences or similarities are present across those guidelines regarding the workup regarding blood tests and NCS?
Therefore, we aim to provide an overview of recommendations for blood tests and NCS in national chronic polyneuropathy guidelines from different countries. We will summarize the methodology used to develop these guidelines and include the strength of evidence of recommendations. The findings serve as a starting point to identify and address knowledge gaps on what constitutes an appropriate and cost‐effective workup of chronic polyneuropathy in the outpatient clinic by neurologists.
2. METHODS
We focused on guidelines for chronic polyneuropathy developed on behalf or endorsed by of national neurological associations, because we considered these as most representative for daily practice and are most likely to be adhered to by the professional neurologists' communities in countries. We did not include expert opinions papers, including reviews or systematic reviews on workup of chronic polyneuropathy, as it is unclear if these are widely used by neurologists in daily practice, in contrast to national guidelines. To identify national guidelines used in the outpatient clinic by neurologists we followed the steps described below.
We searched PubMed with the following search terms and no restrictions for publication year: (polyneuropathies OR polyneuropathy OR neuropathies OR neuropathy) AND (guideline) OR (diagnosis).
We consulted websites of national neurological associations as listed on the website of the World Federation of Neurology. 16 We consulted the guideline reference center of the European Academy of Neurology. 17
If guidelines were not available on the website of the national neurological association or if there was no website, we contacted neurological associations by email to obtain guidelines.
Because not all guidelines were written in English, we used an online translation tool (Google Translate). We evaluated the methodology used to develop the national guidelines, summarized the recommendations and their strength of evidence. We focused on the workup of chronic polyneuropathy, which we defined as presence of distal symmetrical symptoms with slow onset and evolution in months and absence of alarm symptoms. Alarm symptoms were defined by the progression of symptoms in weeks to 6 months resulting in walking problems or limitations of motor function in the arms, asymmetric or non‐length distribution of symptoms, pure motor or motor dominant symptoms, ataxia, autonomic symptoms, and severe neuropathic pain. We did not consider guidelines on small fiber neuropathy or those on specific polyneuropathies (such as chronic inflammatory demyelinating polyradiculoneuropathy or Guillain‐Barre syndrome).
3. RESULTS
Our search strategy identified 10 different national guidelines in eight countries.
We identified
With PubMed; four guidelines in France and the United States. In the United States; one guideline for blood tests and two for NCS.
Via websites of national neurological associations; four guidelines in Germany, the Netherlands, Spain, and Turkey.
- After contacting 115 national neurological associations by email, we identified:
- Two guidelines in Denmark and Norway.
- Representatives from 34 countries reported to have no national guideline, and 79 countries did not respond.
For a schematic representation of identifying the national guidelines, see Figure 1.
FIGURE 1.
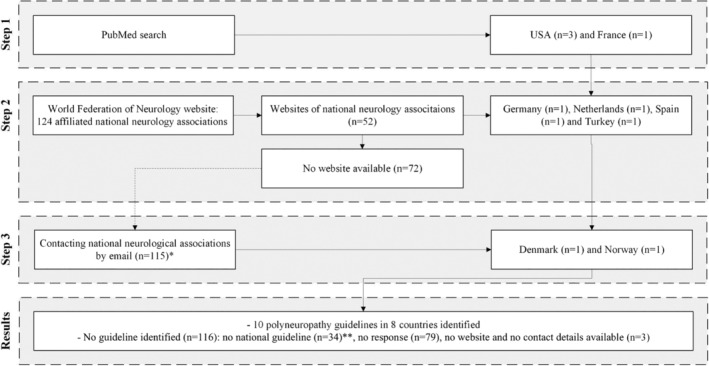
Identification of national polyneuropathy guidelines.
3.1. Development of guidelines
A summary of the methodology used to develop the guidelines is given in Table 1. The American and Dutch guidelines describe methods used to develop the guideline and provide levels of strength to corroborate their recommendations. The other guidelines do not provide development methods and levels of strength with the recommendations.
TABLE 1.
Development of national guidelines.
| Guideline | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Methods | Denmark (2022) | France (2007) | Germany (2019) | Netherlands (2019) | Norway (2022) | Spain a | Turkey (2006) | United States (blood tests, 2009) | United States (NCS, 2012) | United States (NCS, 2017) |
| Developed by | Steering committee consisting of representatives of the Danish neurological society | Work group (neurologists, neuropediatric, neurophysiologists, general practitioners, diabetologists, rheumatologist, infectiologist) | Guideline commission of the German neurological association | Working group (neurologists, neurologists in training, patient representatives) | Two neurologists | Work group of neurologists with specialty in neuromuscular disorders | Panel of experts | Panel of experts | AAN committee | AANEM committee |
| Systematic literature review | Not stated | Yes | Yes | Yes, using PICO | Not stated | Not stated | Not stated | Yes | Not stated | Not stated |
| Number of references | 8 | 113 | 127 | 18 | 20 | Not stated | 9 | 37 | 53 | 24 |
| Assessment of risk of bias (using critical appraisal tool) | Not stated | Not stated | Not stated | Yes (ACROBAT‐NRS for observational studies, QUADAS II for diagnostic studies) | Not stated | Not stated | Not stated | Yes (diagnostic test classification‐of‐evidence scheme) | Not stated | Not stated |
| Determine strength of recommendation | Not stated | Yes (using HAS method) | Not stated | Yes (using GRADE) | Not stated | Not stated | Not stated | Yes (using AAN method) | Not stated | Not stated |
| Other | DELPHI‐method to reach consensus about recommendations | |||||||||
| Classification b | S1 | S1 | S2 | S3 | S1 | S1 | S1 | S3 | S1 | S1 |
Year of publication unknown.
S1, recommendations by experts; S2, structured consensus process or systematic literature review; S3, structured consensus process and systematic literature review.
3.2. Denmark 18
The Danish guideline is developed by the Danish neurological association and is maintained by a steering committee consisting of representatives of the Danish Neurological Society (DNS), Danish Nurses Organization (DNO), and Young Neurologists, Neurosurgeons and Neurophysiologists (YNNN). They strive to update the guidelines yearly. No clear details are given on the methods used for the guideline development.
3.3. France 19
The work group consists of doctors specialized in neurology, neuropediatric, neurophysiology, family medicine, diabetes/diabetology, rheumatology, and infectious diseases. A systematic literature search was performed in Medline, Embase, and Pascal. Other sources such as the Cochrane Library were used. The degree of evidence for the recommendations was ranked based on the type of research. When there was no literature available, the recommendation was based on consensus within the work group.
3.4. Germany 20
The authors of the German guideline were selected by the guideline commission of the German neurological association. The authors performed a literature search using different electronic databases (PubMed, Embase, Cochrane Library, and DIMDI search), which resulted in 127 relevant articles. The DELPHI method was used to reach a consensus about recommendations.
3.5. Netherlands 21
A work group of neurologists and patient representatives created the Dutch guideline in adherence with the requirements of the AGREE II instrument, by constructing clinical research queries with the population/intervention/comparison/outcome (PICO) question. A systemic literature analysis was conducted in Medline, PubMed and Embase database. To assess the risk of bias, the A Cochrane Risk Of Bias Assessment Tool: for Non‐Randomized Studies of Interventions (ACROBAT‐NRS) method was used for observational studies and the Quality Assessment of Diagnostic Accuracy Studies II (QUADAS II) method for diagnostic studies. Evidence was assessed with the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) method.
3.6. Norway 22
The guidelines used by Norwegian neurologists are edited by two neurology professors, who performed a PubMed search and gathered relevant articles. They strive to update the guidelines every year. No details are reported about systematic assessment of risk of bias.
3.7. Spain 23
The guideline of the Spanish neurological association consists of an algorithm for diagnosing patients with chronic polyneuropathy. The algorithm was constructed by a study group of neurologists specialized in neuromuscular disorders. No details are provided on the methods used for the guideline development.
3.8. Turkey 24
The guideline of the Turkish neurological association is published by the faculty of medicine of the Gazi university. Professors of neurology of different universities in Turkey contributed to the guideline. No details are stated about guideline development methods.
3.9. United States 25 , 26 , 27
Regarding the guideline on blood tests, a systematic literature search was conducted by a panel of experts and the relevant literature was assessed by the same panel. The expert panel consisted of 19 physicians with representatives from the American Academy of Neurology (AAN), the American Academy of Neuromuscular and Electrodiagnostic Medicine (AANEM), and the American Academy of Physical Medicine and Rehabilitation (AAPM&R) with comprehensive experience and knowledge in polyneuropathy. The risk of bias was graded using the diagnostic test classification‐of‐evidence scheme. To determine the strength of recommendations, the panel used the AAN method, a stepwise process for developing guidelines using evidence‐based medicine methodology. Regarding the guidelines on nerve conduction studies, no details are reported on the methods used for the guideline development.
3.10. Guideline recommendations
An overview of recommendations from the different guidelines is given in Tables 2 and 3. Below, we made a distinction between guidelines where methods and levels of strength are described versus guidelines which provide no details about development.
TABLE 2.
Suggestions of blood tests.
| Guidelines | ||||||||
|---|---|---|---|---|---|---|---|---|
| Blood tests | Denmark (2022) | France (2007) | Germany (2019) | Netherlands (2019) | Norway (2022) | Spain a | Turkey (2006) | United States (2009) |
| Fasting glucose, HbA1c | Yes | Fasting glucose | HbA1c | Yes | Yes | Yes. If normal, oral glucose tolerance test | If indicated b |
Fasting glucose.∇ If normal, oral glucose tolerance test |
| Blood count (Hb, leukocytes, erythrocytes, platelets) | Hb, leukocytes, platelets | Yes | Yes | Yes | Hb, leukocytes, platelets | Yes | If indicated b | Yes |
| Liver function (GammaGT, ALAT) | Yes | Yes | Yes | Yes | Yes | Yes | If indicated b | Yes |
| Kidney function (creatinine, eGFR) | Creatinine + urea | Yes | Yes | Yes | Yes | Yes | If indicated b | Yes |
| TSH | Yes | Yes | Yes | Yes | TSH and T4 | Yes | If indicated b | Yes |
| Inflammation (CRP, ESR) | CRP | Yes | Yes | ESR | CRP | ESR | If indicated b | Yes |
| Vitamin B1, B6, B12, folic acid | Yes | No | B12 | Yes | B12 | B12 | B12 if indicated b | B12∇ and folic acid |
| Paraprotein | Yes | No | Yes | Yes | Yes | Yes | If indicated b | Yes∇ |
| Other | IgG, IgA, IgM, rheumatoid factor‐IgM, electrolytes, CK | Transferrin | Electrolytes | No | Electrolytes, CK, Urine: free light chain, if normal free light chain serum | CT‐thorax | If indicated; b urea, nitrogen, urine analysis, chest X‐ray, CT‐thorax, CT‐abdomen, serological tests, c cryoglobulin, CSF, skin biopsy, d ACE, heavy metal analysis, treponema pallidum anti‐bodies, quantitative sensory tests, MRI (cranial, spinal, etc.) | Homocysteine, methylmalonic acid∇ |
Abbreviation: ∇, highest yield.
Year of publication unknown.
The Turkish guideline has no standard set of blood tests to perform, but all these tests can be performed when deemed necessary by treating physician.
Anti‐ganglioside antibodies, anti‐HIV, anti‐hu, anti‐MAG, ANA, Rheumatoid factor, ANCA, anti‐La, anti‐Ro, CMV, EBV, hepatitis B and C.
Paraffin sections, teasing, immunohistochemistry, electron microscopy, confocal microscopy.
TABLE 3.
Suggestions nerve conduction studies.
| Guidelines | Denmark (2022) | France (2007) | Germany (2019) | Netherlands (2019) | Norway (2022) | Spain d | Turkey (2006) | United States (2012) | United States (2017) |
|---|---|---|---|---|---|---|---|---|---|
| Indication NCS | Unclear cause or alarm symptoms. a | Not necessary in patients with a clinically distinct homogeneous symmetrical polyneuropathy with a known etiology. b | Every patient. | Unclear cause or alarm symptoms. c | Every patient. | Every patient. | Every patient. | No specific recommendations. | In patients with characteristics corresponding to high yield. e |
| Recommended nerves | No specific recommendations. | Sensory: sural nerve bilaterally, peroneal nerve unilateral, median nerve bilaterally, two measurements of ulnar/radial nerve. Motor: peroneal nerve bilaterally (with F‐waves), H‐reflexes of tibial nerves bilaterally, median nerve bilaterally, ulnar nerve unilateral | Sensory: sural, peroneus superficialis, median, and ulnar nerve. Motor: peroneal, tibial, median and ulnar nerve. | Sensory: sural, radial, median, or ulnar nerve. Motor: peroneal and tibial, median, or ulnar nerve. | No specific recommendations. | No specific recommendations. | No specific recommendations. |
1. Sensory: sural, motor: peroneal 2. If sural or peroneal absent: sensory: ulnar and median, motor: ulnar. 3. If a response is absent for any of the nerve's studies, NCS of contralateral nerve should be performed. 4. If peroneal motor absent: ipsilateral tibial motor. |
No specific recommendations. |
Positive family history, acute onset, course relapsing, non‐length distribution, asymmetry, multiple nerves affected.
Diabetes, renal insufficiency, neurotoxic treatment.
Fast progression (4 weeks–6 months) leading to problems with walking or loss of motor function of arms, asymmetry, pure motor or motor dominant, ataxia, non‐length distribution, early autonomic symptoms, severe pain.
Year of publication unknown.
High yield: (1) the patient's history, physical and standard neuropathy blood tests do not indicate a likely etiology, (2) symptoms and/or physical findings are moderate to severe, (3) atypical presentation (predominantly motor symptoms or findings, proximal deficits or asymmetry), (4) rapid progression of symptoms or signs, (5) presence of symptoms or signs indicating another disorder, such as lumbar radiculopathy, (6) unknown duration or severity of the underlying cause, (7) family history suggesting hereditary neuropathy, (8) exposure to substances known to cause neuropathy, including medications, (9) discrepancy between symptoms and signs.
3.11. Blood tests
3.11.1. Strong guideline development methods
The American and Dutch guideline recommend testing fasting glucose, hemoglobin, leukocytes, platelets, erythrocyte sedimentation rate (ESR), vitamin B12, folic acid, alanine aminotransferase (ALAT), creatinine, thyroid‐stimulating hormone (TSH), and paraprotein. The Dutch guideline also advises to test HbA1c, Vitamin B1 and B6, while the American guideline recommends testing for functional vitamin B12 deficiency by determination of methylmalonic acid and homocysteine. The recommendations from the American guideline are rated at level C, which is the lowest level of recommendation. The Dutch guideline provides no level of recommendation but rate the evidential value as low.
3.11.2. No details stated about guideline development methods
The Danish, French, German, Norwegian, and Spanish guidelines recommend testing fasting glucose, hemoglobin, leukocytes, platelets, ESR or CRP, gammaGT, ALAT, creatinine, and TSH. HbA1c, B12, and paraprotein testing are recommended by all guidelines, except the French guideline. Immunoglobulin levels (IgG, IgA, and IgM) are recommended by the Danish guideline, and testing for transferrin is recommended by the French guideline. The Danish and Norwegian guidelines additionally advise to test for CK. The Danish, Norwegian, and German guidelines recommend testing of electrolytes, without further specification. The Turkish guideline does not recommend a standard set of blood tests but lists a large range of possible blood test.
3.12. Nerve conduction studies (NCS)
3.12.1. Strong guideline development methods
In the American guideline, the recommendation to conduct the NCS is based on the expected diagnostic yield when diagnosing patients with chronic polyneuropathy. When a high diagnostic yield is expected, it is described in Table 3. Diagnostic yield is expected to be low when (1) symptoms and physical findings are mild, (2) findings are symmetric, distal, predominantly sensory, (3) there is a known cause, and (4) there is little suspicion of a coexisting nerve disorder. It is recommended to perform a thorough clinically history and physical examination in deciding whether to conduct NCS or not. The Dutch guideline recommends conducting NCS in all patients with an unclear cause or when alarm symptoms are present, such as a rapid onset or ataxia, whereas NCS is not necessary in patients with a symmetrical sensory polyneuropathy limited to the legs or a characteristic distal symmetric polyneuropathy consistent with the underlying cause.
The American and Dutch guidelines advise to perform sensory NCS of the sural nerve and motor NCS of the peroneal nerve. The American guideline advises to start with sural and peroneal motor nerve recording in one lower extremity and to perform additional NCS. Measurements of upper extremity nerves are recommended by the Dutch guideline. Table 3 provides a more detailed indication description regarding recommendations on NCS. The GRADE level, assigned by the Dutch guideline for the recommendation of conduction of NCS, was low.
3.12.2. No details stated about guideline development methods
The Danish guidelines recommends conducting NCS in all patients with an unclear cause not discovered by blood tests or when red flags are present, such as a rapid onset, ataxia, or familial predisposition to polyneuropathy. NCS is not considered necessary by the French guideline in patients with a symmetrical sensory polyneuropathy limited to the legs or a characteristic distal symmetric polyneuropathy consistent with the underlying cause. The German, Norwegian, Spanish, and Turkish guidelines advise NCS for every patient suspected of polyneuropathy.
The French and German guidelines advise to perform sensory NCS of the sural nerve and motor NCS of the peroneal nerve. The Danish, Norwegian, Spanish, and Turkish guidelines give no specific recommendations on which nerves to investigate. Measurements of upper extremity nerves are recommended by the German and French guideline. The German guideline provides an overview of nerves which should be measured, but without a specified order. The French guideline recommends bilateral measurement of sural, median, peroneal, and tibial nerves. Table 3 provides a more detailed indication description regarding recommendations on NCS.
3.13. Genetic testing
3.13.1. Strong guideline development methods
The American guideline recommends genetic testing if a patient presents with a cryptogenic and classic hereditary neuropathy type. The strength assigned for this recommendation is level C. The Dutch guideline makes no specific recommendations about genetic testing.
3.13.2. No details stated about guideline development methods
The German guideline recommends conducting genetic testing when there is a positive family history of polyneuropathy or if there are typical signs (pes cavus/arched feet, hammer toes) of a hereditary neuropathy. The French, Danish, Norwegian, Spanish, and Turkish guidelines make no specific recommendations about genetic testing. The overlapping recommendations of all guidelines, irrespective of methods of guideline development, for blood tests and NCS are outlined in Table 4.
TABLE 4.
Overlapping recommendations guidelines.
| Most commonly recommended blood tests by all guidelines | Nerves recommended to be tested by guidelines from France, Germany, the Netherlands and the United States |
|---|---|
| Glucose and/or HbA1c, blood count (Hb, leukocytes and platelets), kidney and liver function (ALAT and GGT), TSH, and inflammation parameters (BSE or CRP) |
Sensory: sural nerve. Motor: peroneal nerve. |
4. DISCUSSION
Notwithstanding low‐ to poor‐quality evidence and low grade levels of recommendations, guidelines on the workup of chronic polyneuropathy overlap in recommending blood tests fasting glucose, hemoglobin, leukocytes, platelets, liver (GGT, ALAT) and kidney function (creatinine), vitamin B12, TSH, inflammation parameters (ESR or CRP), and paraprotein. Regarding NCS, these are not addressed in every guideline and recommendations range from performing NCS in every patient to only in patients with alarm symptoms or if the yield is expected to be high. Guidelines that include NCS all recommend investigation of the sural nerve and peroneal motor nerve.
A workup limited to the overlapping recommendations raises the question if this would suffice to reliably establish the etiology and diagnosis. Studies have shown that in two thirds of patients with a new diagnosis of chronic polyneuropathy, the etiology of polyneuropathy was discovered based on medical history before diagnostic testing. 14 The most frequent etiologies of chronic polyneuropathy in patients investigated in outpatient neurology clinics, as well as in a population study, are diabetes, excessive alcohol consumption, and an equal proportion of patients with no apparent etiology and are classified as idiopathic or cryptogenic polyneuropathy. 2 , 28 Based on the frequency of etiologies, testing fasting glucose and HbA1c in patients without a known etiology will likely be the most useful. Other blood tests, such as vitamin B12, TSH and paraprotein, have a much lower yield to reveal an underlying cause. 10 , 13 , 14 , 29 , 30 , 31
A population‐based study showed that 20% of newly diagnosed polyneuropathy patients had more than one risk factor for polyneuropathy. 2 A retrospective hospital based study reported that in two thirds of patients with multiple risk factors, diabetes was one of those risk factors and was already known based on the medical history of the patient. 31 Another retrospective study showed that up to 53% of patients with diabetes had multiple risk factors, which were either discovered with history taking (toxic medication or excessive alcohol consumption) or with extensive blood tests. 32 These studies imply that neurologists should be aware of additional risk factors in patients known to have diabetes or another risk factor.
Studies on the usefulness of NCS showed conflicting results regarding the contribution of NCS to the diagnosis and management of polyneuropathy, as this ranged from 0.5% to 52%. 10 , 14 , 33 , 34 , 35 The different study populations and settings illustrate that NCS are most contributory when referrals are made by non‐neurologists and when the study population is broad and diverse, but appear less contributory after neurological consultation with the clinical suspicion of a distal symmetric polyneuropathy with a known cause. NCS is probably only beneficial in patients with atypical symptoms, but sufficient evidence is lacking. 15 The range of recommendations in national guidelines reflects these differences in the literature. Overall, there is a tendency to define a selection of patients in which the yield of the NCS is expected the highest to enhance the efficacy. Several studies indicate that the sensory sural nerve and motor peroneal nerve are the most sensitive to diagnose polyneuropathy, and this is reflected in the overlapping recommendations of nerves to be examined according to these guidelines. 29 , 36 , 37 , 38
A single multinational guideline with a limited workup would probably be feasible for countries with the same incidence of etiologies, irrespective of differences in health care systems of different countries. Furthermore, since chronic polyneuropathy is such a common disease, a multinational guideline with a standardized approach would also improve research purposes. Low‐ and middle‐income countries would probably benefit more from a national guideline focused on the specific etiologies rather than a multinational guideline based on studies performed in Western countries with different frequency of etiologies.
A limited workup would require a certain level of adherence to guidelines by neurologists, and it is unclear whether this is attainable in daily practice. For example, in the Netherlands, guideline adherence is a regularly ascertained indicator of health care quality by the Dutch neurological association during mandatory quality visitations nationwide in all neurological practices. We did not find studies about guideline adherence and/or monitoring in other countries, but even when guideline adherence is low, a limited workup is possibly still more cost effective without loss of diagnostic reliability. 12 Moreover, a limited workup likely would expose patients less frequently to hospital visits and tests with less loss of productivity and, consequently, could result in a positive effect on patients' opinion of experienced health care.
There are several study limitations to consider. We managed to mainly identify guidelines from European countries and the United States as issued by national neurological associations, despite contacting every neurological association affiliated with the World Federation of Neurology. There may be even more differences with guidelines from non‐Western or low‐income countries because of different prevalence's of various etiologies or risk factors. Recommendations for blood tests are mainly based on the prevalence of the underlying etiology and we realize that the presented guidelines might be less useful in those countries to optimize health care. It is certainly possible that we did not identify all national chronic polyneuropathy guidelines within the area we selected, as some guidelines might only be accessible by members of the neurological association. By contacting different neurological associations via email, we tried to gather as many guidelines as possible. While expert opinion papers or systematic reviews may be used in daily practice by neurologists, we did not include those because we presumed that during the guideline development process by national neurological associations, these are used as relevant literature on which the recommendations are based.
Since interpretation of the patient's history and neurological examination are indispensable when evaluating patients with a suspected chronic polyneuropathy and deciding about further tests, it is unclear if these guidelines developed by neurological associations would be sufficient for other physicians such as general practitioners or internal medicine specialists without specifical neurological training. In countries where patients with a suspected chronic polyneuropathy are not always evaluated by a neurologist, a more extensive guideline might be necessary to ensure a proper diagnosis. Of course, when alarm symptoms are present, the treating neurologists or physicians may perform the tests they deem necessary and thus deviate from recommendations or guidelines.
General medical examination may be an important step when evaluating patients with chronic polyneuropathy. Most of the guidelines do not mention this. Some guidelines however recommend additional examinations, such as a computed tomography (CT)–thorax, or to examine patients for hepatomegaly, splenomegaly, and adenopathy. We agree that unexpected findings from general medical examination could uncover rare potential risk factors for chronic polyneuropathy. Therefore, we emphasize the importance of history taking and general medical examination in patients with a chronic polyneuropathy. This should also be considered when a possible multinational guideline will be developed.
Because the guidelines were written in the national languages, we used online translation tools for languages other than Dutch or English and may have missed some nuances. However, most of the guidelines list recommendations or refer to tables that are stated explicitly.
In summary, there is an overlap between recommendations from national chronic polyneuropathy guidelines, but all evidence is graded as low, and it is not clear which blood tests should be used in the different populations of polyneuropathy patients. There are more variations in recommendations regarding NCS made by the guidelines, and it remains unclear in which patients’ NCS have added value. Knowledge gaps continue to exist about the necessary workup in patients with chronic polyneuropathy that warrants further studies.
ACKNOWLEDGEMENTS
We would like to acknowledge our collaborators as follows:
EXPRESS Study Consortium: A.M. Dekker (Department of Neurology, UMC Utrecht Brain Center, University Medical Center Utrecht), P.T.C. van Doormaal (Department of Neurology, UMC Utrecht Brain Center, University Medical Center Utrecht & Department of Neurology, Tergooi MC), F. Eftimov (Department of Neurology, University Medical Centers, University of Amsterdam), M. Eurelings (Department of Neurology, Spaarne Gasthuis Hoofddorp), M.F.G van der Meulen & L.L. Teunissen (Department of Neurology and Clinical Neurophysiology, St. Antonius Hospital), S. Piepers (Department of Neurology, Meander MC), E. Verstraete (Department of Neurology, Rijnstate Hospital).
Wiersma M, van der Star GM, Notermans NC, van Doorn PA, Vrancken AFJE, The EXPRESS Study Consortium . Knowledge gaps in diagnosing chronic polyneuropathy: Review of national guidelines. J Peripher Nerv Syst. 2024;29(4):383‐392. doi: 10.1111/jns.12667
Contributor Information
M. Wiersma, Email: m.wiersma-3@umcutrecht.nl.
The EXPRESS Study Consortium:
A. M. Dekker, P. T. C. van Doormaal, F. Eftimov, M. Eurelings, M. F. G. van der Meulen, S. Piepers, L. L. Teunissen, and E. Verstraete
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Hanewinckel R, Drenthen J, van Oijen M, Hofman A, van Doorn PA, Ikram MA. Prevalence of polyneuropathy in the general middle‐aged and elderly population. Neurology. 2016;87(18):1892‐1898. doi: 10.1212/wnl.0000000000003293 [DOI] [PubMed] [Google Scholar]
- 2. Taams NE, Drenthen J, Hanewinckel R, Ikram MA, Doorn PAV. Prevalence and risk factor profiles for chronic axonal polyneuropathy in the general population. Neurology. 2022;99(20):e2234‐e2240. doi: 10.1212/wnl.0000000000201168 [DOI] [PubMed] [Google Scholar]
- 3. Hoffman EM, Staff NP, Robb JM, St Sauver JL, Dyck PJ, Klein CJ. Impairments and comorbidities of polyneuropathy revealed by population‐based analyses. Neurology. 2015;84(16):1644‐1651. doi: 10.1212/wnl.0000000000001492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Teunissen LL, Franssen H, Wokke JH, et al. Is cardiovascular disease a risk factor in the development of axonal polyneuropathy? J Neurol Neurosurg Psychiatry. 2002;72(5):590‐595. doi: 10.1136/jnnp.72.5.590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hanewinckel R, Drenthen J, Ligthart S, et al. Metabolic syndrome is related to polyneuropathy and impaired peripheral nerve function: a prospective population‐based cohort study. J Neurol Neurosurg Psychiatry. 2016;87(12):1336‐1342. doi: 10.1136/jnnp-2016-314171 [DOI] [PubMed] [Google Scholar]
- 6. Callaghan BC, Xia R, Reynolds E, et al. Association between metabolic syndrome components and polyneuropathy in an obese population. JAMA Neurol. 2016;73(12):1468‐1476. doi: 10.1001/jamaneurol.2016.3745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Visser NA, Vrancken AF, van der Schouw YT, van den Berg LH, Notermans NC. Chronic idiopathic axonal polyneuropathy is associated with the metabolic syndrome. Diabetes Care. 2013;36(4):817‐822. doi: 10.2337/dc12-0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Callaghan BC, Kerber K, Smith AL, Fendrick AM, Feldman EL. The evaluation of distal symmetric polyneuropathy: a physician survey of clinical practice. Arch Neurol. 2012;69(3):339‐345. doi: 10.1001/archneurol.2011.1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Callaghan B, McCammon R, Kerber K, Xu X, Langa KM, Feldman E. Tests and expenditures in the initial evaluation of peripheral neuropathy. Arch Intern Med. 2012;172(2):127‐132. doi: 10.1001/archinternmed.2011.1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosenberg NR, Portegies P, de Visser M, Vermeulen M. Diagnostic investigation of patients with chronic polyneuropathy: evaluation of a clinical guideline. J Neurol Neurosurg Psychiatry. 2001;71(2):205‐209. doi: 10.1136/jnnp.71.2.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pourmand R. Evaluating patients with suspected peripheral neuropathy: do the right thing, not everything. Muscle Nerve. 2002;26(2):288‐290. doi: 10.1002/mus.10184 [DOI] [PubMed] [Google Scholar]
- 12. Vrancken AF, Kalmijn S, Buskens E, et al. Feasibility and cost efficiency of a diagnostic guideline for chronic polyneuropathy: a prospective implementation study. J Neurol Neurosurg Psychiatry. 2006;77(3):397‐401. doi: 10.1136/jnnp.2005.073239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Callaghan BC, Kerber KA, Banerjee M, et al. The evaluation of distal symmetric polyneuropathy: utilisation and expenditures by community neurologists. J Neurol Neurosurg Psychiatry. 2016;87(1):113‐114. doi: 10.1136/jnnp-2014-307575 [DOI] [PubMed] [Google Scholar]
- 14. Callaghan BC, Kerber KA, Lisabeth LL, et al. Role of neurologists and diagnostic tests on the management of distal symmetric polyneuropathy. JAMA Neurol. 2014;71(9):1143‐1149. doi: 10.1001/jamaneurol.2014.1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Callaghan BC. Test utilization and value in the evaluation of peripheral neuropathies. Continuum (Minneap Minn). 2020;26(5):1384‐1391. doi: 10.1212/con.0000000000000910 [DOI] [PubMed] [Google Scholar]
- 16. Neurology WF. WFN Member Societies. Accessed 05‐08‐2024, https://wfneurology.org/member-societies
- 17. Guideline Reference Center . European Academy of Neurology. Accessed 22‐08‐2024, https://www.ean.org/research/ean‐guidelines/guideline‐reference‐center
- 18. Polyneuropati . Dansk Neurologisk Selskab. 2022. Accessed 10‐07‐2023, https://nnbv.dk/polyneuropati/
- 19. Diagnostic management of peripheral neuropathies (polyneuropathies and multiple mononeuropathies). Rev Neurol (Paris). 2008;164 Spec No 1::F37‐F50; discussion F51‐2. [PubMed] [Google Scholar]
- 20. Diagnostik bei Polyneuropathien . Deutsche Gesellschaft Neurologie. 2019. Accessed 10‐07‐2023, https://dgn.org/leitlinie/123
- 21. Richtlijn Polyneuropathie 2019 . Federatie Medisch Specialisten. 2019. Accessed 10‐07‐2023, https://richtlijnendatabase.nl/richtlijn/polyneuropathie
- 22. Polynevropati (PN) . Norsk Nevrologisk Forening. 2022. Accessed 10‐07‐2023, https://nevrologi.legehandboka.no/handboken/sykdomsgrupper/nevromuskulare‐sykdommer/sykdommer‐og‐symptomer/polynevropati#diagnostikk‐av‐pn
- 23. Algoritmos diagnósticos de enfermedades neuromusculares. Sociedad Espanola de Neurologia. Accessed 10‐07‐2023, http://genm.sen.es/index.php/profesionales/algoritmos-diagnosticos
- 24. Neuromuscular Diseases Diagnosis and Treatment Guide . Turkish Neurology Association. Accessed 22‐08‐2024, https://noroloji.org.tr/menu/151/faydali-linkler-ve-tani-ve-tedavi-rehberi
- 25. AANEM policy statement on electrodiagnosis for distal symmetric polyneuropathy. Muscle Nerve. 2018;57(2):337‐339. doi: 10.1002/mus.26003 [DOI] [PubMed] [Google Scholar]
- 26. Distal Symmetric Polyneuropathy Performance Measurement Set . American Academy of Neurology & American Association of Neuromuscular & Electrodiagnostic Medicine. Distal Symmetric Polyneuropathy Performance Measurement Set; 2019. [Google Scholar]
- 27. England JD, Gronseth GS, Franklin G, et al. Practice parameter: evaluation of distal symmetric polyneuropathy: role of laboratory and genetic testing (an evidence‐based review). Report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of physical medicine and rehabilitation. Neurology. 2009;72(2):185‐192. doi: 10.1212/01.wnl.0000336370.51010.a1 [DOI] [PubMed] [Google Scholar]
- 28. Visser NA, Notermans NC, Linssen RS, van den Berg LH, Vrancken AF. Incidence of polyneuropathy in Utrecht, The Netherlands. Neurology. 2015;84(3):259‐264. doi: 10.1212/wnl.0000000000001160 [DOI] [PubMed] [Google Scholar]
- 29. Smith AG, Singleton JR. The diagnostic yield of a standardized approach to idiopathic sensory‐predominant neuropathy. Arch Intern Med. 2004;164(9):1021‐1025. doi: 10.1001/archinte.164.9.1021 [DOI] [PubMed] [Google Scholar]
- 30. Johannsen L, Smith T, Havsager AM, et al. Evaluation of patients with symptoms suggestive of chronic polyneuropathy. J Clin Neuromuscul Dis. 2001;3(2):47‐52. doi: 10.1097/00131402-200112000-00001 [DOI] [PubMed] [Google Scholar]
- 31. Rudolph T, Farbu E. Hospital‐referred polyneuropathies—causes, prevalences, clinical‐and neurophysiological findings. Eur J Neurol. 2007;14(6):603‐608. doi: 10.1111/j.1468-1331.2007.01758.x [DOI] [PubMed] [Google Scholar]
- 32. Gorson KC, Ropper AH. Additional causes for distal sensory polyneuropathy in diabetic patients. J Neurol Neurosurg Psychiatry. 2006;77(3):354‐358. doi: 10.1136/jnnp.2005.075119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cho SC, Siao‐Tick‐Chong P, So YT. Clinical utility of electrodiagnostic consultation in suspected polyneuropathy. Muscle Nerve. 2004;30(5):659‐662. doi: 10.1002/mus.20119 [DOI] [PubMed] [Google Scholar]
- 34. Kothari MJ, Blakeslee MA, Reichwein R, Simmons Z, Logigian EL. Electrodiagnostic studies: are they useful in clinical practice? Arch Phys Med Rehabil. 1998;79(12):1510‐1511. doi: 10.1016/s0003-9993(98)90411-7 [DOI] [PubMed] [Google Scholar]
- 35. Kothari MJ, Preston DC, Plotkin GM, Venkatesh S, Shefner JM, Logigian EL. Electromyography: do the diagnostic ends justify the means? Arch Phys Med Rehabil. 1995;76(10):947‐949. doi: 10.1016/s0003-9993(95)80072-7 [DOI] [PubMed] [Google Scholar]
- 36. Heise CO, Machado FC, Amorim SC, Toledo SM. Combined nerve conduction index in diabetic polyneuropathy. Arq Neuropsiquiatr. 2012;70(5):330‐334. doi: 10.1590/s0004-282x2012000500005 [DOI] [PubMed] [Google Scholar]
- 37. Vrancken AF, Franssen H, Wokke JH, Teunissen LL, Notermans NC. Chronic idiopathic axonal polyneuropathy and successful aging of the peripheral nervous system in elderly people. Arch Neurol. 2002;59(4):533‐540. doi: 10.1001/archneur.59.4.533 [DOI] [PubMed] [Google Scholar]
- 38. England JD, Gronseth GS, Franklin G, et al. Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of physical medicine and rehabilitation. Neurology. 2005;64(2):199‐207. doi: 10.1212/01.Wnl.0000149522.32823.Ea [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


