Abstract
Aims: We investigate the value of postoperative minimal residual disease (MRD) detection using circulating tumor DNA (ctDNA) in guiding adjuvant therapy for patients with potentially high recurrence risk in non-small cell lung cancer (NSCLC) due to the presence of MRD. Patients and Methods: A randomized controlled trial will enroll stage IA-IIA NSCLC patients with Epidermal Growth Factor Receptor (EGFR) mutation and negative resection margins to evaluate the clinical value of MRD in guiding adjuvant osimertinib. That is, if the patient’s peripheral blood does not show ctDNA (negative) after next generation sequencing (NGS) testing, postoperative observation and follow-up are sufficient. Conversely, if ctDNA is positive, the patient will be randomly assigned to two groups and receive adjuvant treatment with osimertinib or observation and follow-up. In total 1068 postoperative patients should be recruited, finally, 32 MRD positive patients were divided into a treatment group or an observation group. Primary endpoint: progression-free survival (PFS). Secondary endpoints: 2- and 5-year PFS rates, regimen safety, and tolerability. Exploratory indicator in the MRD-positive group: ctDNA clearance rate at 12 and 24 months. Results and conclusions: This study provides crucial insights into therapy guidance for EGFR-mutated NSCLC patients with MRD, potentially enhancing patient outcomes.
Keywords: MRD, EGFR, NSCLC, adjuvant therapy
Introduction
Background and rationale
Lung cancer is the most common cancer worldwide, and NSCLC accounts for about 85% of all lung cancers. In 2020, there were 2.21 million new lung cancer cases and 1.8 million deaths worldwide, which caused an enormous economic burden for patients and society [1]. Data from the National Cancer Center of China corroborates lung cancer’s prevalence as the leading cancer type in China. In 2016, out of 4.064 million new cancer cases, lung cancer represented 828,000, with lung cancer deaths reaching 457,000 out of 2.414 million cancer-related fatalities [2]. For patients with early-stage NSCLC, the main treatment strategy is radical resection of the primary tumor, and some researchers reported that the 5-year overall survival (OS) rate of stage I-IIIa patients is 33%-60% with surgery with curative intent [3], while others reported as 73-90% [4]. For NSCLC patients of stage I, the prognosis is generally favorable with 5-year survival rate ranging from 73% to 90% [5]. The overall survival of patients is mainly affected by tumor recurrence, which may directly lead to death after surgical resection. According to reports, the recurrence rate ranges from 20-37.4% depending on the follow-up time [6]. For stage IB, 1-year overall rate for patients with and without adjuvant chemotherapy were 94% and 94%, respectively. 5 years overall rate were 60% and 58%, respectively [7].
ctDNA contains cancer-associated genetic information derived from tumor cells [8]. It was usually demonstrated several ways that ctDNA originated, including nuclear and mitochondrial DNA released by apoptotic and necrotic cells, and even living tumor cells such as circulating tumor cells (CTCs) present in the blood [8]. The non-invasive analysis of ctDNA offers considerable advantages, including simplified sample collection and the ability to longitudinally track tumor evolution. ctDNA not only provides a broad genetic snapshot but also serves to reduce the impact of tumor heterogeneity on diagnostics. Advances in detection methodologies have propelled the utilization of ctDNA as a complementary, and at times alternative, approach to conventional tissue biopsies. Such applications range from early cancer diagnosis to monitoring for disease recurrence, prognostication, and predicting response to treatment as well as identifying the development of drug resistance [9-11].
Osimertinib, a third-generation EGFR tyrosine kinase inhibitor (EGFR-TKI), is designed to selectively target both EGFR activating and EGFR T790M resistance mutations. It has secured full approval from the US Food and Drug Administration (FDA) for the treatment of metastatic EGFR-mutant NSCLC patients who harbor these mutations and as an adjuvant treatment for early-stage NSCLC (IB-IIIA). Similarly, the National Medical Products Administration (NMPA) of China has sanctioned its use for three specific clinical settings: as a subsequent line of therapy for adult patients with locally advanced or metastatic NSCLC with confirmed EGFR T790M mutation following prior EGFR-TKI treatment; as first-line treatment for adult patients with locally advanced or metastatic NSCLC possessing EGFR exon 19 deletions or exon 21 (L858R) substitution mutations; and as adjuvant therapy after tumor resection in patients with EGFR mutations [12,13].
The ADAURA study, a randomized phase III trial, has scrutinized the efficacy and safety of osimertinib as adjuvant therapy in resected early-stage EGFR-mutated NSCLC. The overall survival analysis from the ADAURA trial was published recently, which indicated that among patients with stage IB to IIIA disease, the 5-year OS was 88% with adjuvant osimertinib compared to 78% with placebo, with a median follow-up for OS of 60.4 months (osimertinib) and 59.4 months (placebo) (HR, 0.49; 95% CI, 0.34 to 0.70; P < 0.0001) [14]. This study demonstrated adjuvant osimertinib displayed an obvious and exciting benefit in resected EGFR-mutated NSCLC. Nevertheless, the overall hazard ratio for death at 0.49 highlights the need for further investigations to refine the eligible patient cohort for maximal benefit. For this purpose, there are two potential research directions for future strategies. One is the application of combination therapy in the adjuvant treatment stage, such as osimertinib combined with chemotherapy or other treatment methods. The other is to improve the benefit of adjuvant osimertinib utilizing specific methods or biomarkers. The evaluation of MRD to guide osimertinib adjuvant therapy appears to be the most promising of these strategies. Therefore, we have designed and registered this clinical trial to propose meaningful directions for the optimization of adjuvant targeted therapy for early-stage NSCLC.
Objectives
This study is a randomized controlled trial (RCT) designed to investigate the utility of ctDNA-based MRD as a guide for postoperative adjuvant therapy with osimertinib in early-stage NSCLC. The primary endpoint is the PFS of the participants. Secondary endpoints include the PFS rate at 2 and 5 years, alongside an evaluation of the treatment’s safety and tolerability. In addition, among the MRD-positive group, the ctDNA clearance rate at 12 and 24 months will serve as an exploratory measure of the adjuvant therapy’s effectiveness.
Trial design
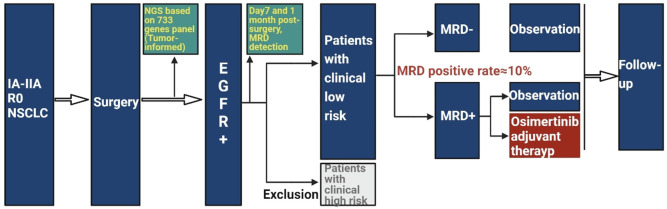
This open-label, multicenter RCT prospectively examines the effectiveness of ctDNA-based MRD in guiding postoperative adjuvant therapy with osimertinib for early-stage NSCLC. Eligible participants include patients with stage IA-IIA NSCLC who have undergone radical surgery yielding negative resection margins (R0), with their tumor samples subjected to NGS detection. Following stratification, patients harboring EGFR mutations will proceed within the study framework. On the seventh day and 1 month postsurgery, peripheral blood samples will be collected for MRD assessment using NGS to detect ctDNA as previously described [15]. Tumor samples obtained from surgical resections are subjected to a comprehensive analysis for somatic mutations using an NGS panel that targets 733 cancer-related genes (3D Medicines Inc., Shanghai, China). Prior to analyzing the tumor sequencing data, germline variants identified by comparing the NGS results with matched peripheral blood leukocytes are systematically excluded. Each patient’s somatic variants are cataloged as individual markers for subsequent ctDNA surveillance. Plasma samples are assessed with a 179-gene NGS panel specialized for MRD detection of NSCLC. Patients are classified as MRD-positive if one or more individual somatic variants are identified in their plasma; those with no detectable variants are deemed MRD-negative. Prospective enrollment will be conducted to evaluate the PFS of the adjuvant osimertinib therapy (Figure 1).
Figure 1.
Flow chart for data analysis in this research.
Methods
Participants
In this study, patients diagnosed with stage IA-IIA EGFR-mutated NSCLC will be recruited (Figure 1) and their baseline characteristics will need to be comprehensively collected (Supplementary Table 1). The sample size calculation is predicated on the assumption that for postoperative MRD-negative patients with clinically low-risk, there is no significant difference in the 2-year survival rate between those who receive adjuvant therapy and those who do not. For patients who are MRD-positive with clinically low-risk however, it is projected that the 2-year PFS rate stands at 45% for the observation group (Group A), and increases to 90% in the osimertinib adjuvant treatment cohort (Group B), as per available data [16]. The intended patient number ratio for the two groups is set to A:B = 1:1 with a statistical power (POWER) of 0.8 and a significance level (α) of 0.05. Utilizing an online statistical calculator (http://powerandsamplesize.com/Calculators/Compare-2-Proportions/2-Sample-Equality) yielded a required sample size of 14 patients for Group B, leading to a total of 28 patients for both groups combined. Accounting for an anticipated dropout rate of 15%, it is determined that 16 patients per group will be necessary. In light of the fact that the average MRD positivity rate post-IA-IIA surgery is documented as 15% [17], and considering 3D Medicine Inc.’s MRD detection strategy that this study will adopt showed a lower positivity rate than previously reported data [18], we have conservatively estimated the MRD positivity rate in our study at 10%. To reach a sufficient sample of MRD-positive patients, we need to include 320 low-risk participants in that category. Given that clinical experience suggests low-risk patients comprise about 60% of our target population, this necessitates the enrollment of approximately 534 postoperative patients positive for EGFR. Furthermore, drawing from previous research findings, the prevalence of EGFR positivity in the Chinese NSCLC patient cohort is around 50% [19,20]. Therefore, we estimate the necessity to recruit upwards of 1068 postoperative patients to meet the requirements of our study.
Inclusion criteria
(1) Adult patients aged over 18-70 years. (2) NSCLC confirmed by histopathology. (3) Patients with stage IA-IIA NSCLC who have undergone curative lung surgery with negative resection margins and possess EGFR mutations, excluding mutations associated with osimertinib resistance (e.g., L718Q, C797S). (4) The Eastern Cooperative Oncology Group (ECOG) performance status score of 0-1, without deterioration in the initial two weeks post-enrollment and an expected survival of at least 12 weeks. (5) Full recovery from surgery with pre-treatment imaging indicating no evidence of tumor relapse or progression. (6) Clinical laboratory parameters within the specified ranges: (a) Platelet count ≥ 100 × 109/L; (b) Absolute neutrophil count (ANC) ≥ 1.5 × 109/L or absolute white blood cell count (WBC) ≥ 3.5 × 109/L; (c) Hemoglobin (Hgb) ≥ 100 g/L (no blood transfusion or use of erythropoietin within 4 weeks); (d) Total bilirubin ≤ 1.5 × the upper limit of normal value (ULN) (or ≤ 2.5 × ULN with liver metastasis); (e) Alanine transaminase (ALT) and cereal straw transaminase (AST) ≤ 2.5 × ULN; (f) Creatinine ≤ 1.5 × ULN or creatinine clearance rate ≥ 50 mL/min (according to Cockcroft Fault formula); (g) Serum amylase ≤ 2 × ULN or pancreatic amylase ≤ 1.5 × ULN; (h) Serum lipase ≤ 1.5 × ULN; (i) The subjects who did not take anticoagulants, or those who had previously used anticoagulants met the requirement of stopping the medication for 28 days before enrollment, with an international standardized ratio (INR) ≤ 1.5 and an activated partial thromboplastin time (APTT) ≤ 1.5 × ULN. (7) Able to swallow and take oral medication. (8) Comply with the informed consent form.
Exclusion criteria
(1) Tumor histology indicative of small cell lung cancer, neuroendocrine tumors, sarcoma, or mixed pathology. (2) Prior systemic anti-cancer treatments and postoperative therapy such as chemotherapy, radiation, targeted therapy, or immunotherapy. (3) Clinically evident disease recurrence prior to adjuvant therapy, as demonstrated via pathological or imaging techniques. (4) Previous history of interstitial lung disease (ILD), evidence of drug-induced ILD, or any clinically active ILD, and the baseline CT scan showed the presence of idiopathic pulmonary fibrosis. (5) Clinically uncontrollable pleural/abdominal effusion. (6) High risk factors include the degree of differentiation is low, postoperative pathology suggests cancer thrombus in the vessels, pathology suggests involvement of the visceral pleura, the number of detected lymph nodes is too small, and the pathological subtype is micropapillary. (7) Received glucocorticoid treatment 28 days before the first administration (equivalent dose of > 10 mg prednisone per day). (8) History of active autoimmune diseases requiring systemic treatment (i.e. the use of disease-regulating drugs, corticosteroids, or immunosuppressive drugs) within the last two years. (9) Allergy to osimertinib. (10) Allogeneic tissue/solid organ transplantation has been carried out. (11) Except for hair loss and stable peripheral neurotoxicity below level 2, any clinical toxicity related to previous treatment before enrollment has not returned to pre-treatment levels or level 1. (12) Current pregnancy (evidenced by positive serum β-HCG) or lactation. (13) Incomplete clinical pathological data or follow-up information. (14) Suspected or confirmed immune deficiency or active infection. (15) As assessed by the investigator, unsuitability for trial participation for any reason. (16) Known psychiatric disorders or substance abuse that could affect protocol adherence.
Interventions
In this study, we focus on early-stage (IA-IIA) non-small cell lung cancer (NSCLC) in adult patients with EGFR mutations but without clinical high-risk factors, which are clinically classified as low-risk individuals. After surgical resection, patients who tested positive for MRD are assigned to one of two groups: the observation group or the adjuvant osimertinib treatment group. In the adjuvant treatment group, patients receive a daily dose of 80 mg of osimertinib for a duration of 3 years, unless treatment is prematurely discontinued due to disease progression or intolerance. Conversely, for patients with EGFR mutations who are clinically low-risk and MRD-negative, the decision to pursue adjuvant osimertinib therapy or undergo postoperative observation is determined according to the patient’s preferences.
Outcomes
(1) Primary endpoint: Evaluation of PFS among patients with early-stage EGFR-mutated NSCLC undergoing MRD-guided osimertinib adjuvant therapy, assessed according to RECIST 1.1 criteria. (2) Secondary endpoints: 2-year and 5-year PFS rates and subjects’ quality of life (QoL). QoL was assessed using the EORTC QLQ-C30 questionnaire. (3) Exploratory endpoint: ctDNA clearance rate at 12 and 24 months after surgery. (4) Safety: The overall incidence of adverse events (AEs); the incidence of AE at level 3 or higher; the incidence of serious adverse events (SAEs); the incidence of AEs leading to the termination of experimental medication; and the incidence of AEs leading to the suspension of experimental medication.
Data statistics and analysis
Patients with a cancer event unrelated to the original tumor such as a ‘second primary’ tumor were considered censored for purposes of PFS, but not for OS. Death was included as an event for OS and PFS. Hazard Ratios and significance were obtained using the R package ‘survival’ based on the time from the end of treatment. Kaplan-Meier and log rank tests were adopted to evaluate the predictive significance of ctDNA/MRD detection.
Discussion
The 5-year overall survival rate of NSCLC is still very low with considerable variation across stages. A European study involving 2098 lung cancer patients indicated 5-year recurrence and metastasis rates for stages IA, IB, and IIA as 33.9%, 37.8%, and 61.2%, respectively. Contrastingly, research from Taiwan revealed only about a 50% 5-year DFS rate for stage I lung cancer post-surgery, implying over 40% of these patients experience recurrence or disease progression within five years [21,22]. Therefore, postoperative adjuvant therapy for early-stage patients with a high risk of recurrence is very necessary and important.
According to the latest version of National Comprehensive Cancer Network (NCCN) guidelines, patients with stage IB and IIA can choose observation or chemotherapy for high-risk patients or atezolizumab (for stage IIA) or osimertinib (EGFR exon 19 deletion or L858R). However, the LACE meta-analysis suggests a modest increase of 5.2% (P < 0.0001) in 5-year DFS and 5.3% (P = 0.0043) in 5-year OS with adjuvant chemotherapy, with potential drawbacks for stage IA patients. Specifically, adjuvant chemotherapy correspondingly diminishes the mortality risk by merely 8% in stage IB and 17% in stages II-III [23]. Furthermore, studies have revealed that adjuvant chemotherapy benefits patients with micropapillary lung adenocarcinoma at stage IA. However, the benefits among NSCLC patients with various pathological subtypes at stage IB differ; those with micropapillary/parenchymal patterns experience advantages from adjuvant chemotherapy, unlike those with the acinar subtype [20,24]. These findings underscore the necessity of a judicious approach to adjuvant therapy for stages IIA and earlier, avoiding one-size-fits-all strategies and overtreatment, which can lead to both patient burden and healthcare resource wastage.
Selecting patients for adjuvant therapy through biomarkers is vital, especially with emerging targeted treatments. Notably, adjuvant osimertinib has shown promise for EGFR-positive patients (EGFR exon 19 deletion or L858R). The approval of osimertinib as adjuvant therapy for EGFR-positive patients is based on the data of the ADAURA study, a global multicenter, randomized, double-blind, phase III clinical trial, demonstrating a staggering 90% versus 44% two-year DFS rate between the osimertinib and placebo groups for stage II to IIIA patients (hazard ratio for disease recurrence or death, 0.17; 99.06% CI, 0.11 to 0.26; P < 0.001) [16]. The approval of this targeted adjuvant therapy has expanded the benefit patient group of osimertinib from advanced patients to early resectable NSCLC patients, revolutionizing the treatment mode of early-stage NSCLC and breaking through the standard of radical surgery and adjuvant chemotherapy treatment mode. Additionally, adjuvant targeted therapy after surgery can further improve the curative effect, which is of great significance for reducing the risk of postoperative recurrence and improving the prognosis and long-term survival of patients with EGFR mutations.
Detection of ctDNA using NGS is a type of the liquid biopsies currently employed for MRD assessment in various solid tumors. Particularly, ctDNA-based MRD monitoring during postoperative follow-up can allow for an earlier prediction of disease relapse compared with current imaging modalities. Thereby, precise MRD monitoring could be pivotal in the effective management and potential eradication of tumors in the future [25,26]. Moreover, the value of MRD monitoring is not only in the prediction of patients’ postoperative recurrence risk but also in guiding postoperative treatment decisions [26-28]. Several research studies focusing on MRD in resectable NSCLC have been published, and almost all of these studies have shown that in patients with stage II-III NSCLC, ctDNA-based MRD contributes more significantly to the prediction of recurrence-free survival (RFS) than other variables, including TNM staging [26-28]. Furthermore, MRD status was associated with patient benefit from adjuvant therapy; MRD-positive patients could benefit from adjuvant therapies, while MRD-negative patients showed a low risk of relapse regardless of whether they received adjuvant therapy [26,27]. However, in these studies, adjuvant therapy mainly refers to adjuvant chemotherapy; the significance of MRD monitoring in guiding the decision-making for postoperative targeted therapy in NSCLC patients with EGFR mutations remains unclear. Therefore, we have designed and are actively promoting this study to address these academic and clinical questions and to enrich the literature in this field.
Conclusion
This study aims to evaluate the clinical value of MRD detected through ctDNA in guiding adjuvant therapy for patients with EGFR-mutated early-stage NSCLC. The findings from this study have the potential to significantly impact clinical practice by providing valuable information on postoperative recurrence risk prediction and guidance for therapy strategy in EGFR-mutated NSCLC patients with MRD, ultimately improving patient outcomes and survival rates.
Acknowledgements
This research was supported by the Clinical Research Special Fund of Wu Jieping Medical Foundation (320.6575.2021-17-5).
Informed consent will be obtained from the patients/participants.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent. 2022;2:1–9. doi: 10.1016/j.jncc.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 4.Qian JY, Li ZX, Wu LL, Song SH, Li CW, Lin WK, Xu SQ, Li K, Xie D. A clinical risk model for assessing the survival of patients with stage IA-IIA non-small cell lung cancer after surgery. J Thorac Dis. 2022;14:4285–4296. doi: 10.21037/jtd-22-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. 2017;151:193–203. doi: 10.1016/j.chest.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Schuchert MJ, Normolle DP, Awais O, Pennathur A, Wilson DO, Luketich JD, Landreneau RJ. Factors influencing recurrence following anatomic lung resection for clinical stage I non-small cell lung cancer. Lung Cancer. 2019;128:145–151. doi: 10.1016/j.lungcan.2018.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strauss GM, Herndon JE 2nd, Maddaus MA, Johnstone DW, Johnson EA, Harpole DH, Gillenwater HH, Watson DM, Sugarbaker DJ, Schilsky RL, Vokes EE, Green MR. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the cancer and leukemia group B, radiation therapy oncology group, and north central cancer treatment group study groups. J. Clin. Oncol. 2008;26:5043–5051. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10:472–484. doi: 10.1038/nrclinonc.2013.110. [DOI] [PubMed] [Google Scholar]
- 9.Horn L, Whisenant JG, Wakelee H, Reckamp KL, Qiao H, Leal TA, Du L, Hernandez J, Huang V, Blumenschein GR, Waqar SN, Patel SP, Nieva J, Oxnard GR, Sanborn RE, Shaffer T, Garg K, Holzhausen A, Harrow K, Liang C, Lim LP, Li M, Lovly CM. Monitoring therapeutic response and resistance: analysis of circulating tumor DNA in patients with ALK+ lung cancer. J Thorac Oncol. 2019;14:1901–1911. doi: 10.1016/j.jtho.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Liu JB, Hou LK, Yu F, Zhang J, Wu W, Tang XM, Sun F, Lu HM, Deng J, Bai J, Li J, Wu CY, Lin QL, Lv ZW, Wang GR, Jiang GX, Ma YS, Fu D. Liquid biopsy in lung cancer: significance in diagnostics, prediction, and treatment monitoring. Mol Cancer. 2022;21:25. doi: 10.1186/s12943-022-01505-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricciuti B, Jones G, Severgnini M, Alessi JV, Recondo G, Lawrence M, Forshew T, Lydon C, Nishino M, Cheng M, Awad M. Early plasma circulating tumor DNA (ctDNA) changes predict response to first-line pembrolizumab-based therapy in non-small cell lung cancer (NSCLC) J Immunother Cancer. 2021;9:e001504. doi: 10.1136/jitc-2020-001504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koch AL, Vellanki PJ, Drezner N, Li X, Mishra-Kalyani PS, Shen YL, Xia H, Li Y, Liu J, Zirkelbach JF, Palazov E, Gamarian A, Choo Q, Gircys A, Rohr UP, Fesenko N, Spillman D, Pazdur R, Beaver JA, Singh H. FDA approval summary: osimertinib for adjuvant treatment of surgically resected non-small cell lung cancer, a collaborative project orbis review. Clin Cancer Res. 2021;27:6638–6643. doi: 10.1158/1078-0432.CCR-21-1034. [DOI] [PubMed] [Google Scholar]
- 13.Remon J, Steuer CE, Ramalingam SS, Felip E. Osimertinib and other third-generation EGFR TKI in EGFR-mutant NSCLC patients. Ann Oncol. 2018;29:i20–i27. doi: 10.1093/annonc/mdx704. [DOI] [PubMed] [Google Scholar]
- 14.Tsuboi M, Herbst RS, John T, Kato T, Majem M, Grohe C, Wang J, Goldman JW, Lu S, Su WC, de Marinis F, Shepherd FA, Lee KH, Le NT, Dechaphunkul A, Kowalski D, Poole L, Bolanos A, Rukazenkov Y, Wu YL ADAURA Investigators. Overall survival with osimertinib in resected EGFR-mutated NSCLC. N Engl J Med. 2023;389:137–147. doi: 10.1056/NEJMoa2304594. [DOI] [PubMed] [Google Scholar]
- 15.Gao Z, Huang D, Chen H, Yang Y, An K, Ding C, Yuan Z, Zhai Z, Niu P, Gao Q, Cai J, Zeng Q, Wang Y, Hong Y, Rong W, Huang W, Lei F, Wang X, Chen S, Zhao X, Bai Y, Gu J. Development and validation of postoperative circulating tumor DNA combined with clinicopathological risk factors for recurrence prediction in patients with stage I-III colorectal cancer. J Transl Med. 2023;21:63. doi: 10.1186/s12967-023-03884-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M, Goldman JW, Laktionov K, Kim SW, Kato T, Vu HV, Lu S, Lee KY, Akewanlop C, Yu CJ, de Marinis F, Bonanno L, Domine M, Shepherd FA, Zeng L, Hodge R, Atasoy A, Rukazenkov Y, Herbst RS ADAURA Investigators. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2020;383:1711–1723. doi: 10.1056/NEJMoa2027071. [DOI] [PubMed] [Google Scholar]
- 17.Peng M, Huang Q, Yin W, Tan S, Chen C, Liu W, Tang J, Wang X, Zhang B, Zou M, Li J, Su W, Wang L, Chin L, Yu F. Circulating tumor DNA as a prognostic biomarker in localized non-small cell lung cancer. Front Oncol. 2020;10:561598. doi: 10.3389/fonc.2020.561598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yue D, Liu W, Chen C, Zhang T, Ma Y, Cui L, Gu Y, Bei T, Zhao X, Zhang B, Bai Y, Romero A, Xu-Welliver M, Wang C, Zhang Z, Zhang B. Circulating tumor DNA predicts neoadjuvant immunotherapy efficacy and recurrence-free survival in surgical non-small cell lung cancer patients. Transl Lung Cancer Res. 2022;11:263–276. doi: 10.21037/tlcr-22-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lei Y, Wang K, Liu Y, Wang X, Xiang X, Ning X, Ding W, Duan J, Li D, Zhao W, Li Y, Zhang F, Luo X, Shi Y, Wang Y, Huang D, Bai Y, Zhang H. Various subtypes of EGFR mutations in patients with NSCLC define genetic, immunologic diversity and possess different prognostic biomarkers. Front Immunol. 2022;13:811601. doi: 10.3389/fimmu.2022.811601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C, Yang J, Lu M. Micropapillary predominant lung adenocarcinoma in stage IA benefits from adjuvant chemotherapy. Ann Surg Oncol. 2020;27:2051–2060. doi: 10.1245/s10434-019-08113-0. [DOI] [PubMed] [Google Scholar]
- 21.Consonni D, Pierobon M, Gail MH, Rubagotti M, Rotunno M, Goldstein A, Goldin L, Lubin J, Wacholder S, Caporaso NE, Bertazzi PA, Tucker MA, Pesatori AC, Landi MT. Lung cancer prognosis before and after recurrence in a population-based setting. J Natl Cancer Inst. 2015;107:djv059. doi: 10.1093/jnci/djv059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hung JJ, Jeng WJ, Hsu WH, Chou TY, Huang BS, Wu YC. Predictors of death, local recurrence, and distant metastasis in completely resected pathological stage-I non-small-cell lung cancer. J Thorac Oncol. 2012;7:1115–1123. doi: 10.1097/JTO.0b013e31824cbad8. [DOI] [PubMed] [Google Scholar]
- 23.Goldstraw P, Ball D, Jett JR, Le Chevalier T, Lim E, Nicholson AG, Shepherd FA. Non-small-cell lung cancer. Lancet. 2011;378:1727–1740. doi: 10.1016/S0140-6736(10)62101-0. [DOI] [PubMed] [Google Scholar]
- 24.Hung JJ, Wu YC, Chou TY, Jeng WJ, Yeh YC, Hsu WH. Adjuvant chemotherapy improves the probability of freedom from recurrence in patients with resected stage IB lung adenocarcinoma. Ann Thorac Surg. 2016;101:1346–1353. doi: 10.1016/j.athoracsur.2015.10.075. [DOI] [PubMed] [Google Scholar]
- 25.Pantel K, Alix-Panabieres C. Liquid biopsy and minimal residual disease - latest advances and implications for cure. Nat Rev Clin Oncol. 2019;16:409–424. doi: 10.1038/s41571-019-0187-3. [DOI] [PubMed] [Google Scholar]
- 26.Zhang JT, Liu SY, Gao W, Liu SM, Yan HH, Ji L, Chen Y, Gong Y, Lu HL, Lin JT, Yin K, Jiang BY, Nie Q, Liao RQ, Dong S, Guan Y, Dai P, Zhang XC, Yang JJ, Tu HY, Xia X, Yi X, Zhou Q, Zhong WZ, Yang XN, Wu YL. Longitudinal undetectable molecular residual disease defines potentially cured population in localized non-small cell lung cancer. Cancer Discov. 2022;12:1690–1701. doi: 10.1158/2159-8290.CD-21-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu B, Guo W, Zhang F, Lv F, Ji Y, Peng Y, Chen X, Bao H, Xu Y, Shao Y, Tan F, Xue Q, Gao S, He J. Dynamic recurrence risk and adjuvant chemotherapy benefit prediction by ctDNA in resected NSCLC. Nat Commun. 2021;12:6770. doi: 10.1038/s41467-021-27022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia L, Mei J, Kang R, Deng S, Chen Y, Yang Y, Feng G, Deng Y, Gan F, Lin Y, Pu Q, Ma L, Lin F, Yuan Y, Hu Y, Guo C, Liao H, Liu C, Zhu Y, Wang W, Liu Z, Xu Y, Li K, Li C, Li Q, He J, Chen W, Zhang X, Kou Y, Wang Y, Wu Z, Che G, Chen L, Liu L. Perioperative ctDNA-based molecular residual disease detection for non-small cell lung cancer: a prospective multicenter cohort study (LUNGCA-1) Clin Cancer Res. 2022;28:3308–3317. doi: 10.1158/1078-0432.CCR-21-3044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.