Abstract
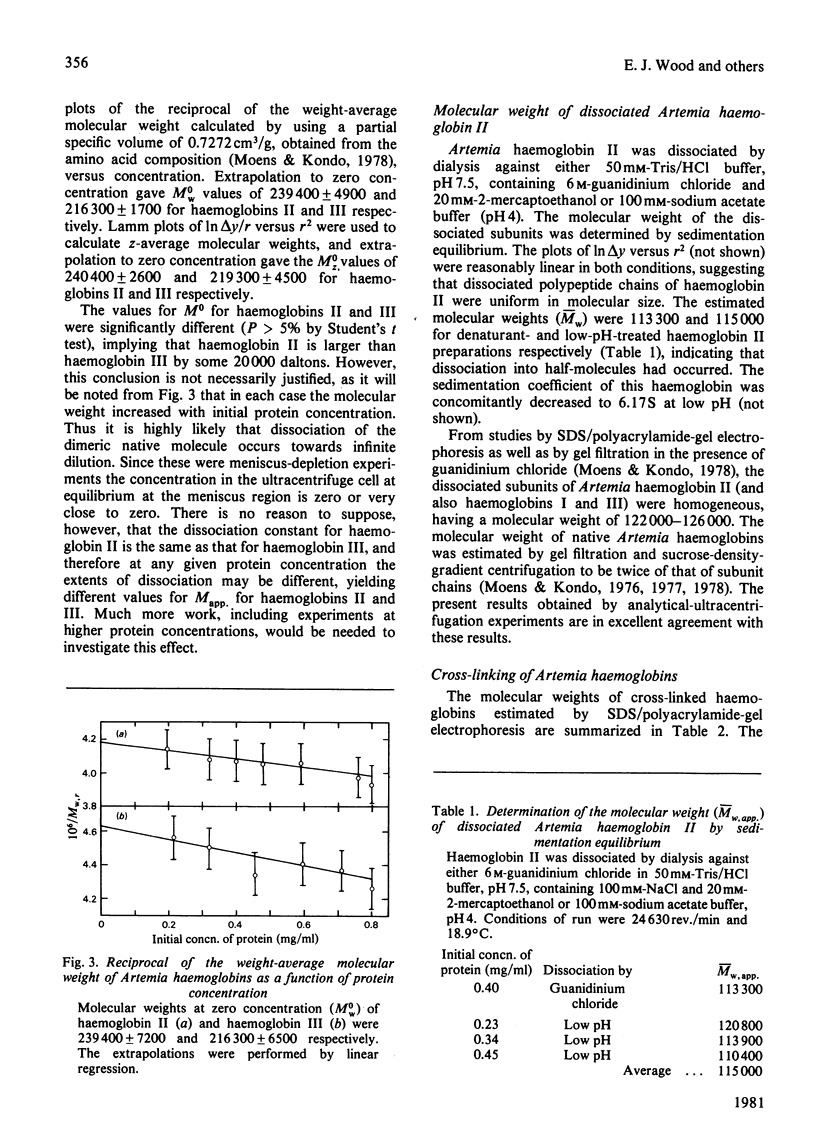
Sedimentation coefficients (s0 20,w) of 11.57 +/- 0.10 S and 11.52 +/- 0.09 S were assigned for Artemia salina (L.) extracellular haemoglobins II and III respectively. These values are not significantly different. The molecular weights, M0w and M0z, of the native haemoglobins as determined by the high-speed sedimentation-equilibrium method were for haemoglobin II 239 400 +/- 7200 and 240 400 +/- 2600 respectively, and for haemoglobin III 216 300 +/- 6500 and 219 300 +/- 4500 respectively. The observed increase of Mapp. with concentration suggested that association was occurring over the concentration range investigated. Exposure of haemoglobin II to either 6 M-guanidinium chloride or to low pH (pH 4) resulted in dissociation to units of approximately half the size of the native protein, with molecular weights approx. 115 000. Electron-microscopic observations indicated a molecular structure composed of two stacked lobed discs. These results strongly support the dimeric model for Artemia haemoglobins proposed by Moens & Kondo [(1978) Eur. J. Biochem. 82, 65-72].
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowen S. T., Moise H. W., Waring G., Poon M. C. The hemoglobins of Artemia salina--III. Characterization. Comp Biochem Physiol B. 1976;55(1):99–103. doi: 10.1016/0305-0491(76)90180-2. [DOI] [PubMed] [Google Scholar]
- David M. M., Schejter A., Daniel E., Ar A., Ben-Shaul Y. Subunit structure of hemoglobin from the clam shrimp Cyzicus. J Mol Biol. 1977 Apr;111(2):211–214. doi: 10.1016/s0022-2836(77)80125-3. [DOI] [PubMed] [Google Scholar]
- Heip J., Moens L., Joniau M., Kondo M. Ontogenetical studies on extracellular hemoglobins of Artemia salina. Dev Biol. 1978 May;64(1):73–81. doi: 10.1016/0012-1606(78)90061-1. [DOI] [PubMed] [Google Scholar]
- Ilan E., Daniel E. Haemoglobin from the tadpole shrimp, Lepidurus apus lubbocki Characterization of the molecule and determination of the number of polypeptide chains. Biochem J. 1979 Nov 1;183(2):325–330. doi: 10.1042/bj1830325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen R., Kondo M., Römer W., Weissmann C. Reconstitution of Q replicase lacking subunit with protein-synthesis-interference factor i. Eur J Biochem. 1972 Nov 21;31(1):44–51. doi: 10.1111/j.1432-1033.1972.tb02498.x. [DOI] [PubMed] [Google Scholar]
- LEVIN O. Electron microscope observations on some 60 s erythrocruorins and their split products. J Mol Biol. 1963 Jan;6:95–101. doi: 10.1016/s0022-2836(63)80084-4. [DOI] [PubMed] [Google Scholar]
- Manwell C. A simplified electrophoretic system for determining molecular weights of proteins. Biochem J. 1977 Sep 1;165(3):487–495. doi: 10.1042/bj1650487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens L., Kondo M. Characterization of the extracellular haemoglobins of Artemia salina. Biochem J. 1977 Jul 1;165(1):111–119. doi: 10.1042/bj1650111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens L., Kondo M. Evidence for a dimeric form of Artemia salina extracellular hemoglobins with high-molecular-weight subunits. Eur J Biochem. 1978 Jan 2;82(1):65–72. doi: 10.1111/j.1432-1033.1978.tb11997.x. [DOI] [PubMed] [Google Scholar]
- Moens L., Kondo M. The structure of Artemia salina haemoglobins. A comparative characterisation of four naupliar and adult heamoglobins. Eur J Biochem. 1976 Aug 16;67(2):397–402. doi: 10.1111/j.1432-1033.1976.tb10704.x. [DOI] [PubMed] [Google Scholar]
- Shlom J. M., Vinogradov S. N. A study of the subunit structure of the extracellular hemoglobin of Lumbricus terrestris. J Biol Chem. 1973 Nov 25;248(22):7904–7912. [PubMed] [Google Scholar]
- Terwilliger N. B., Terwilliger R. C., Schabtach E. The quaternary structure of a molluscan (Helisoma trivolvis) extracellular hemoglobin. Biochim Biophys Acta. 1976 Nov 26;453(1):101–110. doi: 10.1016/0005-2795(76)90254-3. [DOI] [PubMed] [Google Scholar]
- Waxman L. The structure of annelid and mollusc hemoglobins. J Biol Chem. 1975 May 25;250(10):3790–3795. [PubMed] [Google Scholar]
- Wood E. J., Mosby L. J. Physicochemical properties of Planorbis corneus erythrocruorin. Biochem J. 1975 Aug;149(2):437–445. doi: 10.1042/bj1490437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E. J., Mosby L. J., Robinson M. S. Characterization of the extracellular haemoglobin of Haemopsis sanguisuga (L.). Biochem J. 1976 Mar 1;153(3):589–596. doi: 10.1042/bj1530589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Young R. A., Blumenthal T. Phage Q-beta ribonucleic acid replicase. Subunit relationships determined by intramolecular cross-linking. J Biol Chem. 1975 Mar 10;250(5):1829–1832. [PubMed] [Google Scholar]




