Abstract
In 2022, citrus fruits were the second most widely produced fruit globally, highlighting their significant role in the fruit industry. However, due to their clonal propagation, these fruits are highly susceptible to viral infections, posing challenges for growers. In response to the booming nursery market, the Korean plant quarantine station reported over 80 million sapling stocks, with 15% being discarded after rigorous inspection due to contamination or disease. As the global nursery trade continues to expand, there is an urgent need for a fast and accurate diagnostic tool to ensure the health of plant stocks. In this study, we developed a TaqMan-based real-time reverse transcription-quantitative PCR assay specifically designed to detect two critical citrus viruses: citrus psorosis virus and citrus leprosis virus C. Our assay demonstrated the capability to detect virus sequences with as few as 30 copies, maintaining high PCR efficiency with RNA extracted from both twig and leaf tissues. Additionally, we incorporated an artificial sequence into the positive controls, which effectively served as a marker for detecting potential sample contamination. This comprehensive diagnostic system promises to enhance plant quarantine measures and phytosanitation practices, providing a reliable and efficient solution to safeguard citrus crops from viral threats.
Keywords: citrus, multiplex qPCR, quarantine, TaqMan assay, virus detection
Citrus trees and citrus fruits are of significant economic and agricultural importance worldwide. Economically, citrus fruits such as oranges, lemons, limes, and grapefruits contribute significantly to the agricultural sectors of many countries and drive local economies through both domestic sales and international exports. Citrus is an important source of income for farmers and provides employment opportunities for growing, harvesting, processing, and distribution. Global citrus fruit production has been remarkable in recent years. It is estimated to reach 103.7 million metric tons in 2023, making it the second most produced fruit after bananas and plantains (Food and Agriculture Organizations of the United Nations, 2023; Gonzatto and Santos, 2023; United States Department of Agriculture, 2024). China remains the leading producer, with an output of approximately 48.8 million metric tons in 2022, accounting for nearly 29.25% of global citrus production. Other major producers include Brazil, India, Mexico, and Spain, accounting for more than half of global citrus production (United States Department of Agriculture, 2024). This substantial production supports local economies through domestic sales and contributes significantly to international trade. Citrus fruits such as oranges, tangerines, mandarins, lemons, and grapefruits are major export commodities, with countries like Egypt and South Africa achieving record export levels (United States Department of Agriculture, 2024).
Citrus plants are mainly propagated clonally, as they are generally interspecific hybrids (Rao et al., 2021). It also allows the long juvenile phase to be bypassed and is the main propagation method (Deng et al., 2020). However, viruses can spread much more easily in tissue culture than in seed, and the homogeneity of the population makes it more susceptible to infestation. Many viruses have been reported in citrus (Kim et al., 2024; Lee, 2015). Diseases such as citrus tristeza virus (CTV) and citrus greening (Huanglongbing, HLB) can severely affect the health and productivity of citrus trees, resulting in reduced fruit yield and quality; therefore, greater attention is required to prevent these diseases through quarantine. In Korea, citrus trees and citrus fruits, especially mandarins (locally known as "gamgyul"), are of great economic and agricultural importance. Disease management in citrus is, therefore, critical because of its potentially devastating impact on the industry's economic and agricultural aspects.
Citrus psorosis virus (CPsV) and cytoplasmic-type citrus leprosis virus (CiLV-C) are important due to their detrimental effects on citrus trees, affecting economic viability and agricultural sustainability. CPsV, which belongs to the genus Ophiovirus, can be transmitted by grafting, and yield losses of up to 72% have been reported (Achachi et al., 2014). CPsV infection exhibits a wide range of symptoms, leading to it being the causal agent of notorious diseases such as citrus psorosis and citrus ringspot disease (Barthe et al., 1998). CiLV-C, on the other hand, shares its characteristic disease symptoms with an unrelated virus, the nuclear leprosy virus (Roy et al., 2013). While CiLV-C cannot move systemically within plants, it does produce necrotic, chlorotic lesions where mites have fed (Pascon et al., 2006). Management of CPsV and CiLV-C is essential to protect the economic interests of citrus growers by ensuring high yields and quality fruit, reducing management costs, and maintaining market access. Effective virus management strategies include using virus-free planting material, implementing strict sanitation practices, monitoring and controlling vectors, removing infected trees, and preventing the introduction of these viruses through the quarantine process to mitigate the impact of these viruses.
Several methods are available for CPsV and CiLV-C, including biological indexing (Roistacher, 1991), reverse transcription-polymerase chain reaction (RT-PCR) (Barthe et al., 1998; Locali et al., 2003), enzyme-linked immunosorbent assay (Alioto et al., 1999; Calegario et al., 2013), and transmission electron microscopy (Kitajima et al., 2003). None of the above, except for RT-PCR, suits the plant virus quarantine criteria, which require high sensitivity with the simple and inexpensive method for mass screening. Moreover, it should be flexible with the source tissue as the plants are often asymptomatic or imported without leaves to diagnose symptoms. Therefore, we developed a TaqMan-based real-time qPCR assay for detecting CPsV and CiLV-C. A sequence-specific probe labeled with fluorescent dye not only enhances the specificity over RT-PCR but also enables quantification.
In 2015, the first TaqMan assays for each virus were published (Choudhary et al., 2015; Osman et al., 2015). Since then, many additional viral isolates have been discovered. For instance, a novel lineage of CiLV-C, named SJP, was identified in Brazil in 2016 (Ramos-González et al., 2016). A new primer-probe set, specialized for the SJP lineage, was developed in 2023 (Arena et al., 2023). This set targets the RNA-dependent RNA polymerase region of CiLV-C, thereby avoiding the overrepresentation of subgenomic RNA. As a result, it provides a more accurate quantification of the virus, despite compensating the nucleotide sequence identity with other lineages. TaqMan assay for nine citrus viruses, including CiLV-C and CPsV, was developed earlier this year (Yan et al., 2024). Yan et al. (2024) designed primer sets by selecting target regions that were most conserved and/or had the most sequence data available and selected the capsid protein (CP) of RNA 3 for CPsV, and the movement protein of RNA 2 for CiLV-C. While all these reports have some advantages, we aimed to design highly specific and robust primers and probes for all reported isolates and field applications and selected the CP regions for both viruses. In addition, we presented two probes for each virus and utilized the multiplexity of the TaqMan assay.
Materials and Methods
Sample collection and RNA extraction
All virus-free and infected tissues come from peach and two citrus cultivars (Table 1). Total RNA was extracted from the plant's leaves and branches using the IQeasy plus Plant RNA Extraction Mini Kit (iNtRON, Seongnam, Korea). The primers for reference genes were used to check the quality of branch RNA samples and to adjust the concentration for its optimal PCR efficiency (He et al., 2022).
Table 1.
List of plant and virus materials and their Cq value from the detection set
| Plant species | Pathogen | Abbreviation | Cq value | Source | |
|---|---|---|---|---|---|
|
| |||||
| CiLV-C(1/2) | CPsV(1/2)a | ||||
| Satsuma mandarin (Citrus unshiu Marc. var. Miyagawa-wase) | Healthy | NA/NA | NA/39.67 ± 0.23 | Citrus Research Center, Korea | |
| Citrus tatter leaf virus | CTLV | NA/NA | NA/39.47 ± 0.32 | ||
| Citrus tristeza virus | CTV | NA/NA | NA/39.02 ± 0.11 | ||
| Satsuma dwarf virus | SDV | NA/NA | NA/39.34 ± 0.26 | ||
| Hallabong Tangor (Citrus kiyomi × ponkan) | Healthy | NA/NA | NA/NA | ||
| Citrus mosaic sadwavirus | CiMV | NA/NA | NA/39.44 ± 0.14 | ||
| Peach (Prunus persica) | Hop stunt viroid | HSVd | NA/NA | NA/39.09 ± 0.28 | NIHHS, Korea |
NA, amplification below the detection threshold; NIHSS, National Institute of Horticultural and Herbal Science.
The mean value with standard deviation is presented from 3 technical repeats.
Primer and probe design
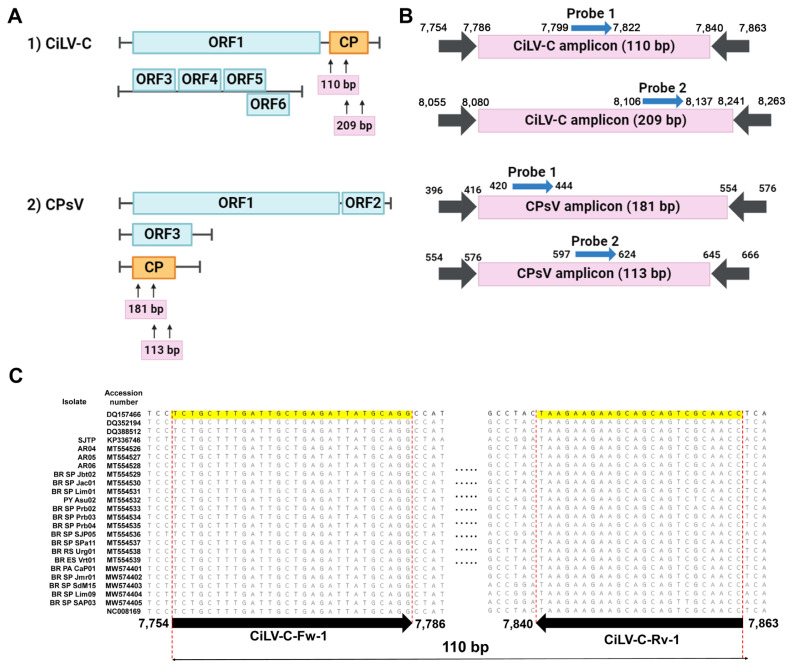
The genome sequences of each virus were retrieved from the GenBank database (http://www.ncbi.nlm.nih.gov). Independent isolates were analyzed to avoid overrepresentation, resulting in 24 for CiLV-C and 108 for CPsV. The sequences were aligned by ClustalW using MEGA X (Kumar et al., 2018). Primers and probes were designed on the CP coding region with high conserveness (Fig. 1A). Probes were labeled with Cyanine5 (Cy5) at the 5' end and Black Hole Quencher (BHQ) 2 at the 3' end. Fluorescein phosphoramidite (FAM) and carboxy tetramethylrhodamine (TAMRA) were tagged on the 5' and 3' end of the CPsV probe candidates when testing multiplexity. For, internal control, hexachloro-fluorescein (HEX) and BHQ1 were labeled each end. The final selection of primers and probes is listed in Table 2.
Fig. 1.
Designing of the primers and probes. (A) The coat protein coding region of 24 cytoplasmic-type CiLV-C isolates was aligned. Arrows indicate primer positions, and numbers indicate nucleotide positions on the consensus sequence. The size of the amplicon is shown below each alignment. (B) Genome organization of CiLV-C and CPsV. The CP region is boxed in orange, and arrows indicate the primer binding sites. (C) Detailed amplicons of each detection set. The binding sites of primers and probes are numbered based on the genomic location and orientation. CiLV-C, citrus leprosis virus; CP, capsid protein; CPsV, citrus psorosis virus; ORF, open reading frame.
Table 2.
List of primers and probes with their amplicon size
| Target virus | Primer (5′ → 3′) | Probe (5′ → 3′) | Amplicon size (bp) |
|---|---|---|---|
| CiLV-C | TCTGCTTTGATTGCTGAGATTATGCAGG | GTGTCTGTTCCTGAAGGTCTGCGT | 110 |
| GGTTGCGACTGCTGCTTCTTCTTA | |||
| CAGAAGCAGATAGGGATGACATATTA | CCTGATTTTAAGGTAATGGAAGCTTCTGAGGA | 209 | |
| ACAATGTAGTGATCACTGAACTC | |||
| CPsV | CAGCAGGGGAYAACTTTTGCT | TCTGAAAACTGCTTGCAAACCCAGC | 181 |
| GAGGARTTGAGCCATGCTCCAAC | |||
| GTTGGAGCATGGCTCAAYTCCTC | CATCACCATCATACAGCTTCGATTTGAC | 113 | |
| CTTCCTGGAAAAGCCGATGACA |
CiLV-C, cytoplasmic-type citrus leprosis virus; CPsV, citrus psorosis virus.
Positive control construction
Viral consensus sequences were synthesized as DNA fragments by L&C Bio, Seongnam, Korea. These fragments were cloned into a pGEM-Teasy vector (Promega, Madison, WI, USA) and then subjected to site-directed mutagenesis to insert an artificial sequence (CAGTCATGCGATCTAG) as a contamination indicator (Supplementary Table 1). The plasmid constructs were subsequently transcribed into RNA in vitro using the MEGAscript T7 Transcription Kit (Invitrogen, Carlsbad, CA, USA).
Real-time reverse transcription-quantitative PCR assay
The reaction mixture of 20 μl contained real-time reverse transcription-quantitative PCR (RT-qPCR) 2× Master Mix (Biocube, Suwon, Korea), 500 nM of each primer and probe. Host plants’ total RNA had the optimal amplification and PCR efficiency around 2 ng/μl, thereby adopted in the experiments. The RT-qPCR was run with a CFX96 real-time thermocycler (Bio-Rad, Hercules, CA, USA). The condition consists of reverse transcription at 42°C for 30 min, initial denaturation at 95°C for 10 min, followed by 40 cycles at 95°C for 10 s, 55°C for 30 s, and 72°C for 1 min.
PCR efficiency and detection limit analysis
We used a ten-fold dilution series of DNA fragments or RNA transcripts as PCR templates. For each detection point, we plotted the corresponding concentration. The qPCR efficiency was calculated based on the slope of the regression line. The viral copy number at the detection threshold of 30 cycles was established as the effective detection limit for the assay.
Results
Screening of primers and probes
The primers and probes targeted conserved regions encoding CPs (Fig. 1A and B). These candidates underwent multiple rounds of screening. Initially, primers were tested using a 10-fold dilution series of DNA templates to check for issues such as primer dimers. Next, RNA templates were mixed with total host RNA to eliminate non-specific amplification. We tested two different natural host species: Hallabong Tangor (Citrus kiyomi × ponkan) and Satsuma mandarin (Citrus unshiu Marc. var. Miyagawa-wase), as well as Nicotiana benthamiana. Plant tissues infected with other viruses and a viroid were also tested to assess potential cross-contamination. These included citrus mosaic sadwavirus (CiMV), citrus tatter leaf virus (CTLV), CTV, hop stunt viroid (HSVd), and satsuma dwarf virus (SDV). The results regarding specificity are summarized in Table 1. The two best-performing primer-probe sets for each virus were selected and are detailed in Table 2 and Fig. 1C.
Highly sensitive and robust assay
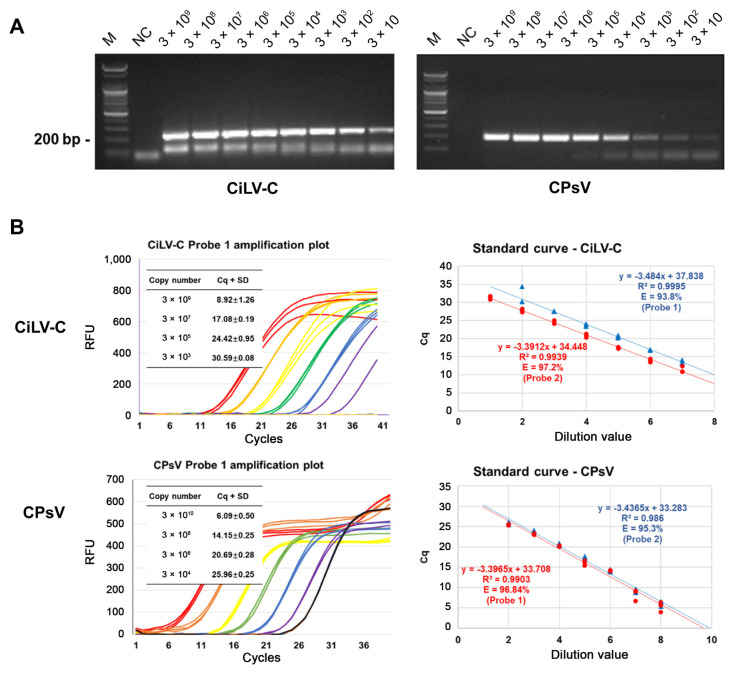
To test the assay's versatility, RNA transcripts were mixed with RNA extracted from woody branches. Despite the presence of high concentrations of host RNA, which delayed the detection time point, the assay remained functional. At a final concentration of 2 ng/μl, host genes were amplified with a quantification cycle (Cq) value of 30 (data not shown), while the assay's overall performance was maintained (Fig. 2A). Cq values and viral concentrations were plotted on a logarithmic scale, producing a standard regression curve (Fig. 2B). All selected primer-probe sets achieved R2 values over 0.98 and PCR efficiency exceeding 95%. We established Cq = 30 as the critical threshold for virus detection, where amplification below this value indicates a genuine infection signal. The corresponding virus copy numbers were 5331 and 6631 for the CiLV-C sets 1 and 2 and 3707 and 2706 for CPsV sets 1 and 2. RT-PCR results were visualized via gel electrophoresis (Fig. 2C), with bands of the correct amplicon sizes confirmed in samples containing as few as 30 initial copies.
Fig. 2.
TaqMan probe-based real-time reverse transcription-quantitative PCR detection of virus sequence in mixture with host RNAs extracted from branch tissue. (A) Reverse transcription-polymerase chain reaction products were electrophoresed on a 1.5% agarose gel stained with ethidium bromide. The negative control (NC) consisted only of total RNA from the host plants. (B) Amplification curves from the Cy5 channel were plotted. From red to navy, each color gradient represents 10 times the dilution of the previous one. The regression lines were generated from each quantification cycle (Cq) value by its concentration. The incline informs the efficiency of the qPCR. CiLV-C, citrus leprosis virus; CPsV, citrus psorosis virus; RFU, relative fluorescence unit.
Multiplexing enables simultaneous detection of viruses and contamination monitoring
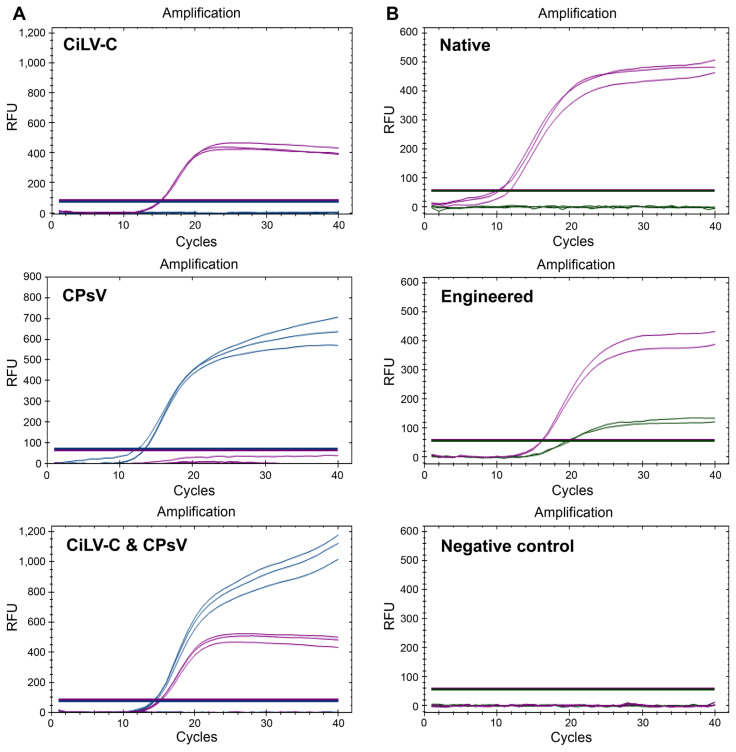
By differentiating fluorescence emission channels, the TaqMan assay can be performed in multiplex mode. CiLV-C and CPsV Using Cy5 and FAM fluorophores for CiLV-C and CPsV, respectively, both viruses were detected simultaneously without significant interference (Fig. 3A). Next, we incorporated a 16-mer artificial sequence into the amplicon sequence. A probe with a complementary sequence, tagged with HEX, was added to the reaction mixture (Fig. 3B). Since this probe cannot bind to natural virus sequences, it functions as a positive control for contamination. The concentration of the HEX probe was adjusted to 50 nM, 1/10 of Cy5, due to its higher sensitivity to the reader machine.
Fig. 3.
Multiplex assay using different fluorescent channels. (A) Both CiLV-C and CPsV set1 were mixed in the real-time reverse transcription-quantitative PCR reactant. Templates are marked on the top left corner. The CiLV-C signal was read from the Cy5 channel, whereas the CPsV signal was read from FAM. (B) Duplex assay of internal control. The positive control vector was left with an artificial sequence trace to discern itself from the virus. The HEX-tagged probe detected the artificial sequence in the engineered vector but not the native virus sequence. CiLV-C, citrus leprosis virus; CPsV, citrus psorosis virus; FAM, fluorescein phosphoramidite; HEX, hexachloro-fluorescein; RFU, relative fluorescence unit.
Discussion
Although several TaqMan RT-qPCR assays have been developed for CiLV-C and CPsV (Arena et al., 2023; Choudhary et al., 2015; Osman et al., 2015; Yan et al., 2024), the detection primer sets described in this study could offer improved and reliable virus diagnosis in comparison. First, the sets were designed to identify a broad range of isolates within the species, aiming to capture nearly all reported variants. The core structural protein, CP, is highly conserved, making it an ideal target for virus detection and identification. After aligning the up-to-date sequences of virus isolates, primers and probes were designed within the CP coding region, with amplicon sizes ranging from 100 to 200 bp. The primers and probe from the earliest CiLV-C assay (Choudhary et al., 2015) were based on a reference isolate resulting in a shortcoming in the coverage of lineage SJP, which was first identified after the study was published. On the other hand, Arena et al. (2023) targeted their assay to lineage SJP. The sequence identities between the primers or probes to the lineage CRD matched between 65% to 93%. Since the focus of this study was on accurate detection and quantification, sequences designed from this study matched more than 90%, especially being identical for 15 nucleotides on 3′ termini, to all reported isolates.
Next, we evaluated the PCR efficiency for each primer and probe set. Using the 10-fold dilution series, each detection point was plotted with its corresponding concentration. Efficiency was calculated from the slope of the regression line, with 100% efficiency indicated by a one-cycle delay in Cq when the initial template concentration is halved. Deviations from 100% efficiency suggest potential issues with primer or probe design. The standard curve also provided insights into the effective detection limit. Although viral DNA or RNA was detectable down to 30 copies when extending the reaction to 50 cycles, false signals from non-specific amplifications increased over time. A Cq value of 30 was used as a reference point for definitive virus detection, with high sensitivity minimizing false negatives. All our selected sets showed ample reaction with less than 7,000 copies, corresponding to 10 pg of the RNA transcript in the total RNA solution from the woody tissue. For CiLV-C, the sensitivity is significantly higher than the report by Choudhary et al. (2015). It was comparable or even to that of Arena et al. (2023) or Yan et al. (2024), which were measured from plasmid DNA or in an RNA mixture from leaf tissue, respectively. Similarly, the limit of detection of the current assay for the CPsV is 30 virus copies which was a couple of times higher than those of Osman et al. (2015) and Yan et al. (2024) assays while also being designed to detect all presently known isolates.
Specificity is another crucial factor, as it helps reduce false positives. We tested the reactivity with the host transcriptome to eliminate candidates with non-specific amplification. Since viruses can exhibit similar symptoms and complex infections can occur, we tested our primers against four viruses and one viroid that share the host: CiMV, CTLV, CTV, HSVd, and SDV. The selected primers did not show significant cross-reactivity, ensuring accurate differentiation of target viruses. For field application, RNA was extracted from woody parts of saplings, as leaves may not be available due to overwintering, stress during shipping, or mechanical damage. Branch tissue presents challenges due to high PCR inhibitor content and low RNA yield (Rowhani et al., 1995). In the optimized condition, the PCR efficiency and detection limits were maintained with RNAs extracted from woody parts of saplings.
The TaqMan probe system enables multiplex assays using different fluorescent dyes. We demonstrated two applications: simultaneous detection to save time and materials and contamination control. We used the Cy5 channel for CiLV-C and the FAM channel for CPsV. The duplex assay had a higher Cq value of about 0.38 ± 0.07 over the simplex assay. The delay is assumed to be from having an additional detection set, as the presence of the matching sequence was irrelevant to the outcome. Next, we integrated a reporter system to manage contamination. An artificial sequence was inserted into the amplicon on the positive control vector, allowing it to be distinguished by a HEX-tagged probe with a complementary sequence. This provides a surveillance system, reducing the risk of misinterpreting control contamination. We hope this work will support clean and safe shoot production and nursery trade.
Acknowledgments
We thank Citrus Research Center (National Institute of Horticultural and Herbal Science, South Korea) for providing us with citrus saplings (healthy and virus-infected). This work was supported in part by grants from the Animal and Plant Quarantine Agency (RQ20231B004) and the Agriculture and Food Convergence Technologies Program for Research Manpower Development, funded by the IPET, the Ministry of Agriculture, Food and Rural Affairs (No. RS-2024-00398300).
Footnotes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Electronic Supplementary Materials
Supplementary materials are available at The Plant Pathology Journal website (http://www.ppjonline.org/).
References
- Achachi A., Ait Barka E., Ibriz M. Recent advances in citrus psorosis virus. VirusDisease. 2014;25:261–276. doi: 10.1007/s13337-014-0199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alioto D., Gangemi M., Deaglio S., Sposato P., Noris E., Luisoni E., Milne R. G. Improved detection of citrus psorosis virus using polyclonal and monoclonal antibodies. Plant Pathol. 1999;48:735–741. [Google Scholar]
- Arena G. D., Ramos-González P. L., Tassi A. D., Machado M. A., Freitas-Astúa J. A TaqMan RT-qPCR assay for absolute quantification of citrus leprosis virus C lineage SJP: disclosing the subgenomic/genomic ratio in plant and mite vector, plant organ-specific viral loads, and the kinetics of viral accumulation in plants. Trop. Plant Pathol. 2023;48:30–41. [Google Scholar]
- Barthe G. A., Ceccardi T. L., Manjunath K. L., Derrick K. S. Citrus psorosis virus: nucleotide sequencing of the coat protein gene and detection by hybridization and RT-PCR. J. Gen. Virol. 1998;79:1531–1537. doi: 10.1099/0022-1317-79-6-1531. [DOI] [PubMed] [Google Scholar]
- Calegario R. F., Locali E. C., Stach-Machado D. R., Peroni L. A., Caserta R., Salaroli R. B., Freitas-Astúa J., Machado M. A., Kitajima E. W. Polyclonal antibodies to the putative coat protein of citrus leprosis virus C expressed in Escherichia coli: production and use in immunodiagnosis. Trop. Plant Pathol. 2013;38:188–197. [Google Scholar]
- Choudhary N., Wei G., Govindarajulu A., Roy A., Li W., Picton D. D., Nakhla M., Levy L., Brlansky R. H. Detection of citrus leprosis virus C using specific primers and TaqMan probe in one-step real-time reverse-transcription polymerase chain reaction assays. J. Virol. Methods. 2015;224:105–109. doi: 10.1016/j.jviromet.2015.08.022. [DOI] [PubMed] [Google Scholar]
- Deng X., Yang X., Yamamoto M., Biswas M. K. Domestication and history. In: Talon M., Caruso M., Gmitter F. G., editors. The genus citrus. Woodhead Publishing; Cambridge, UK: 2020. pp. 33–55. [Google Scholar]
- Food and Agriculture Organizations of the United Nations . Statistical yearbook: world food and agriculture 2023. Food and Agriculture Organizations of the United Nations; Rome, Italy: 2023. p. 384. [Google Scholar]
- Gonzatto M. P., Santos J. S. Citrus research: horticultural and human health aspects. IntechOpen; London, UK: 2023. Introductory chapter: world citrus production and research. [Google Scholar]
- He W., Xie R., Li H., Wang Y., Chen Q., Lin Y., Zhang Y., Luo Y., Zhang Y., Tang H., Wang X. Evaluation of suitable qRT-PCR normalization genes for various citrus rootstocks. Plant Biotechnol. Rep. 2022;16:101–111. [Google Scholar]
- Kim H.-J., Choi S.-R., Cho I.-S., Jeong R.-D. Viral metatranscriptomic analysis to reveal the diversity of viruses infecting satsuma mandarin (Citrus unshiu) in Korea. Plant Pathol. J. 2024;40:115–124. doi: 10.5423/PPJ.OA.01.2024.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima E. W., Chagas C. M., Rodrigues J. C. V. Brevipalpus-transmitted plant virus and virus-like diseases: cytopathology and some recent cases. Exp. Appl. Acarol. 2003;30:135–160. doi: 10.1023/b:appa.0000006546.55305.e3. [DOI] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R. F. Control of virus diseases of citrus. Adv. Virus Res. 2015;91:143–173. doi: 10.1016/bs.aivir.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Locali E. C., Freitas-Astua J., de Souza A. A., Takita M. A., Astua-Monge G., Antonioli R., Kitajima E. W., Machado M. A. Development of a molecular tool for the diagnosis of leprosis, a major threat to citrus production in the Americas. Plant Dis. 2003;87:1317–1321. doi: 10.1094/PDIS.2003.87.11.1317. [DOI] [PubMed] [Google Scholar]
- Osman F., Hodzic E., Kwon S.-J., Wang J., Vidalakis G. Development and validation of a multiplex reverse transcription quantitative PCR (RT-qPCR) assay for the rapid detection of citrus tristeza virus, citrus psorosis virus, and citrus leaf blotch virus. J. Virol. Methods. 2015;220:64–75. doi: 10.1016/j.jviromet.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Pascon R. C., Kitajima J. P., Breton M. C., Assumpção L., Greggio C., Zanca A. S., Okura V. K., Alegria M. C., Camargo M. E., Silva G. G. C., Cardozo J. C., Vallim M. A., Franco S. F., Silva V. H., Jordão H., Jr, Oliveira F., Giachetto P. F., Ferrari F., Aguilar-Vildoso C. I., Franchiscini F. J. B., Silva J. M. F., Arruda P., Ferro J. A., Reinach F., da Silva A. C. R. The complete nucleotide sequence and genomic organization of citrus leprosis associated virus, cytoplasmatic type (CiLV-C) Virus Genes. 2006;32:289–298. doi: 10.1007/s11262-005-6913-1. [DOI] [PubMed] [Google Scholar]
- Ramos-González P. L., Chabi-Jesus C., Guerra-Peraza O., Breton M. C., Arena G. D., Nunes M. A., Kitajima E. W., Machado M. A., Freitas-Astúa J. Phylogenetic and molecular variability studies reveal a new genetic clade of citrus leprosis virus C. Viruses. 2016;8:153. doi: 10.3390/v8060153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M. J., Zuo H., Xu Q. Genomic insights into citrus domestication and its important agronomic traits. Plant Commun. 2021;2:100138. doi: 10.1016/j.xplc.2020.100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roistacher C. N. Graft-transmissible diseases of citrus: handbook for detection and diagnosis. Food and Agriculture Organizations of the United Nations; Rome, Italy: 1991. p. 300. [Google Scholar]
- Rowhani A., Maningas M. A., Lile L. S., Daubert S. D., Golino D. A. Development of a detection system for viruses of woody plants based on PCR analysis of immobilized virions. Phytopathology. 1995;85:347–352. [Google Scholar]
- Roy A., Stone A., Otero-Colina G., Wei G., Choudhary N., Achor D., Shao J., Levy L., Nakhla M. K., Hollingsworth C. R., Hartung J. S., Schneider W. L., Brlansky R. H. Genome assembly of citrus leprosis virus nuclear type reveals a close association with orchid fleck virus. Genome Announc. 2013;1:e00519–13. doi: 10.1128/genomeA.00519-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Agriculture Citrus: world markets and trade. 2024. URL https://apps.fas.usda.gov/psdonline/circulars/citrus.pdf [3 September 2024]
- Yan J., Tang J., Perez-Egusquiza Z., Thompson J. R. Development of TaqMan RT-qPCR for the detection of regulated citrus viruses and viroids in Aotearoa, New Zealand. J. Virol. Methods. 2024;327:114950. doi: 10.1016/j.jviromet.2024.114950. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.