Abstract
It has been claimed that microRNA 503-5p (miR-503-5p) is the key to the future diagnosis and treatment of cardiac hemangioma (CH), but the relationship between the two has not been fully validated. In this study, we analyzed the effect of miR-503-5p targeting type IA bone morphogenetic protein receptor (BMPR1A) on CH to inform future diagnosis and treatment of CH. First, miR-503-5p and BMPR1A abnormal expression sequences (vectors) were transfected into human hemangioma-derived endothelial cells (HemECs) and human umbilical vein endothelial cells (HUVECs) to observe alterations in cell biological behavior, adhesion, and epithelial mesenchymal transition (EMT). We found enhanced proliferative, invasive and migrating abilities of HemECs and HUVECs after silencing miR-503-5p or increasing BMPR1A, accompanied by reduced apoptosis, elevated intercellular cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1), and accelerated EMT; after increasing miR-503-5p or silencing BMPR1A, the cells exhibited reduced apoptosis, elevated ICAM-1 and VCAM-1, and accelerated EMT (P<0.05). Subsequently, a dual-luciferase reporter assay was performed to analyze the targeting relationship between miR-503-5p and BMPR1A. The results showed that miR-503-5p inhibited BMPR1A-wild type (WT) fluorescence activity (P<0.05). Through the rescue experiment, it was observed that the biological behavior of the cells with simultaneous elevation or simultaneous silencing of miR-503-5p and BMPR1A was not different from that of cells transfected with BMPR1A empty vector (P>0.05), indicating that the effect of BMPR1A on cells was reversed by miR-503-5p. Finally, in the analysis of clinical records, we found that CH cases exhibited lower miR-503-5p and higher BMPR1A levels than healthy controls (P<0.05). The expression of the two genes was negatively correlated (P<0.05). These results suggest that miR-503-5p participates in CH growth by targeted sponging of BMPR1A.
Keywords: miR-503-5p, heart neoplasms, hemangioma, BMPR1A, vascular endothelial cell, epithelial-mesenchymal transition
Introduction
Cardiac hemangioma (CH) is a rare benign tumor of the heart that can occur at any age or in any part of the heart [1]. Cardiac blood vessels are the main blood vessels supplying blood to the heart - the body’s power system. Small-volume CH may not cause obvious symptoms [2]. CH is basically asymptomatic, however, as the CH size increases, there may be blood circulation disorders, resulting in insufficient blood supply to other tissues and organs of the body and causing danger [3]. In addition, too strong myocardial contractility may cause CH ruptures, leading to the rapid entry of blood into the chest or abdominal cavity, causing severe headaches, accelerated heartbeats, shock, and even cardiac arrest in serious cases [4]. Surgery is the main treatment for CH, but it is very difficult, leading to extremely high risk of death in patients with CH, so the diagnosis and treatment of CH remains a key clinical concern [5]. Because the pathogenesis of CH is still unclear, and the potential threat of CH is not yet fully grasped in the clinic, more research is urgently needed to help understand the development of CH, and the search for new research directions from a molecular perspective is one of the hotspots [6].
With the increasing clinical attention given to the application of microRNAs in tumor diseases in recent years, microRNAs are also considered a breakthrough in the future diagnosis and treatment of tumors [7]. For vascular neoplasms, including CH, the malignant proliferation of vascular endothelial cells is the basic pathological cause [8]. MicroRNA 503-5p (miR-503-5p) has been shown to have the ability to target and regulate the cell cycle of human lung fibroblasts [9]. Another report showed that it regulates epithelial mesenchymal transition (EMT) in hepatocellular carcinoma cells directly through Wee1-like protein kinase (WEE1), thus affecting the malignant progression of hepatocellular carcinoma [10]. In a study on atherosclerosis, Liu et al. also found that miR-503-5p was critical in mediating the proliferation and migration of vascular smooth muscle cells [11]. Meanwhile, miR-503-5p was also mentioned as one of the keys to regulate myocardial ischemia-reperfusion injury [12]. It is well known that CH is precisely composed of reticular vascular lumens formed by the proliferation of vascular endothelial cells [13]. The regulatory effect of miR-503-5p on the biological behavior of vascular cells reveals the diagnostic potential of miR-503-5p in CH. Similarly, miR-503-5p was found to promote pulmonary fibrosis through targeted regulation of type IA bone morphogenetic protein receptor (BMPR1A) [14], which has been confirmed to not only regulate cardiopulmonary vascular remodeling but also affect the over-transformation of vascular endothelial cells [15,16]. Recently, Guo et al. even found that miR-503-5p affected myocardial ischemia-reperfusion injury by mediating mitochondrial apoptosis [12], further suggesting the potential close link between miR-503-5p and the development of cardiovascular diseases. Meanwhile, the relationship between BMPR1A, a classical cellular differentiation protein in modern tumor disease research, and cardiac function has been well documented [15]. Unfortunately, the functional role and molecular mechanism of miR-503-5p in CH are currently unknown.
Based on these previous studies, we hypothesize that miR-503-5p may have an important influence on the biological behavior of vascular endothelial cells in CH, and the mechanism may be related to BMPR1A. Validation of this idea requires addressing two issues: (1) confirming the exact effect of miR-503-5p on CH; (2) confirmation of the clinical expression of miR-503-5p in CH. Therefore, this work conducted experiments mainly from these two perspectives. Meanwhile, we further observed the effects of miR-503-5p on normal vascular endothelial cells. This study hopes to help fully understand the effect of miR-503-5p on CH, and lay a reliable foundation for finding new diagnostic and treatment options for CH in the future.
Materials and methods
Flow of research
This study is divided into a total of 2 parts: (1) cell assay, which aims to analyze the effects of miR-503-5p, BMPR1A on the biological behavior of CH cells and normal endothelial cells; (2) clinical trials, which aims to confirm the expression of miR-503-5p and BMPR1A in CH. The flow of the study is shown in Figure 1.
Figure 1.
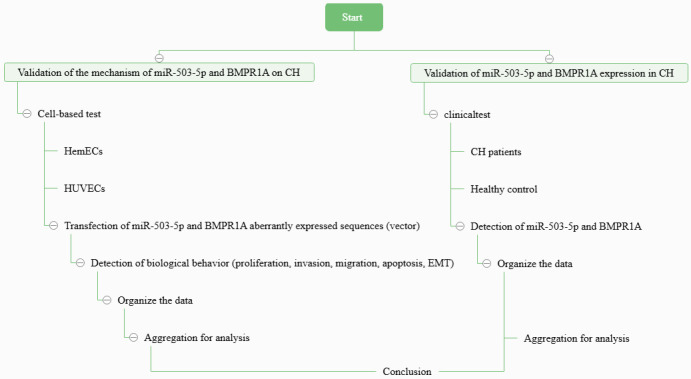
Flowchart of this work. Note: miR-503-5p, microRNA 503-5p; BMPR1A, type IA bone morphogenetic protein receptor; CH, cardiac hemangioma; HemECs, human hemangioma-derived endothelial cells; HUVECs, human umbilical vein endothelial cells; EMT, epithelial mesenchymal transition.
Cell information
To determine the exact mechanism of action of miR-503-5p and BMPR1A in CH, we purchased human hemangioma-derived endothelial cells (HemECs) and human umbilical vein endothelial cells (HUVECs) from the American Tissue Culture Collection (ATCC) (Manassas, VA, USA). Cells were cultured in a Roswell Park Memorial Institute (RPMI)-1640 medium containing 10% fetal bovine serum (FBS) and 1% streptomycin antibody in a 37°C and 5% CO2 incubator (Gibco, USA).
Cell transfection
HemECs and HUVECs were seeded in six-well plates at a density of 1-2 × 105 cells/well and cultured for 1-3 days until 70-80% confluent. Thereafter, the cells were subjected to transfection with mimic, inhibitor, and negative control sequences of miR-503-5p as well as overexpression, silencing, and blank control vectors of BMPR1A following the Lipofectamine 2000 transfection kit instructions (Invitrogen, NY, USA). The transfection efficiencies were validated by real-time quantitative polymerase chain reaction (qRT-PCR). After 48 h of transfection, cells were collected for further experiments.
qRT-PCR
Total ribonucleic acid (RNA) was extracted from plasma or tissues using a FastPure Cell/Tissue Total RNA Isolation Mini Kit (Vazyme, Nanjing, China). RNA was reverse transcribed into cDNA using a one-step reverse transcription HiScript II 1st Strand cDNA Synthesis Kit (Vazyme, China). qRT-PCR was carried out following the SYBR Premix Ex Taq kit instructions. The reaction conditions (40 cycles) were 95°C/10 min (pre-denaturation), 95°C/15 s (denaturation), 60°C/30 s (annealing), and 72°C/30 s (extension). GAPDH and U6 were used as the endogenous controls, and the relative miR-503-5p and BMPR1A levels were quantified using the 2-ΔΔCt method. The primer sequences are given in Table 1.
Table 1.
Primer sequences used in the study
| F (5’-3’) | R (5’-3’) | |
|---|---|---|
| miR-503-5p | GGTCCGCGTAAGTGCAGAAGA | GAGGTTCCGCGCTAGATCCGTC |
| U6 | ATGATAGTCGCTAGTCTGATC | TTGACCGTAGCTGTATTTTGA |
| BMPR1A | TCTCAAGCAGACGTCGTTAC | CCGGACCATCTGAATCTGTT |
| GAPDH | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG |
Note: miR-503-5p, microRNA 503-5p; BMPR1A, type IA bone morphogenetic protein receptor.
Cell colony formation assay for measuring cell proliferation
Cells were inoculated into a 6-well culture plate (500 cells per well) for continuous culture, and the culture medium was changed once every 2 days. Forty-eight hours later, cells were fixed with 4% paraformaldehyde for 30 min and stained with 1% crystal violet for 30 min. After drying, the cells were photographed, and the number of cell clones was counted.
Transwell assay for measuring cell invasion
Totally 3 × 105 cells were inoculated in the upper chamber of a Transwell after cell resuspension in serum-free medium, and 20% FBS-supplemented RPMI-1640 medium was placed in the lower chamber as a chemotactic agent. The upper chamber was removed following 24 hours of incubation in the cell culture incubator. Cells that had invade into the lower chamber were fixed using 4% paraformaldehyde and subsequently stained with 0.1% crystal violet. A minimum of five visual fields were randomly chosen, and the number of invaded cells was microscopically observed (Olympus, Japan) and counted using ImageJ software.
Wound healing assay for measuring cell migration
Cells were seeded onto 24-well plates at 10 × 105 cells/mL and cultured in 10% FBS-supplemented RPMI 1640 medium for 24 hours. Then, the tip of a 10 μL pipette was used to make vertical scratches. Thereafter, the cells were incubated for additional 24 hours. After that, cells were washed with phosphate buffered saline (PBS). After removing the culture solution, the cell mobility was calculated by observing and taking pictures with an inverted microscope. Cell migration rate = (initial scratch width - final scratch width)/initial scratch width × 100%.
Flow cytometry for measuring cell apoptosis
Cell apoptosis was measured using an Annexin V-Fluorescein Isothiocyanate (FITC)/propidium iodide (PI) Apoptosis Detection Kit (Beyotime, China). Briefly, transfected/naïve cells were collected and washed with PBS, after which 1 × 106 cells/mL were resuspended in 1× binding buffer solution. The cells were subsequently subjected to staining using Annexin V/FITC solution, consisting of 5 µL of FITC Annexin V, as well as 5 µL of PI. Following this, the cells were incubated at room temperature for a duration of 30 minutes, while being kept in a dark environment. Cells were then acquired using BD FACS sorter (BD Biosciences®, USA), and apoptosis rate was calculated using FlowJo software. Apoptosis rate = Q2 (early apoptosis rate) + Q4 (late apoptosis rate).
Western blot for protein expression
Total protein was isolated from cells using Radio-Immunoprecipitation Assay (RIPA) lysis buffer (Thermo Fisher), and its concentration was measured using Enhanced Bicinchoninic Acid (BCA) Protein Assay Kit (Beyotime). Then equal protein was loaded onto sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by transfer to polyvinylidene fluoride (PVDF) membranes (Millipore). The membranes were then blocked for 1 hour at room temperature with 5% fat-free milk and then incubated overnight at 4°C with the primary antibodies (BMPR1A 1:1000, Intercellular cell adhesion molecule-1 (ICAM-1) 1:1000, Vascular cell adhesion molecule-1 (VCAM-1) 1:1000, N-cadherin 1:1000, E-cadherin 1:1000, and vimentin 1:1000). The next morning, after washing the membrane with washing buffer 3 times, the membranes were incubated with the secondary antibody (1:2000) for 1 hour at room temperature. Finally, the Electrochemiluminescence chemiluminescent detection reagent was applied to visualize the proteins, which were then quantified using ImageJ software.
Validation of the targeting relationship between miR-503-5p and BMPR1A
The downstream potential target genes of miR-503-5p were predicted and analyzed by Targetscan (URL: https://www.targetscan.org/vert_72/), miRWalk (URL: http://mirwalk.umm.uni-heidelberg.de/), miRBD (URL: https://mirdb.org/mirdb/index.html), and Starbase databases (URL: https://starbase.sysu.edu.cn/), and a Wayne diagram was plotted to observe whether all the four databases contained BMPR1A in the analyzed results. The BMPR1A 3’UTR, synthesized by Shanghai Generay Biotech, was inserted into the pGL3-promoter plasmid vector, named BMPR1A-wild type (WT). The BMPR1A 3’UTR containing the miR-503-5p binding site was mutated by site-specific mutagenesis and then introduced into the pGL3-promoter plasmid vector, named BMPR1A-mutant (MUT). BMPR1A-WT and BMPR1A-MUT were cotransfected with miR-mimics and miR-NC into HemECs. Luciferase activities were determined according to the operating instructions of a dual-luciferase reporter (DLR) assay kit.
Sample size calculation
CH patients from The People’s Hospital of Wuqia County from March 2022 to January 2024 were used as potential study subjects. According to the random sampling formula N = Z2σ2/E2, we included a total of 28 CH patients as the study subjects in this study. Patient data showed that the gender distribution was relatively uniform and that the age span was large, with the youngest patient being 18 and the oldest patient being 71 years old. The right ventricle was the most common site of tumor onset, although tumors were found elsewhere as well. Most of the lesions were single, and only 2 patients developed multiple lesions. The tumor volume distribution also spanned a large area, ranging from 0.8 × 0.5 × 0.5 cm3 to 11.3 × 5.2 × 1.5 cm3, with sponginess as the main pathomorphological feature (Table 2). At the same time, we selected 34 healthy controls (HCs) in the same period according to a ratio of 1:1. The current study was approved by the Ethics Committee of the People’s Hospital of Wuqia County (20220914-039y), and all subjects signed an informed consent form. In addition, this study was conducted in strict compliance with the Declaration of Helsinki.
Table 2.
Clinicopathological characteristics of CH patients
| Number | Age | Gender | CH site | Number of lesions | Volume (cm3) | Pathomorphological features |
|---|---|---|---|---|---|---|
| 1 | 18 | Female | Right ventricle | Single | 6 × 4 × 2 | Spongy |
| 2 | 69 | Female | Right ventricle | Single | 5 × 4 × 2 | Spongy |
| 3 | 24 | Female | Left atrial surface | Single | 11.3 × 5.2 × 1.5 | Spongy+Partial capillary type |
| 4 | 67 | Male | Apical surface of the heart | Single | 4 × 3 × 2 | Spongy |
| 5 | 65 | Male | Left atrial surface | Single | 5.5 × 3.5 × 2 | Spongy |
| 6 | 34 | Female | Right ventricle | Single | 3 × 2 × 0.8 | Spongy |
| 7 | 70 | Male | Right ventricle | Single | 0.8 × 0.5 × 0.5 | Spongy+Small amount of intravenous type |
| 8 | 39 | Male | Right ventricle | Single | 6 × 6 × 8 | Spongy+Partial capillary type |
| 9 | 69 | Male | Left atrial surface | Single | 4.5 × 3 × 2 | Spongy |
| 10 | 74 | Male | Apical surface of the heart | Single | 3.5 × 2.2 × 2 | Spongy |
| 11 | 42 | Female | Right atrium | Single | 1 × 1 × 0.5 | Spongy+Partial capillary type |
| 12 | 45 | Male | Right ventricle | Multiple | 4.5 × 4 × 2 | Spongy |
| 13 | 65 | Male | Right atrium | Single | 3 × 2.4 × 0.8 | Intravenous |
| 14 | 71 | Female | Right atrium | Single | 5 × 4.5 × 3.6 | Spongy |
| 15 | 63 | Male | Right ventricle | Single | 9.5 × 5.5 × 2.4 | Spongy+Small amount of intravenous type |
| 16 | 67 | Female | Left ventricular surface | Single | 4.5 × 3 × 1 | Spongy |
| 17 | 67 | Male | Left ventricular surface | Single | 3.5 × 2.2 × 1.6 | Spongy+Partial capillary type |
| 18 | 61 | Female | Right ventricle | Single | 3.1 × 2.6 × 1.3 | Spongy |
| 19 | 59 | Male | Right ventricle | Single | 8.4 × 2.4 × 2 | Spongy |
| 20 | 46 | Male | Left atrial surface | Single | 3.5 × 2.3 × 2.8 | Spongy+Partial capillary type |
| 21 | 58 | Male | Right ventricle | Single | 6 × 4 × 1.4 | Spongy |
| 22 | 62 | Male | Apical surface of the heart | Single | 3.7 × 0.6 × 1.5 | Intermuscular capillary type |
| 23 | 71 | Female | Left atrial surface | Single | 4 × 1.2 × 2.6 | Intravenous |
| 24 | 67 | Female | Right atrium | Multiple | 2.4 × 6 × 1.5 | Spongy+Small amount of intravenous type |
| 25 | 64 | Male | Apical surface of the heart | Single | 4 × 6.2 × 1.5 | Spongy+Partial capillary type |
| 26 | 69 | Female | Right ventricle | Single | 0.7 × 0.7 × 2.2 | Spongy |
| 27 | 63 | Male | Right ventricle | Single | 2.8 × 3.1 × 0.7 | Spongy |
| 28 | 71 | Male | Left atrial surface | Single | 3.4 × 0.8 × 1.6 | Spongy |
Note: CH, cardiac hemangioma.
Selection criteria for the enrolled individuals
CH patients were included if they were pathologically confirmed as having CH in our hospital, agreed to participate in this study, and had complete medical records. Those with other tumors, cardio-cerebrovascular diseases, immunodeficiency, mental diseases, organ failure, an estimated survival time <3 months, or structural cardiac abnormalities were excluded. Healthy individuals were selected and enrolled in the study if they had complete medical records, no previous major medical history, and normal physical examination results.
Sample collection and testing
The peripheral blood samples from both groups were collected upon admission, and the plasma and serum were separated through high-speed centrifugation. The collected samples were refrigerated at -80°C for analysis. Subsequently, miR-503-5p and BMPR1A expression were measured by qRT-PCR as described above.
Statistical analyses
Data were analyzed using the SPSS 25.0 software. The distribution of the continuous data was first tested for normality to confirm that it conformed to a normal distribution. Count data were expressed as (%), and continuous data were expressed as (x̅±s). A Chi-square test was performed to identify differences between the groups. For inter-group comparisons, an independent sample t test was performed, while one-way or two-way Analysis of Variance (ANOVA) followed by Bonferroni post hoc test was used for multi-group comparisons. Pearson correlation coefficient analysis was used for correlation analyses. Each biological sample was run in triplicate, and experiments were independently repeated three times (n = 3). P<0.05 was considered statistically significant.
Results
Inhibition of miR-503-5p expression promotes HemEC and HUVEC growth, and EMT
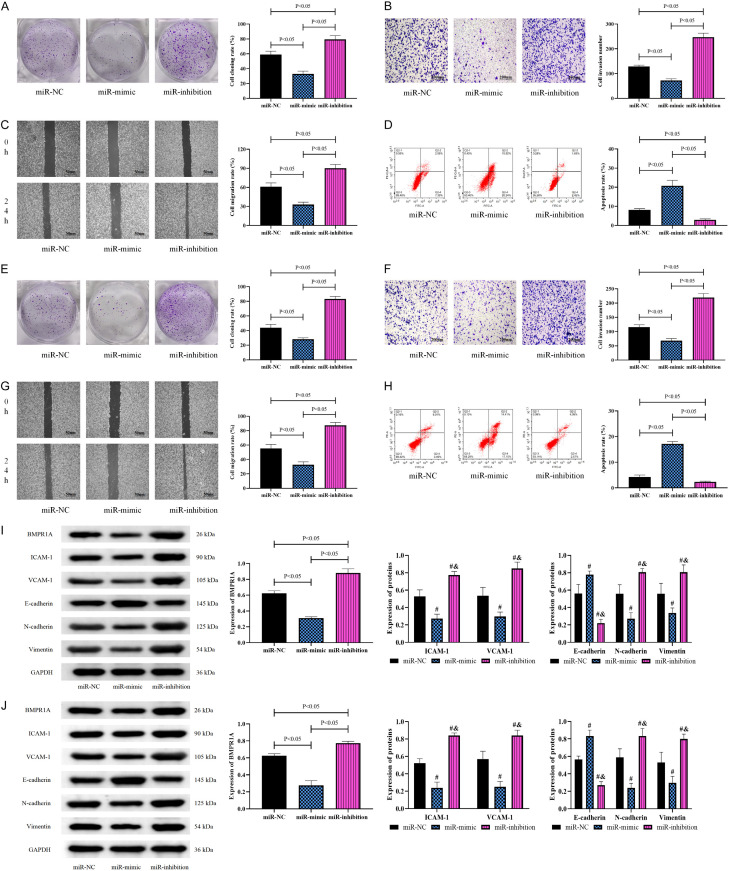
To confirm the effect of miR-503-5p on the activity of CH cells, we constructed a miR-503-5p aberrant expression vector and transfected it into HemECs. Biological tests on HemECs after transfection of abnormal miR-503-5p expression sequences showed that the proliferation, invasion, and migration capacities of the miR-inhibition group were significantly enhanced (Figure 2A-C), while the apoptosis rate was reduced (P<0.05) (Figure 2D). However, the miR-mimic group had lower proliferating, invading, and migrating capacities and higher apoptosis than the miR-NC group (P<0.05). Thus, inhibiting miR-503-5p promoted CH cell growth, while increasing its expression inhibited CH cell growth (Figure 2A-D). Subsequently, to further observe the effect of miR-503-5p on HUVECs, the aberrant expression of miR-503-5p was transfected into HUVECs as above, and their biological behaviors were examined. The biological behavior test results of HUVECs were consistent with the above findings, namely, the miR-inhibition group showed the most potent proliferation (Figure 2E), invasion (Figure 2F), and migration (Figure 2G) abilities and the lowest apoptosis (Figure 2H) among the three groups (P<0.05), while cell activity was lower and apoptosis was higher in the miR-mimic group than in the miR-NC group (P<0.05) (Figure 2E-H). Neovascularization and EMT are both important pathological processes in CH. To confirm the effects of miR-503-5p on angiogenic capacity and EMT in HemECs and HUVECs, we examined the expression of ICAM-1, VCAM-1, and EMT-expressing proteins. Western blot results showed that in HemECs and HUVECs, ICAM-1, VCAM-1, N-cadherin, and vimentin protein levels were markedly elevated in the miR-inhibition group, while E-cadherin protein expression was reduced (P<0.05); the opposite was observed in the miR-mimic group, that is, ICAM-1, VCAM-1, N-cadherin, and vimentin decreased, while E-cadherin increased. Furthermore, the miR-inhibition group was found to have higher BMPR1A protein levels than the miR-mimic and miR-NC groups, while the miR-mimic group had lower BMPR1A levels than the miR-NC group (P<0.05) (Figure 2I, 2J).
Figure 2.
Influence of miR-503-5p on CH cell and HUVEC biological behavior. A: Effect of miR-503-5p on the clonogenic ability of HemECs. B: Effect of miR-503-5p on the invasion ability of HemECs (200×). C: Effect of miR-503-5p on the migration ability of HemECs (50×). D: Effect of miR-503-5p on the apoptosis of HemECs. E: Effect of miR-503-5p on the clonogenic ability of HUVECs. F: Effect of miR-503-5p on the invasion ability of HUVECs (200×). G: Effect of miR-503-5p on the migration ability of HUVECs (50×). H: Effect of miR-503-5p on the apoptosis of HUVECs. I: Effect of miR-503-5p on protein expression in HemECs. J: Effect of miR-503-5p on protein expression in HUVECs. Note: vs. miR-NC #P<0.05, vs. miR-mimic &P<0.05. CH, cardiac hemangioma; BMPR1A, type IA bone morphogenetic protein receptor; ICAM-1, intercellular cell adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1.
Targeted regulatory relationship between miR-503-5p and BMPR1A
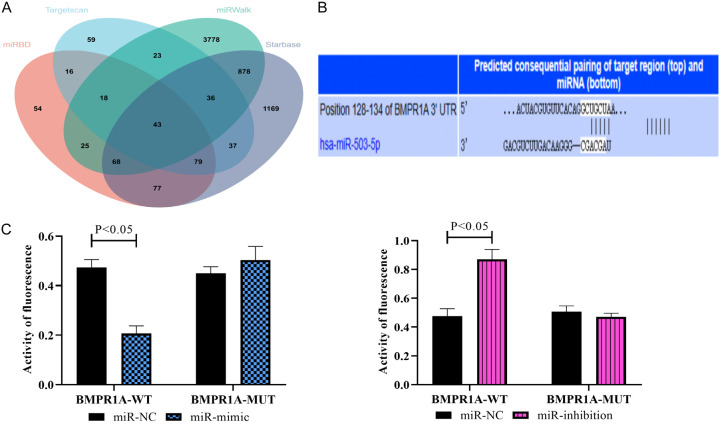
In the above experiments, we have preliminarily demonstrated that miR-503-5p has an important effect on CH and that the expression of BMPR1A is also affected by miR-503-5p. However, the relationship between miR-503-5p and BMPR1A still needs more experimental verification. Thus, we first needed to confirm whether there was a targeting relationship between the two. All the databases (TargetScan, miRWalk, miRBD, and Starbase) identified BMPR1A as one of the potential downstream target genes of miR-503-5p (Figure 3A). The binding complementary sites between them are shown in Figure 3B. As indicated by the DLR assay, BMPR1A-WT fluorescence activity was obviously inhibited after miR-mimic transfection, while BMPR1A-MUT fluorescence activity was enhanced by miR inhibition (P<0.05) (Figure 3C), indicating a targeted regulatory relationship between the two.
Figure 3.
Validation of the targeting relationship between miR-503-5p and BMPR1A. A: Analysis of miR-503-5p target genes in the online database. B: Binding complementary sites of miR-503-5p and BMPR1A. C: Results of the DLR assay. Note: DLR, dual-luciferase reporter; BMPR1A, type IA bone morphogenetic protein receptor.
BMPR1A reverses the effect of miR-503-5p on HemECs and HUVECs
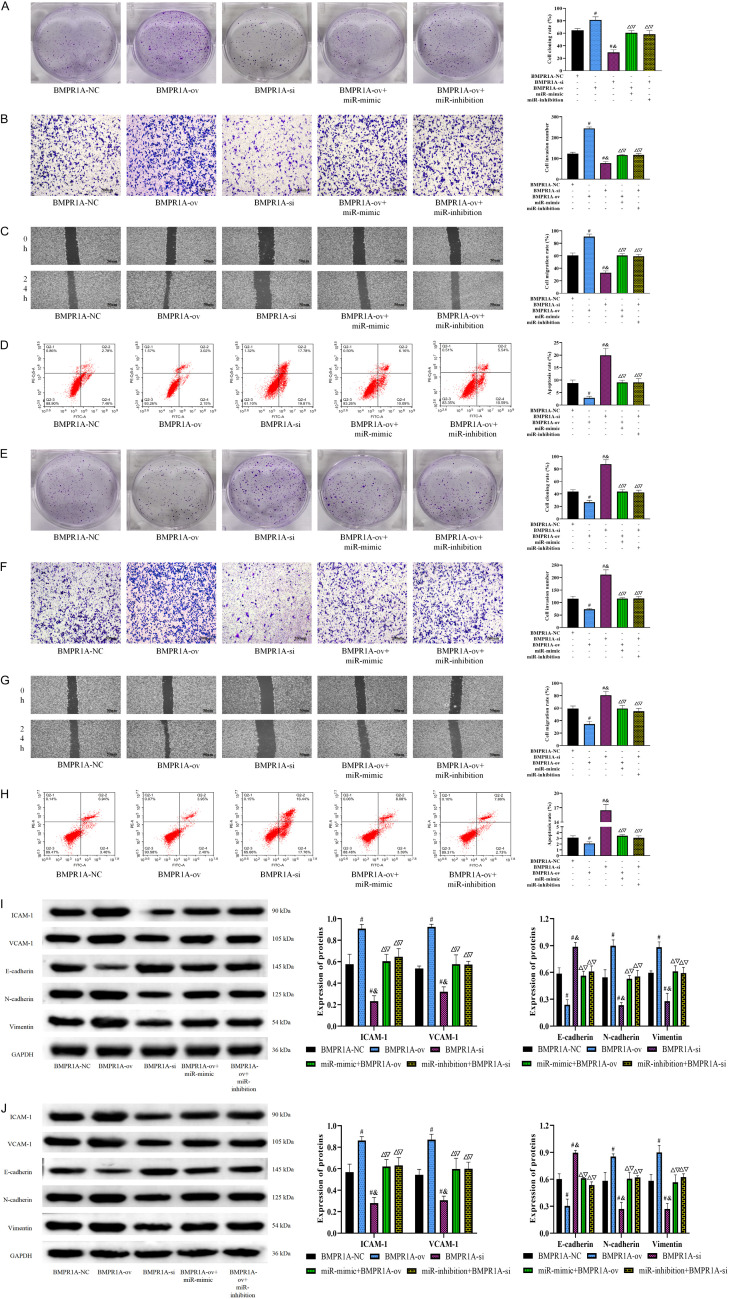
Although we confirmed a targeting relationship between miR-503-5p and BMPR1A, more experiments are needed to verify that miR-503-5p influenced CH progression through BMPR1A. Thus, we observed the altered biological behavior of HemECs by simultaneously regulating the expression of miR-503-5p and BMPR1A. BMPR1A-ov transfection led to obviously enhanced proliferative, invasive, and migrating capacities of HemECs, as well as reduced apoptosis. Decreased HemEC activity and increased apoptosis were observed after BMPR1A-si transfection (P<0.05). However, after the simultaneous transfection of BMPR1A-ov+miR-mimic or BMPR1A-si+miR-inhibition, there were no significant differences in the biological behavior of HemECs compared to BMPR1A-NC-transfected cells (P>0.05), which shows that the influence of BMPR1A on the biological behavior of HemECs was completely reversed by miR-503-5p (Figure 4A-D). Moreover, we further observed the altered biological behavior of HUVECs by simultaneously regulating the expression of miR-503-5p and BMPR1A. In HUVECs, the cell proliferation, invasion and migration capacities of the BMPR1A-ov group were also significantly enhanced, and apoptosis was inhibited. In contrast, the BMPR1A-si group had decreased proliferation, invasion and migration, and increased apoptosis (P<0.05). Similarly, both BMPR1A-ov+miR-mimic and BMPR1A-si+miR-inhibition cotransfection contributed to various biological behaviors that were similar to those in the BMPR1A-NC group (P>0.05) (Figure 4E-H). For the angiogenic capacity and EMT of HemECs and HUVECs, experiments are likewise needed to validate the BMPR1A-targeted regulatory effect of miR-503-5p. ICAM-1, VCAM-1, N-cadherin, and vimentin protein levels were found to be higher in the BMPR1A-ov group than in the BMPR1A-NC group, while E-cadherin was lower. The BMPR1A-si group had lower ICAM-1, VCAM-1, N-cadherin, and vimentin protein levels and higher E-cadherin levels than the BMPR1A-NC group (P<0.05). However, the BMPR1A-ov+miR-mimic group and BMPR1A-si+miR-inhibition group showed no differences in the protein expression of ICAM-1, VCAM-1, N-cadherin, E-cadherin, and vimentin compared with the BMPR1A-NC group (P>0.05) (Figure 4I, 4J).
Figure 4.
Effect of miR-503-5p adsorption of BMPR1A by sponging on the biological behavior of CH cells and HUVECs. A: Results of the cell cloning assay. B: Results of the Transwell assay (200×). C: Results of the wound healing assay (50×). D: Results of flow cytometry assay. E: Results of the cell cloning assay. F: Results of the Transwell assay (200×). G: Results of the wound healing assay (50×). H: Results of flow cytometry assay. I: Protein expression in HemECs. J: Protein expression in HUVECs. Note: vs. BMPR1A-NC #P<0.05, vs. BMPR1A-ov &P<0.05, vs. BMPR1A-si ΔP<0.05, vs. BMPR1A-ov+miR-mimic &P<0.05, vs. BMPR1A-si+miR-inhibition ΔP<0.05. CH, cardiac hemangioma; BMPR1A, type IA bone morphogenetic protein receptor; HUVECs, human umbilical vein endothelial cells.
Comparison of clinical data
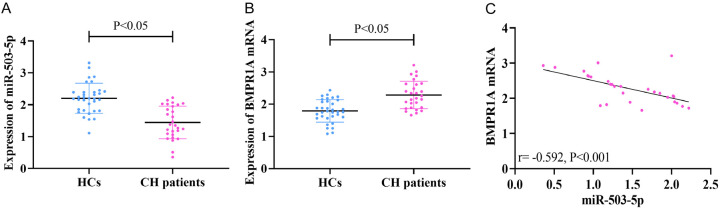
The clinical parameters of the enrolled individuals are shown in Table 3. There was no significant difference in the baseline data between the HC patients and the HCs (P>0.05). First, to confirm the expression of miR-503-5p and BMPR1A in CH, we examined their expression in peripheral blood of CH patients and HCs. The CH patients showed lower peripheral blood miR-503-5p expression (1.44±0.51) (Figure 5A) and higher BMPR1A mRNA (2.28±0.43) (Figure 5B) than the HCs (P<0.05). Pearson correlation coefficients identified a negative connection between miR-503-5p and BMPR1A expression in peripheral blood in the CH patients (P<0.05, Figure 5C).
Table 3.
Baseline parameters of the enrolled subjects
| Group | n | Age | BMI (kg/m2) | Male/Female | Smoking | Family history of CH |
|---|---|---|---|---|---|---|
| Yes/No | Yes/No | |||||
| HCs | 34 | 60.88±8.29 | 22.09±5.56 | 22 (64.71)/12 (35.29) | 15 (44.12)/19 (55.88) | 0 (0.0)/34 (100.0) |
| CH patients | 28 | 58.57±14.98 | 22.07±3.50 | 17 (60.71)/11 (39.29) | 15 (53.57)/13 (46.43) | 2 (7.14)/26 (92.86) |
| t (or χ2) | 0.769 | 0.014 | 0.105 | 0.550 | 2.510 | |
| P | 0.445 | 0.989 | 0.746 | 0.459 | 0.113 |
Note: HCs, health controls; CH, cardiac hemangioma; BMI, body mass index.
Figure 5.
Clinical expression of miR-503-5p and BMPR1A. qRT-PCR was conducted to measure (A) miR-503-5p and (B) BMPR1A mRNA levels in the blood samples of HCs and CH patients. (C) Pearson correlation coefficients was performed to identify the correlation between miR-503-5p and BMPR1A mRNA in peripheral blood samples of CH patients. CH, cardiac hemangioma.
Discussion
As a rare benign cardiac tumor, CH has a much lower incidence rate than myxoma but is extremely concealed. Once CH ruptures, there is a great risk of death, so the diagnosis and treatment of CH are still worthy of clinical attention [17]. In this study, we found that miR-503-5p was significantly aberrantly expressed in CH and had an important impact on the biological behaviors of both CH cells and HUVECs, which Figure 6 helps us visualize the process more.
Figure 6.
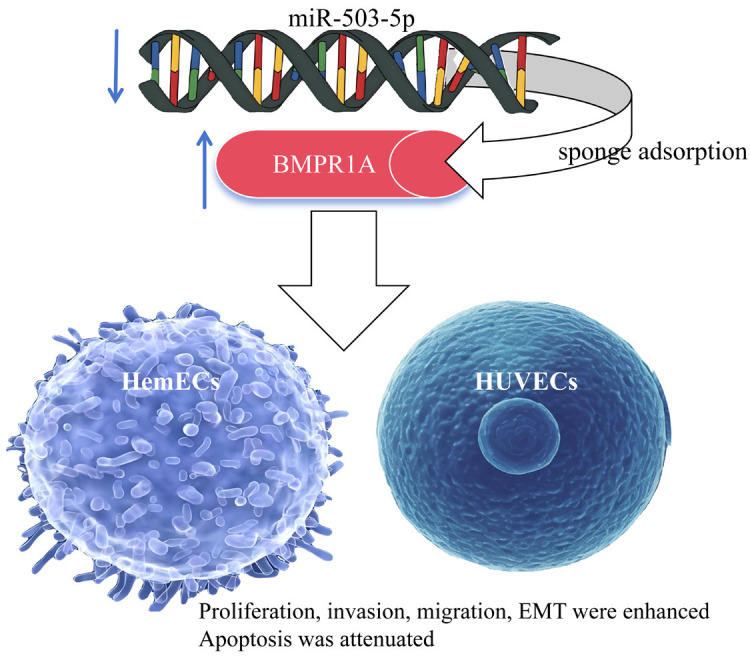
Principle of this work. miR-503-5p promotes proliferation, invasion and EMT of HemECs and HUVECs by targeting BMPR1A. Note: BMPR1A, type IA bone morphogenetic protein receptor; HemECs, human hemangioma-derived endothelial cells; HUVECs, human umbilical vein endothelial cells.
Reviewing previous research on miR-503-5p, we found that miR-503-5p was involved in the development of tumor diseases such as osteosarcoma and oral cancer [18,19], but its action mechanism in CH remains unclear. Therefore, it is necessary to confirm its mechanism in CH through in vitro experiments. By transfecting abnormal miR-503-5p expression sequences into HemECs and detecting cell biological behavior changes, we found that silencing miR-503-5p promoted HemEC proliferation, invasion, and migration and inhibited apoptosis, which suggests that low miR-503-5p expression in CH can accelerate CH development. Similarly, previous studies found that silencing miR-503-5p accelerated hepatocellular carcinoma cell proliferation [20], which supports our experimental results and indicates that miR-503-5p has similar biological effects in other neoplastic diseases. However, after increasing miR-503-5p, CH activity was obviously weakened, and apoptosis was activated. This suggests that immunotherapy by increasing miR-503-5p may be a new treatment option for CH, which is of great significance for CH that is currently extremely difficult to treat surgically. As mentioned above, there is a close relationship between the development of CH and the formation of cardiac vascular endothelial cells or smooth muscle cells with excessive proliferation [13]. Therefore, we not only need to focus on the effect of miR-503-5p on CH cells, but also need to understand the regulatory effect of miR-503-5p on normal vascular cells in order to fully explain the mechanism of miR-503-5p’s action in CH. Therefore, we also observed the effect of miR-503-5p on HUVECs, and similar results as those on HemECs were obtained. That is, elevating the expression of miR-503-5p decreased the activity of HUVECs, whereas silencing the expression of miR-503-5p enhanced the activity of HUVECs. Combined with the results of the above experiments, it can be seen that miR-503-5p not only plays a role in promoting tumor cell activity in CH, but also leads to abnormal proliferation of normal vascular cells, thus contributing to the progression of CH. In addition, the silencing of miR-503-5p in HemECs and HUVECs resulted in significantly improved cell adhesion (increased ICAM-1 and VCAM-1 protein levels) and enhanced EMT (increased N-cadherin and vimentin, and decreased E-cadherin). Currently, it has been clinically proven that EMT is the key to enhance the malignant invasion and migration of tumor cells [21]. For normal vascular endothelial cells, EMT plays a role in promoting cell aggregation and formation of intravascular obstruction [22]. That is to say, miR-503-5p can affect the altered biological behavior of vascular endothelial cells, whether they are in a pathological state or in a normal state. This demonstrates that the mechanism of miR-503-5p in CH is related to the promotion of neovascularization on the one hand and the stimulation of the accelerated growth of tumor cells on the other hand, which agrees with the views of previous studies [23].
MicroRNAs usually regulate biological behavior changes in cells through their downstream targeting proteins [24]. Previous studies have confirmed that miR-503-5p affects the progression of colorectal cancer and hepatocellular carcinoma by targeting PDCD4 and WEE1 [10,25], but its pathway of action in CH remains unclear. We speculate that its mechanism may be related to BMPR1A. To verify this hypothesis, we first predicted the potential downstream target genes of miR-503-5p, and found that BMPR1A was one of the genes predicted by all the databases used. In DLR, BMPR1A-WT activity was enhanced by miR-503-5p, while BMPR1A-MUT activity was inhibited, confirming the targeted regulatory relationship between them. After silencing miR-503-5p, the level of BMPR1A increased, while inhibiting miR-503-5p led to the opposite results. These results were consistent with the above clinical trials, indicating that miR-503-5p negatively regulates BMPR1A, that is, the higher the miR-503-5p, the lower the BMPR1A. Previous evidence has preliminarily demonstrated the regulatory effect of BMPR1A on angiogenesis (BMPR1A provides an important function for bone morphogenetic protein-induced retinal angiogenesis [26]), and Xiao et al.’s study even confirmed that blocking the expression of BMPR1A could disrupt angiogenesis in colon cancer cells [27]. However, its role in CH has yet to be confirmed. Therefore, we further used abnormal BMPR1A expression vectors to interfere with HemECs and HUVECs. The experimental results were completely opposite to those of miR-503-5p; that is, increasing BMPR1A promoted HemEC and HUVEC activities, EMT, and cell adhesion and inhibited apoptosis, while silencing BMPR1A inhibited HemEC and HUVEC growth and promoted apoptosis. These results are also consistent with previous studies [28,29], indicating that high expression of BMPR1A plays a role as an oncogene in CH. Similarly, in a previous study, we found that BMPR1A played an important role in the growth and aging process of cardiomyocytes [30], and Wan et al. demonstrated that BMPR1A was involved in azithromycin-induced cardiotoxicity [31], which shows that this protein has an important impact on cardiovascular function. However, after simultaneously increasing or silencing miR-503-5p and BMPR1A, the biological behavior of HemECs and HUVECs was not different from that of BMPR1A-NC-transfected cells, indicating that the influence of BMPR1A on cells can be completely reversed by miR-503-5p. Taken together, miR-503-5p regulates the biological behavior of CH cells through sponge adsorption of BMPR1A.
Of course, cellular and animal assays alone do not fully determine the clinical expression of miR-503-5p in CH. Therefore, in the final step of this study, we included CH clinical cases for analysis. Summarizing the clinical presentations of CH patients, it was found that patients often had pericardial effusion (most of these patients had clinical symptoms), most of which were clear and nonbloody fluids, with no clear correlation with intracavitary space-occupying lesions. The tumor can be small, found occasionally, or it can show a large mass; in some cases, it can bleed, forming a large amount of pericardial effusion, causing cardiac tamponade and even death [32]. All patients with ventricular septal pace-occupying masses were found on physical examination with no clinical symptoms. All the patients with cardiac space-occupying lesions found by physical examination had a single lesion, without pericardial effusion in 80% of them. Most of them had a single lesion, and few had multiple lesions. Sometimes, they may be combined with multiple hemangiomas in other organs, such as the liver. Further relevant examination is needed to determine whether the cardiac occupying is a local manifestation of systemic diseases. The detection of peripheral blood miR-503-5p and BMPR1A expression in CH cases showed that miR-503-5p was underexpressed while BMPR1A was overexpressed in CH, consistent with the results of previous studies. For example, miR-503-5p is also lowly expressed in myocardial ischemia/reperfusion injury and ovarian cancer [16,33], suggesting that they may be involved in the development and progression of CH. miR-503-5p and BMPR1A levels in cancer of CH patients also showed the same mechanism as in peripheral blood, which can validate the accuracy of the above experimental results. Meanwhile, we identified through correlation analysis that the expression of the two was negatively correlated, which indicates that BMPR1A in CH may be negatively regulated by miR-503-5p. The abnormal expression of miR-503-5p and BMPR1A in CH also indicates their potential as disease assessment indicators in the future. However, due to the low incidence of CH and the limited number of cases collected by us, their diagnostic and evaluation significance was not analyzed, which will be investigated in the future.
However, due to the limited experimental conditions, this study still has some limitations that need to be addressed. For example, we need to include more clinical cases to further analyze the role of miR-503-5p and BMPR1A in evaluating the progression of CH. In addition, it is necessary to analyze the pathway through which miR-503-5p influences CH cells to assist in the clinical understanding of the role of miR-503-5p in CH. Moreover, we need to observe the changes in CH after interfering with the expression of miR-503-5p or BMPR1A in vivo to lay the foundation for future treatment of CH based on miR-503-5p and BMPR1A molecularly targeted therapies.
Conclusion
miR-503-5p is poorly expressed and BMPR1A is highly expressed in CH. By targeting BMPR1A, miR-503-5p promotes CH cell growth, accelerates cell adhesion and EMT, and inhibits apoptosis. In the future, molecularly targeted therapy through miR-503-5p or BMPR1A may be a new direction for CH treatment, but more experiments are still needed to further confirm the mechanism of action of both in CH.
Acknowledgements
This study was supported by the Science and Technology Major Project of Changzhou Science and Technology Bureau (No. CE20205047), Natural Science Foundation of Xinjiang Uygur Autonomous Region (No. 2022D01F52), Major Scientific Research Project of Changzhou Municipal Health Commission (No. ZD202220), and Changzhou Municipal Health High-level Talents (No. 2024CZLJ005).
Disclosure of conflict of interest
None.
References
- 1.Berdica L, Kola E, Nakuci D, Horjeti E, Alimehmeti M. Cardiac hemangioma presenting as a primary cardiac tumor. Cardiooncology. 2023;9:3. doi: 10.1186/s40959-023-00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanigawa K, Kawano H, Hayashi T, Hasegawa T, Maemura K, Eishi K. Cardiac angiosarcoma and hepatic hemangioma. Circ J. 2021;85:318. doi: 10.1253/circj.CJ-20-1235. [DOI] [PubMed] [Google Scholar]
- 3.Shah A, Binkovitz L, Layman AJ, Bois MC, Nguyen B. Cardiac hemangioma mimicking a neuroendocrine tumor on (68) Ga Dotatate PET/CT. J Card Surg. 2022;37:2849–2851. doi: 10.1111/jocs.16578. [DOI] [PubMed] [Google Scholar]
- 4.Bassi A, Azzarelli A, Vaccaro A, Mazzatenta C. Infantile hemangioma and cardiac defects: a puzzling association. A single-center experience. Dermatol Pract Concept. 2022;12:e2022150. doi: 10.5826/dpc.1203a150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie T, Masroor M, Chen X, Liu F, Zhang J, Yang D, Liu C, Xiang M. Rheumatism as a cause of cardiac hemangioma: a rare case report and review of literature with special focus on etiology. BMC Cardiovasc Disord. 2023;23:203. doi: 10.1186/s12872-023-03241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topel C, Sevinc S, Onan B, Yildiz M, Guler GB. Cardiac hemangioma in a difficult anatomical location presented with ventricular tachycardia. A rare case report. Echocardiography. 2021;38:118–122. doi: 10.1111/echo.14954. [DOI] [PubMed] [Google Scholar]
- 7.Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234:5451–5465. doi: 10.1002/jcp.27486. [DOI] [PubMed] [Google Scholar]
- 8.Val-Bernal JF, Martino M, Mayorga M, Garijo MF. Prichard’s structures of the fossa ovalis are age-related phenomena composed of nonreplicating endothelial cells: the cardiac equivalent of cutaneous senile angioma. APMIS. 2007;115:1234–1240. doi: 10.1111/j.1600-0643.2007.00756.x. [DOI] [PubMed] [Google Scholar]
- 9.Markopoulos GS, Roupakia E, Tokamani M, Vartholomatos G, Tzavaras T, Hatziapostolou M, Fackelmayer FO, Sandaltzopoulos R, Polytarchou C, Kolettas E. Senescence-associated microRNAs target cell cycle regulatory genes in normal human lung fibroblasts. Exp Gerontol. 2017;96:110–122. doi: 10.1016/j.exger.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Jiang SP, Li ZR. MiR-503-5p regulates cell epithelial-to-mesenchymal transition, metastasis and prognosis of hepatocellular carcinoma through inhibiting WEE1. Eur Rev Med Pharmacol Sci. 2019;23:2028–2037. doi: 10.26355/eurrev_201903_17242. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Zhang X, Yu Z, Zhang T. Exosomes promote atherosclerosis progression by regulating circ_100696/miR-503-5p/PAPPA axis-mediated vascular smooth muscle cells proliferation and migration. Int Heart J. 2023;64:918–927. doi: 10.1536/ihj.23-089. [DOI] [PubMed] [Google Scholar]
- 12.Guo Z, Zhao M, Jia G, Ma R, Li M. LncRNA PART1 alleviated myocardial ischemia/reperfusion injury via suppressing miR-503-5p/BIRC5 mediated mitochondrial apoptosis. Int J Cardiol. 2021;338:176–184. doi: 10.1016/j.ijcard.2021.05.044. [DOI] [PubMed] [Google Scholar]
- 13.Laakkonen JP, Lahteenvuo J, Jauhiainen S, Heikura T, Yla-Herttuala S. Beyond endothelial cells: vascular endothelial growth factors in heart, vascular anomalies and placenta. Vascul Pharmacol. 2019;112:91–101. doi: 10.1016/j.vph.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Thakur D, Taliaferro O, Atkinson M, Stoffel R, Guleria RS, Gupta S. Inhibition of nuclear factor kappaB in the lungs protect bleomycin-induced lung fibrosis in mice. Mol Biol Rep. 2022;49:3481–3490. doi: 10.1007/s11033-022-07185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Bizri N, Wang L, Merklinger SL, Guignabert C, Desai T, Urashima T, Sheikh AY, Knutsen RH, Mecham RP, Mishina Y, Rabinovitch M. Smooth muscle protein 22alpha-mediated patchy deletion of Bmpr1a impairs cardiac contractility but protects against pulmonary vascular remodeling. Circ Res. 2008;102:380–388. doi: 10.1161/CIRCRESAHA.107.161059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HW, Adachi T, Pak B, Park S, Hu X, Choi W, Kowalski PS, Chang CH, Clapham KR, Lee A, Papangeli I, Kim J, Han O, Park J, Anderson DG, Simons M, Jin SW, Chun HJ. BMPR1A promotes ID2-ZEB1 interaction to suppress excessive endothelial to mesenchymal transition. Cardiovasc Res. 2023;119:813–825. doi: 10.1093/cvr/cvac159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anbardar MH, Soleimani N, Mohammadzadeh S. Two cases of cardiac hemangioma in different anatomical locations presenting with chest pain and palpitation. Clin Case Rep. 2022;10:e05495. doi: 10.1002/ccr3.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Zhang F, Li H, Peng F, Wang Z, Peng H, He J, Li Y, He L, Wei L. Circ_0010220-mediated miR-503-5p/CDCA4 axis contributes to osteosarcoma progression tumorigenesis. Gene. 2020;763:145068. doi: 10.1016/j.gene.2020.145068. [DOI] [PubMed] [Google Scholar]
- 19.Fei Y, Shan W, Chen X. MiR-503-5p functions as an oncogene in oral squamous cell carcinoma by targeting Smad7. Histol Histopathol. 2020;35:893–901. doi: 10.14670/HH-18-220. [DOI] [PubMed] [Google Scholar]
- 20.Ju A, Shen Y, Yue A. Circ_0011232 contributes to hepatocellular carcinoma progression through miR-503-5p/AKT3 axis. Hepatol Res. 2022;52:532–545. doi: 10.1111/hepr.13758. [DOI] [PubMed] [Google Scholar]
- 21.Huang X, Wang L, Guo H, Zhang W, Shao Z. Single-cell transcriptomics reveals the regulative roles of cancer associated fibroblasts in tumor immune microenvironment of recurrent osteosarcoma. Theranostics. 2022;12:5877–5887. doi: 10.7150/thno.73714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribatti D. Epithelial-endothelial transition and endothelial-mesenchymal transition. Int J Dev Biol. 2022;66:311–316. doi: 10.1387/ijdb.210234dr. [DOI] [PubMed] [Google Scholar]
- 23.Tang NP, Hui TT, Ma J, Mei QB. Effects of miR-503-5p on apoptosis of human pulmonary microvascular endothelial cells in simulated microgravity. J Cell Biochem. 2019;120:727–737. doi: 10.1002/jcb.27430. [DOI] [PubMed] [Google Scholar]
- 24.Ho PTB, Clark IM, Le LTT. MicroRNA-based diagnosis and therapy. Int J Mol Sci. 2022;23:7167. doi: 10.3390/ijms23137167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen C, Feng X, Yuan H, Gong Y, Wang G. Circ_0003266 sponges miR-503-5p to suppress colorectal cancer progression via regulating PDCD4 expression. BMC Cancer. 2021;21:284. doi: 10.1186/s12885-021-07997-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HW, Chong DC, Ola R, Dunworth WP, Meadows S, Ka J, Kaartinen VM, Qyang Y, Cleaver O, Bautch VL, Eichmann A, Jin SW. Alk2/ACVR1 and Alk3/BMPR1A provide essential function for bone morphogenetic protein-induced retinal angiogenesis. Arterioscler Thromb Vasc Biol. 2017;37:657–663. doi: 10.1161/ATVBAHA.116.308422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao F, Qiu H, Cui H, Ni X, Li J, Liao W, Lu L, Ding K. MicroRNA-885-3p inhibits the growth of HT-29 colon cancer cell xenografts by disrupting angiogenesis via targeting BMPR1A and blocking BMP/Smad/Id1 signaling. Oncogene. 2015;34:1968–1978. doi: 10.1038/onc.2014.134. [DOI] [PubMed] [Google Scholar]
- 28.Han L, Cheng J, Li A. hsa_circ_0072387 suppresses proliferation, metastasis, and glycolysis of oral squamous cell carcinoma cells by downregulating miR-503-5p. Cancer Biother Radiopharm. 2021;36:84–94. doi: 10.1089/cbr.2019.3371. [DOI] [PubMed] [Google Scholar]
- 29.Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, Zhou X, Huang X, Xia Q, Chen Z, Zhang X, Yang D, Geng YJ. Pax8 plays a pivotal role in regulation of cardiomyocyte growth and senescence. J Cell Mol Med. 2016;20:644–654. doi: 10.1111/jcmm.12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan GX, Cheng L, Qin HL, Zhang YZ, Wang LY, Zhang YG. MiR-15b-5p is involved in doxorubicin-induced cardiotoxicity via inhibiting Bmpr1a signal in H9c2 cardiomyocyte. Cardiovasc Toxicol. 2019;19:264–275. doi: 10.1007/s12012-018-9495-6. [DOI] [PubMed] [Google Scholar]
- 32.Mylonas KS, Ziogas IA, Avgerinos DV. Microenvironment in cardiac tumor development: what lies beyond the event horizon? Adv Exp Med Biol. 2020;1226:51–56. doi: 10.1007/978-3-030-36214-0_4. [DOI] [PubMed] [Google Scholar]
- 33.Li B, Lou G, Zhang J, Cao N, Yu X. Repression of lncRNA PART1 attenuates ovarian cancer cell viability, migration and invasion through the miR-503-5p/FOXK1 axis. BMC Cancer. 2022;22:124. doi: 10.1186/s12885-021-09005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]