Abstract
Watermelon mosaic virus (WMV), a member of the genus Potyvirus, causes serious economic losses in cucurbit crops. For molecular biological studies of viruses, it is necessary to construct an infectious clone that can facilitate gene functional analysis. In this study, we constructed an infectious cDNA clone of WMV genomic RNA by Gibson assembly and evaluated its virulence and symptoms on a variety of host plants. A WMV isolate (WMV-IS-me2) collected from melon in Iksan, Jeonbuk Province, Korea in 2015 caused mosaic symptoms on leaves. Four overlapping PCR fragments of the full-length genome of this isolate and the pJL89 binary vector with overhanging ends of 40 bp were amplified by reverse transcription PCR and joined in a single isothermal reaction. The complete nucleotide sequence of the infectious cDNA clone of WMV was determined and the recombinant vector was transformed into Agrobacterium tumefaciens GV3101. Following agro-infiltration, an infectious clone (pWMV-M, KACC95145P) systemically induced mosaic symptoms on watermelon (Citrullus lanatus), zucchini (Cucurbita pepo), melon (Cucumis melo), and Nicotiana benthamiana plants. This infectious clone may be useful for screening cucurbit varieties resistant to WMV, as well as for studying viral gene function.
Keywords: Gibson assembly, infectious cDNA clone, Potyvirus, watermelon mosaic virus
Watermelon mosaic virus (WMV) is a member of the genus Potyvirus in the family Potyviridae with filamentous particles having approximately 750 nm long (Desbiez and Lecoq, 2004; Purcifull et al., 1984). WMV is an economically important virus that infects cucurbit and horticultural crops worldwide (Chare and Holmes, 2006; Desbiez and Lecoq, 2008; Desbiez et al., 2009; Lecoq et al., 2011; Purcifull et al., 1984). It is efficiently transmitted by at least 35 different aphids in a non-persistent manner and induces symptoms such as mosaic, vein banding, stunting, and leaf deformation (Purcifull et al., 1984). The WMV genome is composed of a single-stranded positive-sense RNA molecule of about 10 kb and has a covalently linked 5-terminal protein (viral protein linked genome) and a 3-terminal polyA tail. The viral genome encodes a polyprotein that is proteolytic for about 10 mature proteins, including helper component protease (HC-Pro), cylindrical inclusion (CI), nuclear inclusion A protein (NIa-Pro), coat protein (CP), and others (Desbiez and Lecoq, 2004). The P3 cistron also encodes a second protein, P3N-PIPO, which is generated by transcriptional slippage in the open reading frame of P3 (Olspert et al., 2015). Based on the amino acid (aa) sequences of the CP protein, WMV isolates are classified into three groups, G1 to G3 (Hajizadeh et al., 2017). The G1 (referred to as the “classical” [CL] strain) and G2 groups contain KEA and KET aa motifs, respectively, at positions 3–5 in the N-terminal part of the CP, whereas the G3 group (the “emergent” [EM] strain) has a KEKET aa motif at positions 3–7 (with the insertion of two amino acids) in the N-terminal part of the CP (Hajizadeh et al., 2017). The G3 group has been observed to cause more severe symptoms since the year 2000 and can be divided into four subgroups, EM1 to EM4 based on multiple introductions and recombination events (Bertin et al., 2020; Desbiez et al., 2007, 2009).
For molecular biological studies of viruses, it is necessary to construct an infectious clone. Infectious clones from plant RNA viruses are useful tools for reverse genetics in studies of viral pathogenesis, gene function, and plant–virus interactions, and in applications as vectors for the expression of foreign genes or virus-induced gene silencing (Boyer and Haenni, 1994; Brewer et al., 2018; Fang et al., 2021; Nagyová and Subr, 2007). For decades, the most common approaches to assembly of DNA constructs and infectious clones have taken advantage of restriction endonuclease specificities to create compatible ends that are joined using DNA ligases. The presence or lack of restriction sites in vector backbones and viral sequences are major constraints to the assembly of large and multiple inserts (Nakahara et al., 2015). The construction of full-length infectious clones of RNA viruses is often laborious due to the many cloning steps required. In the case of Potyvirus, cDNA clones are often unstable due to the toxicity of some viral proteins toward Escherichia coli, but this can be overcome by insertion of introns into the virus genes (Desbiez et al., 2012; Gao et al., 2012; Tran et al., 2019). However, intron insertion requires an additional cloning step to establish restriction sites in the viral sequence. To overcome these problems, sequence- and ligation-independent cloning procedures such as in-fusion cloning (Berrow et al., 2009; Li and Elledge, 2012; Tuo et al., 2015), homologous recombination in yeast (Bao et al., 2020; Cui et al., 2018; Desbiez et al., 2012; Youssef et al., 2011), and Gibson assembly (Blawid and Nagata, 2015; Gibson et al., 2009) have been used to construct full-length cDNA clones of plant viruses.
WMV continues to cause damage to cucurbit crops such as watermelon, melon, cucumber, and pumpkin worldwide, including in Korea. However, there is a lack of reverse genetics tools for WMV to explore virus replication, systemic movement, and symptom development. To develop cucurbit varieties resistant to WMV, it is necessary to study the interactions between the virus and the host plant. The construction of infectious clones of WMV is essential for studying these interactions. In 2012, a full-length cDNA clone of WMV was constructed using a recombination technique in yeast and fusion PCR and by inserting introns (Desbiez et al., 2012).
Generally, when inserting large viral genome, such as potyvirus of about 10 kb in length, or more or when performing subsequent modification of clones, the use of a large binary vector (>10 kb) can reduce the efficiency of infectious clones (Lin et al., 2013; Pasin et al., 2018). In addition, clones can become unstable when the expression of unwanted viral genes is increased using plasmid vectors with high copy numbers (Pasin et al., 2018). To overcome these problems, we used Gibson assembly to construct a full-length cDNA clone of the WMV isolate IS-me2 using a small pJL-89 binary vector (Lindbo, 2007) of about 4.7 kb with a low copy number. We evaluated virulence and symptoms of the clone on a variety of host plants.
Materials and Methods
Virus source and RNA extraction
We collected seven melon leaves showing mosaic symptoms (Supplementary Fig. 1A) in the melon greenhouse of Jeonbuk State Agricultural Research & Extension Service in Iksan, Jeonbuk province, Korea in 2015. The samples collected from symptomatic plants were examined by leaf dip using transmission electron microscopy (TEM) and reverse transcription polymerase chain reaction (RT-PCR) using the WMV detection primers (Table 1). The isolate was purified biologically by three times of single local lesion passages using Chenopodium quinoa and were proliferated in zucchini (Cucurbita pepo). Fresh leaf samples were stored at −80°C until use.
Table 1.
Primers used to construct an infectious full-length cDNA clone of WMV
| Primer | Sequence (5′→3′)a | Tm (°C) | Target (amplicon size, bp) |
|---|---|---|---|
| WMV-1F | aaattaaaacaactcataaa | 45 | WMV-frg1 (3,040) |
| WMV-1R | TGATTATGAAAATTTTTGCAACGCTATGTTCCTTATTTATccacatttcgattcccttct | ||
| WMV-2F | ataaataaggaacatagcgt | 53 | WMV-frg2 (3,760) |
| WMV-2R | CATACCCAACTCCAAACATTGTCTCCTTGTGACCATCTGAagagttcgtgagttggcaga | ||
| WMV-3F | tcagatggtcacaaggagac | 53 | WMV-frg3 (3,371) |
| WMV-3Rb | CAGGTCGGACCGCGAGGAGGTGGAGATGCCATGCCGACCCtttttttttttttttttttt | ||
| pJL89-F1 | gggtcggcatggcatctcca | 63 | pJL89 (4,721) |
| pJL89-R1c | CGTTTGTTTTGTTTGATGTCTTTATGAGTTGTTTTAATTTcctctccaaatgaaatgaac | ||
| Detection primer | |||
| WMV-UNI-1F | CAGTTTGAATCATGGTACAGCGC | 55 | WMV (392) |
| WMV-UNI-1R | TGTGCTATTGCTTCTCTTGCCC | ||
Sequences in capital letters represent 40 bp of overlapping nucleotides among the watermelon mosaic virus (WMV) RNAs and the pJL89 vector.
Underlined sequences represent the overlapping region with pJL89 vector nucleotides.
Underlined sequences represent the overlapping region with WMV RNA nucleotides.
Electron microscopy
Quick dip preparation was used for the observation of virus particle morphology in samples collected from the greenhouse and inoculated by infectious clone. Dip preparations were conducted by grinding a small piece (1 × 1 mm) of leaf tissue with 2–3 drops of 2% PTA (phosphotungstic acid), pH 7.0. The extract was mounted on a carbon-stabilized and formvar coated grid (200 mesh). After 1 min later, the excessive sap on grid was filtered off with a piece of filter paper. Electron microscopy was done with 80 KV with LEO 912AB (Carl Zeiss, Jena, Germany).
Total RNA extraction and RT-PCR
Total RNA was extracted from WMV-infected leaves using the EASY-spin (DNA-free) total RNA extraction kit (Intron, Seongnam, Korea) according to the manufacturer’s instructions. Specific primer pairs were designed to construct a full-length cDNA clone of WMV-IS-me2 and detect WMV infection (Table 1). For the amplification of three WMV genome fragments and the backbone pJL89 vector, RT-PCR reactions were carried out using the SuperScript IV One-step RT-PCR System (Invitrogen, Thermo Fisher Scientific, Seoul, Korea). The reaction was performed in a volume of 50 μl containing 1 μl total nucleic acids (0.5 μg/μl), 2.5 μl forward primer (10 μM), 2.5 μl reverse primer (10 μM), 25 μl 2× Platinum SuperFi RT-PCR master mix, 0.5 μl SuperScript IV RT mix, and nuclease-free water. The RT-PCR conditions were as follows: reverse transcription at 50°C for 10 min and RT inactivation/initial denaturation at 98°C for 2 min; amplification of 35 cycles of denaturation at 98°C for 10 s, annealing at 45°C (for WMV-1F/1R), 53°C (for WMV-2F/2R, -3F/3R), or 63°C (for pJL89-F1/R1) for 10 s, and extension at 72°C for 5 min; and a final extension at 72°C for 5 min. The amplified fragments were purified using a MEGAquick-spin agarose gel extraction kit (Intron), and the concentrations of the purified fragments were measured using the Nanodrop 2000c (Thermo Fisher Scientific). For WMV detection, one-step RT-PCR was conducted in a 20 μl final volume. The reaction mixtures included 10 μL of RT-PCR master mix 2× (Genetbio, Daejeon, Korea), 1 μl of total RNA (approx. 0.5 μg), 0.5 μl each of 10 μM forward and reverse primers, and 8 μl of ddH2O. The RT-PCR conditions were as follows: 30 min at 50°C; 10 min at 94°C; and 35 cycles consisting of 30 s at 94°C, 30 s at 55°C, 60 s at 72°C, and 5 min at 72°C. PCR products were analyzed by electrophoresis on a 1% agarose gel at 100 V for 60 min, and DNA bands were visualized using a UV transilluminator.
Construction of a full-length cDNA clone of WMV using Gibson assembly
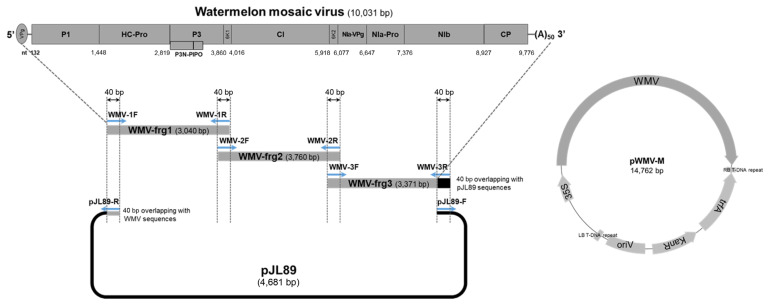
To construct the infectious cDNA clone of WMV, three fragments covering the full-length genomic RNA of WMV-IS-me (GenBank accession no. MN854647, 10,031 nt) were amplified using the primer pairs WMV-1F/1R, −2F/2R, and −3F/3R, and linearized binary vector was prepared from pJL89 (4,707 nt, Addgene, Cambridge, MA, USA) by PCR using the primer pair pJL89-F1/R1 (Table 1). These four fragments share overlapping sequences of 40 bp at both the 5′ and 3′ ends (Table 1, Fig. 1). To generate the recombinant plasmid containing the whole WMV genome, the RT-PCR amplified genomic cDNA fragments of WMV-IS-me and PCR-linearized pJL89 vector were fused using Gibson Assembly Master Mix (New England BioLabs, Hitchin, UK). The reaction was performed in a total volume of 20 μl, containing 1.5 μl (~0.05 pmol) of the gel-purified pJL89 vector (113 ng/μl); 2 μl, 3.5 μl, and 3 μl (0.1 pmol each) of the gel-purified viral fragments for WMV-1F/1R (88 ng/μl), WMV-2F/2R (76 ng/μl), and WMV-3F/3R (77 ng/μl), respectively; and 10 μl of the 2× GA master mix. The reaction mix was incubated at 50°C for 1 h, and then GA products were dialyzed for 15 min using a cellulose membrane (VSWV, 0.025 μm) (Merck-Millipore, Darmstadt, Germany).
Fig. 1.
Strategy for clone construction via Gibson assembly. Three genomic fragments covering the full-length genomic sequence and a fragment of the pJL89 vector were assembled to generate pWMV-M through a GA reaction. Arrows indicate the primers used (Table 1). WMV, watermelon mosaic virus.
E. coli and A. tumefaciens transformation of the full-length cDNA clone of WMV
The dialyzed GA product (2 μl) was used for transformation of competent cells of the E. coli strain DH10B (Thermo Fisher Scientific) by heat shock according to a standard protocol. Positive colonies containing the expected cDNA clone of WMV were screened by kanamycin resistance and confirmed by colony PCR using detection primers (Table 1). The plasmids were extracted from PCR-positive colonies to sequence the full-length cDNA clone of WMV. Constructs of the WMV clone (pWMV-M, 2 μl, 1.7–2.8 μg/μl) were transformed into 100 μl of the A. tumefaciens strain GV3101 using the freeze-thaw method. The transformants were selected on yeast extract peptone (YEP) medium containing 50 mg/l kanamycin and 50 mg/l rifampicin for 2 days at 28°C. In agro-transformation of plasmid pWMV-M, three transformants were selected and confirmed via colony PCR.
A. tumefaciens-mediated agrotransfection
To test the pathogenicity of pWMV-M clone, each of three Agrobacterium cultures was infiltrated into host plants. A positive single colony of Agrobacterium harboring a full-length WMV cDNA clone was grown at 28°C overnight in YEP medium containing kanamycin (50 mg/l), rifampicin (50 mg/l), and acetosyringone (20 μM). The cultures were harvested by centrifugation at 5,000 rpm for 10 min, resuspended in MMA infiltration buffer (10 mM MES, pH 5.6, 10 mM MgCl2, 200 μM acetosyringone) to a final OD600 of 0.6, and incubated at 28°C for 2 h. The resuspended Agrobacterium cultures containing each plasmid were infiltrated into the abaxial surface of cotyledons of melon (C. melo), zucchini (C. pepo), watermelon (C. lanatus), and true leaves of N. benthamiana using a 1-ml syringe without a needle. Transformed Agrobacterium containing only the binary vector pJL-89 were used as a negative control. All of the inoculated plants were kept in an aphid-proof greenhouse at 25–28°C, and after 30 days of inoculation the symptoms of inoculated plant leaves were photographed and subjected to RT-PCR using WMV detection primers (Table 1).
Sequence and phylogenetic analysis of the clone
The sequence of the clone was determined by a commercial company (Bionics, Daejeon, Korea). The sequences were assembled using SeqMan of the DNAStar v. 5.02 program (Lasergene, Madison, WI, USA). The nucleotide sequences and the deduced amino acid sequence of the clone were aligned using the Geneious method in Geneious Pro 10 software and compared with those of WMV-IS-me (wild type) and previously reported WMV isolates. The phylogenetic relationships of the clone were analyzed using the maximum likelihood method in MEGA-X (Kumar et al., 2018) with best-fit nucleotide substitution models (TN93+G+I for the CP gene). Bootstrap values were calculated using 1,000 random replications. All positions containing gaps and missing data were eliminated. A soybean mosaic virus isolate (accession no. NC_002634) was used as an outgroup.
Results
Virus source
The melon samples collected from symptomatic plants were examined by leaf dip using TEM and revealed filamentous virus particles about 700 nm long (Supplementary Fig. 1B). The RT-PCR result using the WMV detection primers (Table 1) showed all tested samples were positive for WMV (Supplementary Fig. 1C). One (WMV-IS-me2) of the seven WMV-positive samples was purified biologically and proliferated in zucchini.
Construction of the clone using Gibson assembly
Four overlapping PCR fragments of the full-length genome of this isolate WMV-IS-me2 and the pJL89 binary vector were assembled in an isothermal GA reaction, and the resulting construct was named pWMV-M (Fig. 1). After E. coli (DH10B strain) transformation of GA reaction product, three colonies were randomly picked and confirmed to be positive for WMV via colony PCR. The sequences of plasmid DNA purified from positive colonies were identical. The full sequence of the cloned pWMV-M was determined using the dideoxy chain termination method and deposited in GenBank (GenBank accession no. OR134098, 10,081 nt [containing polyA]).
Infectivity of the clone in host plants
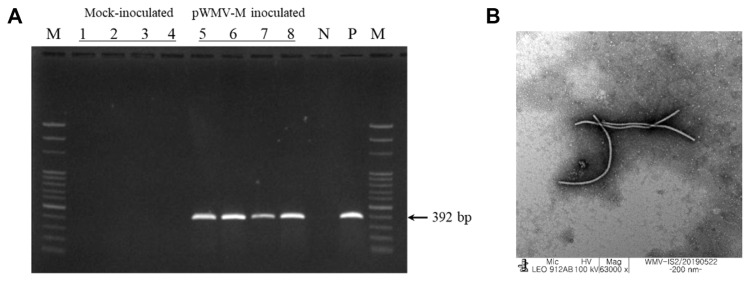
To examine the infectivity of the cloned pWMV-M, agroinfiltration was performed as described previously (Kwak et al., 2016). At 10 days post-inoculation (dpi), the upper leaves of plants agroinfiltrated with pWMV-M began to develop mosaic symptoms. After 3 weeks post-inoculation, N. benthamiana and watermelon showed mild mosaic symptoms, and melon and zucchini showed mosaic symptoms compared to mock-inoculated plants (Fig. 2). No symptoms were observed in the mock-control plants (Fig. 2). To confirm WMV infection of the inoculated plants, total RNAs extracted from the upper non-inoculated leaves were subjected to RT-PCR using the WMV detection primers (Table 1). A band of the predicted size (approximately 392 bp) was observed only from symptomatic plants (Fig. 3A), indicating that these plants were systemically infected with WMV. In addition, the typical WMV virion, a filamentous rod of about 700 nm in length, was visualized by TEM in symptomatic plants (Fig. 3B). Only two of the three agro-transformants were confirmed to infect all four plants with WMV (Table 2, Supplementary Fig. 2A), when the first agro-transformant was re-inoculated into six N. benthamiana plants, five out of the six plants were confirmed to be infected with WMV (Supplementary Fig. 2B). We further evaluated the infectivity of the pWMV-M clone by mechanical inoculation to three identical plants with inoculum from each plant previously agroinoculated by pWMV-M clone. All the plants were infected and confirmed to be positive for WMV detection (Supplementary Fig. 2C). Therefore, the results demonstrated that pWMV-M is fully infectious. The pWMV clone was deposited into the Korean Agricultural Culture Collection (KACC, Wanju, Korea) under accession no. KACC95145P.
Fig. 2.
Symptoms observed after 21 dpi in melon (Cucumis melo), zucchini (Cucurbita pepo), watermelon (Citrullus lanatus), and Nicotiana benthamiana mock-inoculated plants, inoculated with pWMV-M clone, and inoculated with the WMV -IS-Me2 wild-type isolate. WMV, watermelon mosaic virus.
Fig. 3.
(A) Detection by reverse transcription polymerase chain reaction (RT-PCR) of watermelon mosaic virus (WMV) in cucurbit and tobacco plants inoculated with the pWMV-M infectious clone. Total RNAs, isolated from upper non-inoculated leaves of each plant, were analyzed at 30 dpi by RT-PCR. Lanes 1 to 4, mock (empty binary vector)-inoculated plants; lanes 5 to 8, pWMV-M infectious clone-inoculated plants; lanes 1 and 5, watermelon; 2 and 6, zucchini; 3 and 7, melon; 4 and 8, Nicotiana benthamiana. N, negative (healthy) control; P, positive control inoculated with original isolate; M, 100-bp ladder. (B) Filamentous virus particles from pWMV-M inoculated melon leaves.
Table 2.
Symptoms and infectivity on host plants inoculated with the original virus isolate (WMV-IS-me2) and the WMV infectious clone (pWMV-M)
| Province | WMV-IS-me2 (wild-type) | pWMV-M (clone) | ||
|---|---|---|---|---|
|
|
|
|||
| Symptoms | No. of plants infected/no. of plants inoculated | Symptoms | No. of plants infected/no. of plants inoculated | |
| Cucumis melo (melon) | M, VB, VC | 3/3 | M | 2/3 |
| Cucurbita pepo (zucchini) | M, VB | 3/3 | M | 2/3 |
| Citrullus lanatus (watermelon) | M, VB, VC | 3/3 | MM | 2/3 |
| Nicotiana benthamina | - | - | MM | 2/3 |
Symptoms: M, mosaic; MM, mild mosaic; VB, vein banding; VC, vein chlorosis; -, not tested.
WMV, watermelon mosaic virus.
Sequence and phylogenetic analysis of the pWMV clone
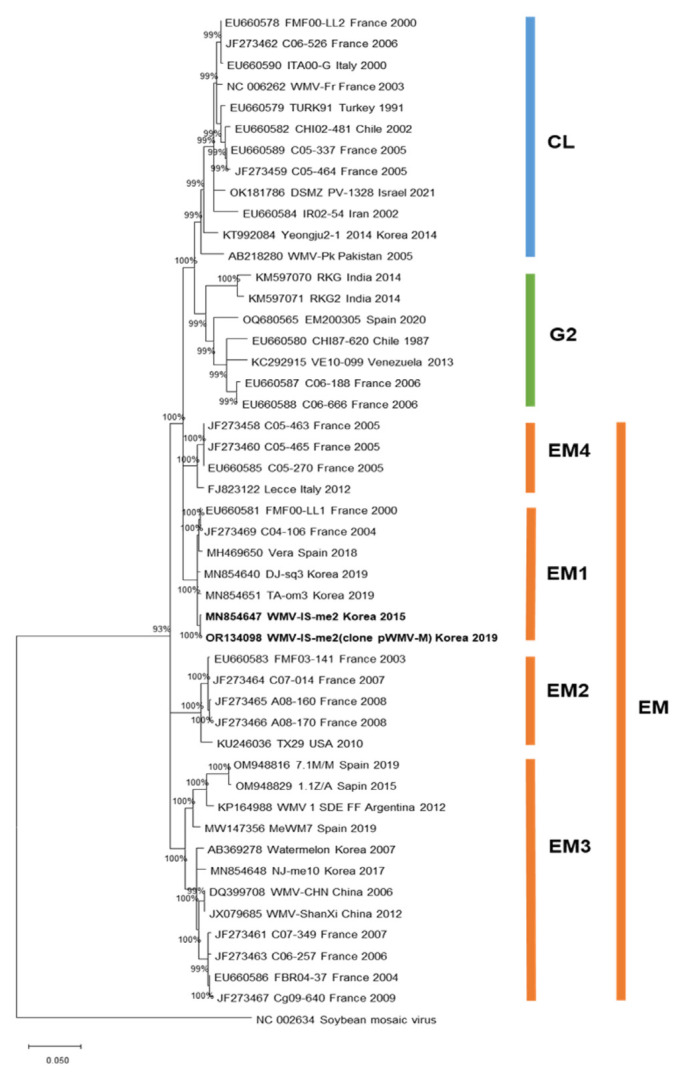
The clone sequence was aligned with those of 46 other WMV isolates, including the WMV-IS-Me2 wild-type isolate, from NCBI GenBank to examine the phylogenetic relationships among WMV isolates. A maximum likelihood tree was constructed based on the CP sequences of 47 WMV isolates; it indicated that the pWMV-M clone and WMV-IS-me isolate clustered with the EM1 subgroup of EM isolates (Fig. 4). The pWMV-M clone had the highest nucleotide sequence identity, 99.8%, with the WMV-IS-Me2 wild-type isolate (MN854647) and showed 95–99% identities with other EM groups and 93–94% identities with the CL and G2 groups.
Fig. 4.
Phylogenetic analysis based on the nucleotide sequences of complete coat protein (CP) genes of the pWMV-M clone, WMV-IS-me isolate, and the watermelon mosaic virus (WMV) isolates previously reported in GenBank. The phylogenetic tree was constructed by maximum likelihood in MEGA X using the TN93+G+I model. The bootstrap values are indicated at the nodes (based on 1,000 replicates). Viruses isolated in this study are shown in bold font. The tree was rooted using soybean mosaic virus isolate (accession no. NC_002634) as an outgroup.
Molecular and pathological comparison between the WMV wild-type and infectious clone
To confirm the pathogenicity and infectivity of the pWMV-M clone, the Agrobacterium culture was repeatedly infiltrated into cucurbit crops and tobacco and then compared to the original wild-type WMV-IS-me2 isolate. The cucurbits melon, zucchini, and watermelon agroinfiltrated with the pWMV-M clone showed milder symptoms of mosaic without vein banding compared to the original wild-type isolate (Table 2, Fig. 2). Full-length sequencing of the clone confirmed that out of 13 nucleotide changes, eight amino acid substitutions were present in seven proteins as compared to the original wild-type WMV-IS-me2 isolate (Table 3).
Table 3.
Amino acid differences between the viral proteins encoded by the original virus isolate (WMV-IS-me2) and the WMV infectious clone (pWMV-M)
| No. | Region | Amino acid position on the polyprotein precursor (on each protein) | WMV-IS-me2a (wild-type) | pWMV-M (clone) |
|---|---|---|---|---|
| 1 | P1 | 135 (135) | Glu (E) | Lys (K) |
| 2 | HC-Pro | 564 (125) | Cys (C) | Tyr (Y) |
| 3 | P3 | 1,059 (163) | Trp (W) | Cys (C) |
| 4 | P3 | 1,060 (164) | Arg (R) | Ala (A) |
| 5 | CI | 1,790 (495) | Ala (A) | Val (V) |
| 6 | NIa-Pro | 2,196 (24) | Ser (S) | Phe (F) |
| 7 | NIb | 2,821 (406) | His (H) | Tyr (Y) |
| 8 | CP | 3,010 (78) | Val (V) | Ile (I) |
WMV, watermelon mosaic virus.
Previously reported WMV-IS-me2 sequence (GenBank accession no. MN854647).
Discussion
WMV is an economically important virus that infects cucurbits and horticultural crops in temperate and Mediterranean climatic regions of the world (Desbiez et al., 2009). Infected plants generally show mosaic, vein banding, vein clearing, and malformation symptoms on leaves. WMV has a wide host range that includes more than 170 species of plants belonging to 27 families (Shukla et al., 1994). In Korea, WMV was first reported in sesame, squash, and cucumber in 1980 (Chang and Lee, 1980) and later in various cucurbits, including melon, watermelon, pumpkin, and gourd (Lee and Lee, 1981). Since then, WMV has become widespread in Korean cucurbit crops (Han et al., 2023; Jin et al., 2009; Kim et al., 2012; Ko et al., 2004, 2006, 2007) and has also been reported in other crops, such as Korean ginseng (Panax ginseng) (Choi et al., 2014; Jung et al., 2013) and cluster mallow (Malva verticillata) (Kim et al., 2019). WMV is one of the most prevalent and widespread viruses, often detected in co-infection with other viruses in cucurbit crops. Especially, WMV exhibits synergistic symptoms in cucurbit crops during mixed infections with cucumber green mottle mosaic virus compared to single infections, which can progress to vascular wilt and plant death in some cases (Kim et al., 2000; Moradi and Jafarpour, 2010; Wang et al., 2017).
In this work, a full-length cDNA clone of a WMV isolate from melon was successfully constructed with a pJL89 binary vector using Gibson assembly. Gibson assembly allows the simultaneous and directional cloning of multiple viral fragments of the complete genomes of viruses in any vector without restriction, digestion, or ligation. The complete nucleotide sequence of the full-length cDNA clone pWMV-M was determined and deposited in GenBank (OR134098) and in KACC (KACC95145P).
The pWMV-M clone and WMV-IS-me isolate were phylogenetically grouped with G3 (EM) group based on their CP sequences (Fig. 4). Emergent strains of WMV have been reported to cause severe symptoms in cucurbits (Desbiez et al., 2009). Recent studies on genetic diversity of WMV revealed that all 56 Chinese isolates (Wang et al., 2017) and 57 U.S. isolates (Abdalla and Ali, 2021) also belong to the G3 group. The pWMV-M clone induced mosaic symptoms on three cucurbit crops (melon, zucchini, and watermelon) and on N. benthamiana (Table 2). However, these symptoms were milder than those caused by the wild-type WMV-IS-me2 isolate. Eight amino acid mutations in seven proteins, including P1, HC-Pro, P3, CI, Pro, RdRp, and CP, were found in the pWMV-M clone comparing to WMV-IS-me2 isolate. Thus, these 8 amino acid sequence differences may function in symptomatology of WMV.
There are currently no reports indicating that mutations in the amino acids of WMV alter its symptoms. However, the key amino acids that influence symptom development in the Potyvirus genus are well-documented. Specifically, the P1, HC-Pro, and P3 proteins play crucial roles in determining the symptoms and pathogenicity. Single amino acid mutations of these proteins can shift the symptoms from severe to mild or even result in no symptoms at all (Urcuqui-Inchima et al., 2001). The FRNK and CDN motifs of HC-Pro in Potyvirus are highly conserved and involved in symptom severity and virulence (Atreya et al., 1992; Desbiez et al., 2010; Gao et al., 2012; Seo et al., 2011).
It remains to be investigated which genes of WMV derived from the infectious full-length clone are involved in the expression of different symptoms. We will study the role of key amino acids in WMV pathogenicity and symptom development using the pWMV-M infectious clone. This clone provides a powerful tool to study gene function, pathogenicity, and virus-host interactions, and may contribute to a better understanding of the molecular biology of WMV.
Acknowledgments
This research was supported by grants from the Agenda Program (PJ01491302), funded by the Rural Development Administration of Republic of Korea.
Footnotes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Electronic Supplementary Materials
Supplementary materials are available at The Plant Pathology Journal website (http://www.ppjonline.org/).
References
- Abdalla O. A., Ali A. Genetic variability and evidence of a new subgroup in Watermelon mosaic virus isolates. Pathogens. 2021;10:1245. doi: 10.3390/pathogens10101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atreya C. D., Atreya P. L., Thornbury D. W., Pirone T. P. Site-directed mutations in the potyvirus HC-Pro gene affect helper component activity, virus accumulation, and symptom expression in infected tobacco plants. Virology. 1992;191:106–111. doi: 10.1016/0042-6822(92)90171-k. [DOI] [PubMed] [Google Scholar]
- Bao W., Yan T., Deng X., Wuriyanghan H. Synthesis of full-length cDNA infectious clones of Soybean mosaic virus and functional identification of a key amino acid in the silencing suppressor Hc-Pro. Viruses. 2020;12:886. doi: 10.3390/v12080886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrow N. S., Alderton D., Owens R. J. The precise engineering of expression vectors using high-throughput In-Fusion PCR cloning. Methods Mol. Biol. 2009;498:75–90. doi: 10.1007/978-1-59745-196-3_5. [DOI] [PubMed] [Google Scholar]
- Bertin S., Manglli A., McLeish M., Tomassoli L. Genetic variability of watermelon mosaic virus isolates infecting cucurbit crops in Italy. Arch. Virol. 2020;165:937–946. doi: 10.1007/s00705-020-04584-9. [DOI] [PubMed] [Google Scholar]
- Blawid R., Nagata T. Construction of an infectious clone of a plant RNA virus in a binary vector using one-step Gibson Assembly. J. Virol. Methods. 2015;222:11–15. doi: 10.1016/j.jviromet.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Boyer J. C., Haenni A. L. Infectious transcripts and cDNA clones of RNA viruses. Virology. 1994;198:415–426. doi: 10.1006/viro.1994.1053. [DOI] [PubMed] [Google Scholar]
- Brewer H. C., Hird D. L., Bailey A. M., Seal S. E., Foster G. D. A guide to the contained use of plant virus infectious clones. Plant Biotechnol. J. 2018;16:832–843. doi: 10.1111/pbi.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M. U., Lee C. U. A virus disease of sesame (Sesamum idicum L.) caused by watermelon mosaic virus (WMV) Korean J. Plant. Prot. 1980;19:193–198. (in Korean) [Google Scholar]
- Chare E. R., Holmes E. C. A phylogenetic survey of recombination frequency in plant RNA viruses. Arch. Virol. 2006;151:933–946. doi: 10.1007/s00705-005-0675-x. [DOI] [PubMed] [Google Scholar]
- Choi S.-K., Cho I.-S., Chung B.-N., Kim M.-K., Jung W.-K., Choi G.-S. Characteristics of watermelon mosaic virus transmission occurring in Korean ginseng. Res. Plant Dis. 2014;20:206–210. (in Korean) [Google Scholar]
- Cui T., Bin Y., Yan J., Mei P., Li Z., Zhou C., Song Z. Development of infectious cDNA clones of Citrus yellow vein clearing virus using a novel and rapid strategy. Phytopathology. 2018;108:1212–1218. doi: 10.1094/PHYTO-02-18-0029-R. [DOI] [PubMed] [Google Scholar]
- Desbiez C., Chandeysson C., Lecoq H., Moury B. A simple, rapid and efficient way to obtain infectious clones of potyviruses. J. Virol. Methods. 2012;183:94–97. doi: 10.1016/j.jviromet.2012.03.035. [DOI] [PubMed] [Google Scholar]
- Desbiez C., Costa C., Wipf-Scheibel C., Girard M., Lecoq H. Serological and molecular variability of Watermelon mosaic virus (genus Potyvirus) Arch. Virol. 2007;152:775–781. doi: 10.1007/s00705-006-0899-4. [DOI] [PubMed] [Google Scholar]
- Desbiez C., Girard M., Lecoq H. A novel natural mutation in HC-Pro responsible for mild symptomatology of Zucchini yellow mosaic virus (ZYMV, Potyvirus) in cucurbits. Arch. Virol. 2010;155:397–401. doi: 10.1007/s00705-009-0569-4. [DOI] [PubMed] [Google Scholar]
- Desbiez C., Joannon B., Wipf-Scheibel C., Chandeysson C., Lecoq H. Emergence of new strains of Watermelon mosaic virus in South-eastern France: evidence for limited spread but rapid local population shift. Virus Res. 2009;141:201–208. doi: 10.1016/j.virusres.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Desbiez C., Lecoq H. The nucleotide sequence of Watermelon mosaic virus (WMV, Potyvirus) reveals interspecific recombination between two related potyviruses in the 5′ part of the genome. Arch. Virol. 2004;149:1619–1632. doi: 10.1007/s00705-004-0340-9. [DOI] [PubMed] [Google Scholar]
- Desbiez C., Lecoq H. Evidence for multiple intraspecific recombinants in natural populations of Watermelon mosaic virus (WMV, Potyvirus) Arch. Virol. 2008;153:1749–1754. doi: 10.1007/s00705-008-0170-2. [DOI] [PubMed] [Google Scholar]
- Fang L., Wei X.-Y., Liu L.-Z., Zhou L.-X., Tian Y.-P., Geng C., Li X.-D. A tobacco ringspot virus-based vector system for gene and microRNA function studies in cucurbits. Plant Physiol. 2021;186:853–864. doi: 10.1093/plphys/kiab146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R., Tian Y.-P., Wang J., Yin X., Li X.-D., Valkonen J. P. T. Construction of an infectious cDNA clone and gene expression vector of Tobacco vein banding mosaic virus (genus Potyvirus) Virus Res. 2012;169:276–281. doi: 10.1016/j.virusres.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Gibson D. G., Young L., Chuang R.-Y., Venter J. C., Hutchison C. A., 3rd, Smith H. O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- Hajizadeh M., Bahrampour H., Abdollahzadeh J. Genetic diversity and population structure of Watermelon mosaic virus. J. Plant Dis. Prot. 2017;124:601–610. [Google Scholar]
- Han J.-W., Park Y.-U., Youn C.-K., Lee S.-H., Jeong T.-G., Choi H.-S., Kim M.-K. Incidence of virus diseases in major cultivated areas of watermelon and melon in Chungbuk province. Res. Plant Dis. 2023;29:88–93. (in Korean) [Google Scholar]
- Jin T.-S., Kim S.-M., Ko S.-J., Lee S.-H., Choi H.-S., Park J.-W., Cha B.-J. Occurrence of Papaya ringspot virus infecting cucurbit crops in Korea. Korean J. Pestic. Sci. 2009;13:298–308. [Google Scholar]
- Jung W.-K., Nam M., Lee J. H., Park C. Y., Kim B. H., Park E. H., Lee M.-A., Kim M.-K., Choi H.-S., Lee J. S., Kim J.-S., Choi J. K., Kwon T. R., Lee K.-W., Lee S.-H. Novel pathogenic strain of Watermelon mosaic virus occurred on Insam (Panax ginseng) Res. Plant Dis. 2013;19:331–337. (in Korean) [Google Scholar]
- Kim J.-E., Kwak H.-R., Choi H.-S., Kim M., Jung W.-K., Seo J.-K., Kim J.-S., Cha B. First report of Watermelon mosaic virus on Malva verticillata in Korea. Plant Dis. 2019;103:380. [Google Scholar]
- Kim J. S., Cho J. D., Choi H. S., Kim K. S. Ultrastructural aspects of the mixed infections of Watermelon mosaic potyvirus and Cucumber green mottle mosaic tobamovirus isolated from watermelon. Plant Pathol. J. 2000;16:211–215. [Google Scholar]
- Kim J.-S., Lee S.-H., Choi H.-S., Kim M.-K., Kwak H.-R., Kim J.-S., Nam M., Cho J.-D., Cho I.-S., Choi G.-S. 2007–2011 Characteristics of plant virus infections on crop samples submitted from agricultural places. Res. Plant Dis. 2012;18:277–289. (in Korean) [Google Scholar]
- Ko S.-J., Lee Y.-H., Cha K.-H., Lee S.-H., Choi H.-S., Choi Y.-S., Lim G.-C., Kim K.-H. Incidence and distribution of virus diseases on cucumber in Jeonnam province during 1999–2002. Plant Pathol. J. 2006;22:147–151. [Google Scholar]
- Ko S.-J., Lee Y.-H., Cha K.-H., Park J.-W., Lee S.-H., Yang K.-Y. Virus diseases occurred on watermelon in Jeonnam province. Res. Plant Dis. 2004;10:39–43. (in Korean) [Google Scholar]
- Ko S.-J., Lee Y.-H., Cho M.-S., Park J.-W., Choi H.-S., Lim G.-C., Kim K.-H. The incidence of virus diseases on melon in Jeonnam province during 2000–2002. Plant Pathol. J. 2007;23:215–218. [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak H.-R., Lee Y.-J., Kim J., Kim M.-K., Kim J.-S., Choi H.-S., Seo J.-K. A determinant of disease symptom severity is located in RNA2 of broad bean wilt virus 2. Virus Res. 2016;211:25–28. doi: 10.1016/j.virusres.2015.09.018. [DOI] [PubMed] [Google Scholar]
- Lecoq H., Fabre F., Joannon B., Wipf-Scheibel C., Chandeysson C., Schoeny A., Desbiez C. Search for factors involved in the rapid shift in Watermelon mosaic virus (WMV) populations in South-eastern France. Virus Res. 2011;159:115–123. doi: 10.1016/j.virusres.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Lee H. S., Lee K. W. Incidence of Watermelon mosaic virus in cucurbits. Korean J. Plant. Prot. 1981;20:191–195. (in Korean) [Google Scholar]
- Li M. Z., Elledge S. J. SLIC: a method for sequence- and ligation-independent cloning. Methods Mol. Biol. 2012;852:51–59. doi: 10.1007/978-1-61779-564-0_5. [DOI] [PubMed] [Google Scholar]
- Lin Y.-Y., Fang M.-M., Lin P.-C., Chiu M.-T., Liu L.-Y., Lin C.-P., Lin S.-S. Improving initial infectivity of the Turnip mosaic virus (TuMV) infectious clone by an mini binary vector via agro-infiltration. Bot. Stud. 2013;54:22. doi: 10.1186/1999-3110-54-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindbo J. A. TRBO: a high-efficiency tobacco mosaic virus RNA-based overexpression vector. Plant Physiol. 2007;145:1232–1240. doi: 10.1104/pp.107.106377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi Z., Jafarpour B. Mixed infections of Watermelon mosaic potyvirus and Cucumber green mottle mosaic tobamovirus in cucurbit hosts. Plant Prot. J. 2010;2:333–346. [Google Scholar]
- Nagyová A., Subr Z. Infectious full-length clones of plant viruses and their use for construction of viral vectors. Acta Virol. 2007;51:223–237. [PubMed] [Google Scholar]
- Nakahara K. S., Nishino K., Uyeda I. Construction of infectious cDNA clones derived from the potyviruses Clover yellow vein virus and Bean yellow mosaic virus. Methods Mol. Biol. 2015;1236:219–227. doi: 10.1007/978-1-4939-1743-3_16. [DOI] [PubMed] [Google Scholar]
- Olspert A., Chung BY-W, Atkins J. F., Carr J. P., Firth A. E. Transcriptional slippage in the positive-sense RNA virus family Potyviridae. EMBO Rep. 2015;16:995–1004. doi: 10.15252/embr.201540509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasin F., Tseng X.-A., Bedoya L. C., Heydarnejad J., Deng T.-C., García J. A., Chen Y.-R. Streamlined generation of plant virus infectious clones using the pLX mini binary vectors. J. Virol. Methods. 2018;262:48–55. doi: 10.1016/j.jviromet.2018.09.007. [DOI] [PubMed] [Google Scholar]
- Purcifull D., Hiebert E., Edwardson J. R. CMI/AAB descriptions of plant viruses. No. 293. Association of Applied Biology; Wellesbourne, UK: 1984. p. 7. [Google Scholar]
- Seo J.-K., Sohn S.-H., Kim K.-H. A single amino acid change in HC-Pro of soybean mosaic virus alters symptom expression in a soybean cultivar carrying Rsv1 and Rsv3. Arch. Virol. 2011;156:135–141. doi: 10.1007/s00705-010-0829-3. [DOI] [PubMed] [Google Scholar]
- Shukla D. D., Ward C. W., Brunt A. A. The Potyviridae. CAB International; Wallingford, UK: 1994. p. 516. [Google Scholar]
- Tran P.-T., Fang M., Widyasari K., Kim K.-H. A plant intron enhances the performance of an infectious clone in planta. J. Virol. Methods. 2019;265:26–34. doi: 10.1016/j.jviromet.2018.12.012. [DOI] [PubMed] [Google Scholar]
- Tuo D., Shen W., Yan P., Li X., Zhou P. Rapid construction of stable infectious full-length cDNA clone of papaya leaf distortion mosaic virus using in-fusion cloning. Viruses. 2015;7:6241–6250. doi: 10.3390/v7122935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuqui-Inchima S., Haenni A. L., Bernardi F. Potyvirus proteins: a wealth of functions. Virus Res. 2001;74:157–175. doi: 10.1016/s0168-1702(01)00220-9. [DOI] [PubMed] [Google Scholar]
- Wang D., Li G., Du S. S. Occurrence of viruses infecting melon in Xinjiang of China and molecular characterization of Watermelon mosaic virus isolates. Eur. J. Plant Pathol. 2017;147:919–931. [Google Scholar]
- Youssef F., Marais A., Faure C., Gentit P., Candresse T. Strategies to facilitate the development of uncloned or cloned infectious full-length viral cDNAs: Apple chlorotic leaf spot virus as a case study. Virol. J. 2011;8:488. doi: 10.1186/1743-422X-8-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.