Abstract
Immune checkpoint inhibitor (ICI) has changed the situation of anti-tumor therapy. Several phase I/II clinical trials explored ICI-based combinations in microsatellite stable (MSS) metastatic colorectal cancer (mCRC) with mixed outcomes. However, real-world data regarding ICI-based combinations in this population is lacking. This retrospective study aimed to evaluate the efficacy and safety of ICI in MSS mCRC patients in third-line or above setting. A total of 143 eligible patients who received third-line or above ICI monotherapy or ICI-based combinations at the Cancer Center of Renmin Hospital of Wuhan University from June 2019 to April 2024 were included in this study. The primary endpoints were real-world median progression-free survival (PFS) and overall survival (OS), and the secondary endpoints included objective response rate (ORR), disease control rate (DCR), safety and prognostic analyses. Results showed that the median PFS was 4.6 months, and the median OS was 11.8 months, with an ORR of 11.2% and a DCR of 72.7%. ICI plus small molecule tyrosine kinase inhibitors have become the most popular combination for MSS mCRC patients at third-line or above setting with a median PFS of 4.4 months and OS of 10.1 months. The subgroup of patients with liver metastasis had worse clinical outcomes and liver metastasis was an independent prognostic factor for PFS (HR = 2.35, 95% CI, 1.54-3.59; P = 0.000) and OS (HR = 1.77, 95% CI, 1.06-2.96; P = 0.030). Forty-eight patients received cross-line ICI and obtained significantly improved OS (15.8 months vs 10.2 months; HR = 0.59, 95% CI, 0.38-0.89; P = 0.017). No new safety concerns were detected. Grade 3/4 treatment-related adverse events were generally controllable, with an incidence of 39.9%. To conclude, ICI-based combinations provide survival benefits for these heavily pretreated MSS mCRC patients with manageable safety, which is worthy of further study.
Keywords: Metastatic colorectal cancer, microsatellite stable, immune checkpoint inhibitor, efficacy, safety
Introduction
Colorectal cancer (CRC) is the third most common cancers globally and ranks in the top two leading cause of cancer-related deaths [1]. According to the 2020 GLOBOCAN report, CRC has become the highest total lifetime risk of developing and dying from gastrointestinal cancers [2]. China is a country with high incidence of CRC and heavy disease burden. Since 2000, the annual incidence and mortality of CRC in China have demonstrated a steady upward trend, with 517,000 new cases and 240,000 deaths in 2022 [3].
Yet, statistically, 25% of patients are diagnosed with metastatic CRC (mCRC) at their initial diagnosis, and almost 50% of patients with localized CRC will eventually develop mCRC. The 5-year overall survival (OS) rate for patients with mCRC is only about 12% [4]. Fluorouracil based doublet or triplet chemotherapy combined with anti-VEGF(R) or anti-EGFR targeted therapy is the standard first- and second-line treatment for mCRC patients recommended by current guidelines. In contrast, the options for third-line or above treatment for mCRC patients are limited. Only regorafenib, fruquintinib and TAS-102 are recommended, and the median progression-free survival (PFS) is only 1.9 to 5.6 months, which is far from meeting the clinical needs [5-8].
Recently, the widespread administration of immune checkpoint inhibitors (ICIs) has revolutionized clinical cancer therapy, dramatically improving the survival and prognosis to specific patient populations. Patients with mismatch repair-deficient/microsatellite instability-high (dMMR/MSI-H) mCRC were found to benefit significantly from ICIs, with prolonged PFS and long disease-free survival [9]. However, only 5% of mCRC patients were dMMR/MSI-H phenotype. The remaining 95% patients were mismatch repair-proficient/microsatellite-stable (pMMR/MSS) phenotype and generally had poor response to ICIs.
ICI-based combination therapies, such as ICI combined with targeted agents (regorafenib, fruquintinib, bevacizumab, etc.), chemotherapy, radiotherapy, biotherapy or other cytotoxic agents, have gained increasing attention in the later-line treatment of mCRC, and has achieved mixed results in a series of exploratory studies [10-15]. This study evaluated the efficacy and safety of ICI-based regimens in third-line and above treatment in MSS mCRC patients through a real-world retrospective study, with a view to providing more clinical evidence for theses heavily pretreated patients.
Methods
Patients and treatment
This study was a retrospective, real-world study conducted at the Renmin Hospital of Wuhan University, China. The study included the database of patients who were treated at the cancer center of Renmin Hospital of Wuhan University from June 2019 to April 2024. Briefly, eligible patients were aged ≥ 18 years, had histologically or cytologically confirmed metastatic and/or progressive pMMR/MSS colorectal adenocarcinoma, had received at least second-line of prior systemic therapy, had at least one measurable/evaluable lesion according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, had an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0 to 2, had adequate organ function, and had life expectancy of 3 months or longer. MSI status was tested using immunohistochemistry and/or polymerase chain reaction or next-generation sequencing. Patients were excluded if they had incomplete medical data, a history of active autoimmune disease or organ transplantation, or were receiving immunosuppressive therapy. The study was approved by the institutional ethics committee of Renmin Hospital of Wuhan University. Informed consent was exempt given the retrospective nature of the study. This study followed the Declaration of Helsinki and adhered to the Guidelines for Good Clinical Practice.
All data were retrospectively collected from medical records and laboratory results. Patients were prescribed ICI or ICI-based combinations in routine clinical practice. The specific administration schedule, dose modification, discontinuation and efficacy evaluation were determined by clinicians.
Outcome
The primary end points of the present study were PFS and OS, and the secondary end points were objective response rate (ORR), disease control rate (DCR), safety and prognostic analyses. PFS was defined as the time since third-line or above treatment initiation to disease progression or death from any cause. OS was measured from the third-line or above treatment initiation to death or the last follow-up.
All patients who received at least one dose of ICI were included in the efficacy and safety analyses. Tumor responses of target and non-target lesions were assessed according to the RECIST v1.1, as follows: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) by computed tomography, magnetic resonance imaging and/or other imageological examinations. Efficacy was evaluated every 2 to 3 treatment cycles. The ORR included CR and PR, and the DCR included CR, PR, and SD. The study was followed up until April 10, 2024.
Treatment-related adverse events (TRAEs) and immune-related adverse events (irAEs) were collected according to the Medical Dictionary for Regulatory Activities v23.0. The severity of any adverse event (AE) was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v5.0. Through the medical electronic system, the researchers collected the patients’ laboratory examinations and tests results, including blood routine, liver and kidney function and other biochemical indicators, urine routine, coagulation function, thyroid function, myocardial enzyme profile, etc., to record the AEs of patients in detail. In addition, data on the duration of medication were collected by telephone visits, outpatient visits or inpatient visits. IrAEs were followed up to 3 months after the end of patients’ current treatment.
Statistical analyses
Chi-square test or Fisher exact test were used to compare categorical variables. For continuous data, t test or Mann-Whitney U test were performed, depending on the normality of the data. Median follow-up was determined using the reverse Kaplan-Meier estimator. The PFS and OS were plotted using the Kaplan-Meier method. The log-rank test was used to compare Kaplan-Meier curves between subgroups, and Cox proportional hazards regression model was adopted to determine the hazard ratio (HR) and bilateral 95% confidence interval (CI). Demographic characteristics, safety, and other clinical data were summarized descriptively. All statistical analyses were performed using SPSS statistical software version 26.0. A P-value of < 0.05 was considered statistically significant.
Results
Patient characteristics
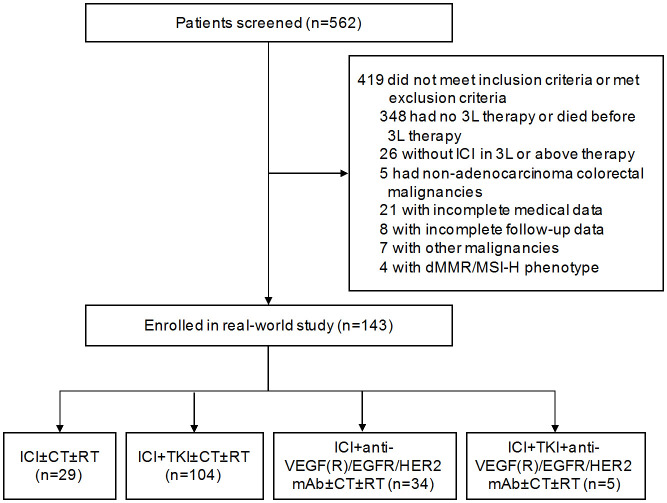
A total of 143 heavily pretreated patients with MSS mCRC from June 2019 to April 2024 at Renmin Hospital of Wuhan University were enrolled and analyzed with a median age of 59 years (IQR 51-67 years) (Figure 1). Of the entire cohort, 98 (68.5%) patients were male, 133 (93.0%) had an ECOG PS of 0-1, 110 (76.9%) had primary tumor located in left-side colon and rectum, 108 (75.5%) had more than 2 metastatic organs and 93 (65.0%) had liver metastasis. All patients received at least second-line of prior chemotherapy, 119 (83.2%) underwent surgical resection of the primary tumor, 65 (45.5%) had received radiotherapy, most patients (92.3%) had previously treated with targeted therapy. Overall, 20 (14.0%) patients previously received ICI at their first- or second-line therapy, and 88 (61.5%) patients underwent initiated ICI treatment at third-line setting. Genetic mutation data were available for 104 (72.7%) patients, of whom 56 (39.1%) were RAS/BRAF mutants. All patients were pMMR and/or MSS phenotypes. The baseline characteristics of patients are summarized in the Table 1.
Figure 1.
Flowchart depicting the patient selection process for the study. CT, chemotherapy; dMMR/MSI-H, mismatch repair-deficient/microsatellite instability-high; ICI, immune checkpoint inhibitor; RT, radiotherapy; TKI, tyrosine kinase inhibitor; 3L, third-line.
Table 1.
Patient demographics and baseline characteristics
| Characteristic | Patients, No. (%) |
|---|---|
| Age, median (IQR), y | 59 (51-67) |
| > 60 | 60 (42.0) |
| ≤ 60 | 83 (58.0) |
| Sex | |
| Male | 98 (68.5) |
| Female | 45 (31.5) |
| ECOG PS score | |
| 0-1 | 133 (93.0) |
| 2 | 10 (7.0) |
| Primary site | |
| Left-sided | 110 (76.9) |
| Right-sided | 33 (23.1) |
| Number of metastatic organs | |
| 1 | 35 (24.5) |
| ≥ 2 | 108 (75.5) |
| Type of metastasis | |
| With liver metastasis | 93 (65.0) |
| With lung metastasis | 86 (60.1) |
| RAS/BRAF gene status | |
| Available | 104 (72.7) |
| RAS/BRAF mutated type | 56 (39.1) |
| RAS/BRAF wild type | 48 (33.6) |
| Unavailable | 39 (27.3) |
| MMR/MSI status | |
| pMMR/MSS | 143 (100.0) |
| Prior treatment | |
| Median lines (range) | 2 (2-6) |
| Surgery of primary lesion | 119 (83.2) |
| Chemotherapy | 143 (100.0) |
| Radiotherapy | 65 (45.5) |
| Immunotherapy | 20 (14.0) |
| Targeted drugsa | 132 (92.3) |
| Anti-EGFR mAb | 28 (19.6) |
| Anti-VEGF(R) mAb | 113 (79.0) |
| TKIs | 50 (35.0) |
| Current line of ICI | |
| 3 line | 88 (61.5) |
| > 3 line | 55 (38.5) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; ICI, immune checkpoint inhibitor; IQR, interquartile range; mAb, monoclonal antibody; mCRC, metastatic colorectal cancer; MMR, mismatch repair; MSI, microsatellite instability; MSS, microsatellite stable; pMMR, mismatch repair proficient; TKIs, tyrosine kinase inhibitors; VEGF(R), vascular endothelial growth factor (receptor).
Targeted drugs included anti-EGFR mAb (Cetuximab and Nimotuzumab), anti-VEGF(R) mAb (Bevacizumab and Ramucirumab) and TKIs (small molecular inhibitors against VEGFR, eg., Regorafenib, Fruquintinib, Apatinib, Anlotinib, inhibitors against BRAF, eg., Dabrafenib, Vemurafenib, and inhibitors against MEK, eg., Trametinib).
Treatment regimens
Different ICI combination strategies are shown in Table 2. ICI combined with tyrosine kinase inhibitor (TKI) has become the most popular combination at third-line or above treatment, accounting for 72.7%. Among them, 79 (55.2%) patients chose the two-drug combination of ICI and TKI, and 27 (18.9%) received ICI plus TKI and chemotherapy. In addition, 20 (14.0%) received ICI plus large molecular targeted drugs such as bevacizumab or cetuximab and systematic chemotherapy, 19 (13.3%) received ICI and chemotherapy without any targeted agents, and 7 (4.9%) received ICI alone.
Table 2.
Treatment patterns
| Regimens | Patients, n (%) |
|---|---|
| ICI±CT±RT | 29 (19.6) |
| ICI alonea | 7 (4.9) |
| ICI+CT | 19 (13.3) |
| ICI+RT | 2 (1.4) |
| ICI+CT+RT | 4 (2.8) |
| ICI+TKI±CT±RT | 104 (72.7) |
| ICI+TKIb | 79 (55.2) |
| ICI+TKI+CT | 23 (16.1) |
| ICI+TKI+RT | 10 (7.0) |
| ICI+TKI+CT+RT | 4 (2.8) |
| ICI+anti-VEGF(R)/EGFR/HER2 mAb±CT±RT | 34 (23.8) |
| ICI+anti-VEGF(R)/EGFR/HER2 mAbc | 9 (6.3) |
| ICI+anti-VEGF(R)/EGFR/HER2 mAb+CT | 20 (14.0) |
| ICI+anti-VEGF(R)/EGFR/HER2 mAb+RT | 1 (0.7) |
| ICI+anti-VEGF(R)/EGFR/HER2 mAb+CT+RT | 7 (4.9) |
| ICI+TKI+anti-VEGF(R)/EGFR/HER2 mAb±CT±RT | 5 (3.5) |
| ICI+TKI+anti-VEGF(R)/EGFR/HER2 mAb | 3 (2.1) |
| ICI+TKI+anti-VEGF(R)/EGFR/HER2 mAb+CT | 1 (0.7) |
| ICI+TKI+anti-VEGF(R)/EGFR/HER2 mAb+RT | 1 (0.7) |
| ICI+TKI+anti-VEGF(R)/EGFR/HER2 mAb+CT+RT | 0 (0) |
Abbreviations: CT, chemotherapy; EGFR, epidermal growth factor receptor; ICI, immune checkpoint inhibitor; mAb, monoclonal antibody; RT, radiotherapy; TKI, tyrosine kinase inhibitor; VEGF(R), vascular endothelial growth factor (receptor); HER2, human epidermal growth factor receptor 2.
ICI: anti-PD-1 mAb (Pembrolizumab, Nivolumab, Camrelizumab, Toripalimab, Sintilimab, Tislelizumab, Penpulimab), anti-PD-L1 mAb (Atezolizumab, Durvalumab, Envafolimab, Adebrelimab) and anti-PD-1/CTLA4 mAb (Cadonilimab).
TKI: VEGFR inhibitor (Regorafenib, Fruquintinib, Sulfatinib, Apatinib, Anlotinib), BRAF inhibitor (Dabrafenib, Vemurafenib), MEK inhibitor (Trametinib), HER2 inhibitor (Pyrotinib, Lapatinib, Tucatinib), ALK inhibitor (Lorlatinib).
Anti-VEGF mAb (Bevacizumab); anti-VEGFR mAb (Ramucirumab); anti-EGFR mAb (Cetuximab, Nimotuzumab); anti-HER2 mAb (Trastuzumab, Pertuzumab); anti-HER2 ADC (Disitamab vedotin, Trastuzumab Emtansine).
Efficacy
As of April 10, 2024, only 56 of the 143 enrolled patients were still alive, of which 26 patients did not have PD. Another 117 patients developed PD, of which 30 were still alive, 14 continued to receive crossline immunotherapy, and 9 received non-immunotherapy. For 87 patients who had died, the main causes of death included disease progression and emergencies such as COVID-19 and acute cardiovascular and cerebrovascular events. In the intention to treat (ITT) population, the ORR was 11.2% (95% CI, 6.7-17.8) and the DCR was 72.7% (95% CI, 64.5-79.7), including 16 (11.2%) confirmed PR and 88 (61.5%) SD (Table 3). Specifically, the ORR was 12.5% (95% CI, 6.7-21.7) and DCR was 79.6% (95% CI, 69.4-87.1) in the third-line (3L) cohort, including 11 (12.5%) cases of PR and 59 (67.0%) cases of SD. As expected, the ORR and DCR in the fourth-line and above (≥ 4L) cohort were lower than those in the 3L cohort, but these differences were not statistically significant (P > 0.05). In the efficacy-evaluable population, a reduction in the size of target lesions was achieved in 58 (41.1%) patients (Figure 2).
Table 3.
Treatment efficacy
| Response | ITT (n = 143) | 3L (n = 88) | ≥ 4L (n = 55) |
|---|---|---|---|
| Best response to treatment, No. (%) | |||
| CR | 0 | 0 | 0 |
| PR | 16 (11.2) | 11 (12.5) | 5 (9.1) |
| SD | 88 (61.5) | 59 (67.0) | 29 (52.7) |
| PD | 37 (25.9) | 17 (19.3) | 20 (36.4) |
| NE | 2 (1.4) | 1 (1.1) | 1 (1.8) |
| ORR, % (95% CI) | 11.2 (6.7-17.8) | 12.5 (6.7-21.7) | 9.1 (3.4-20.7) |
| DCR, % (95% CI) | 72.7 (64.5-79.7) | 79.6 (69.4-87.1) | 61.8 (47.7-74.3) |
Abbreviations: CI, Confidence interval; CR, Complete response; CT, chemotherapy; DCR, Disease control rate; ITT, Intention to treat population; NE, Not evaluable; ORR, Objective response rate; PD, Progressive disease; PR, Partial response; SD, Stable disease; 3L, third-line therapy; ≥ 4L, fourth-line and above therapy.
Figure 2.
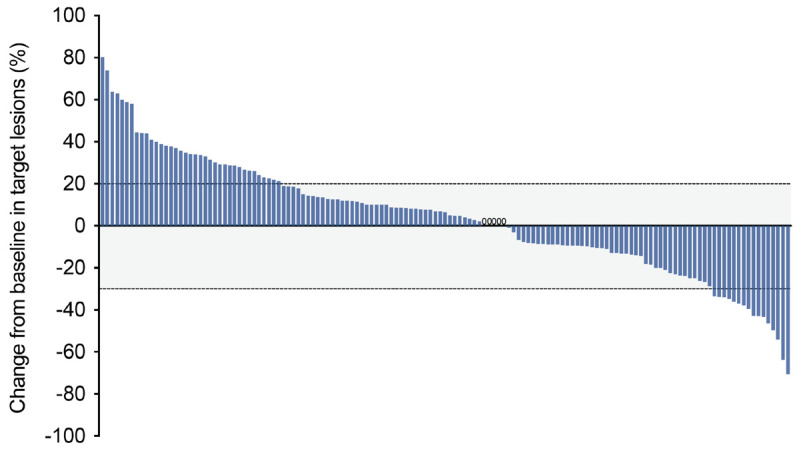
Tumor response in the efficacy-evaluable population (n = 141). Waterfall plot showing the best percent change in the size of target lesions from baseline. The dashed lines at +20% and -30% indicate thresholds for progressive disease and partial response, respectively, according to RECIST v1.1.
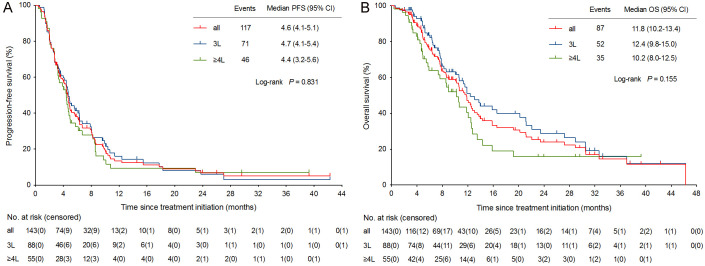
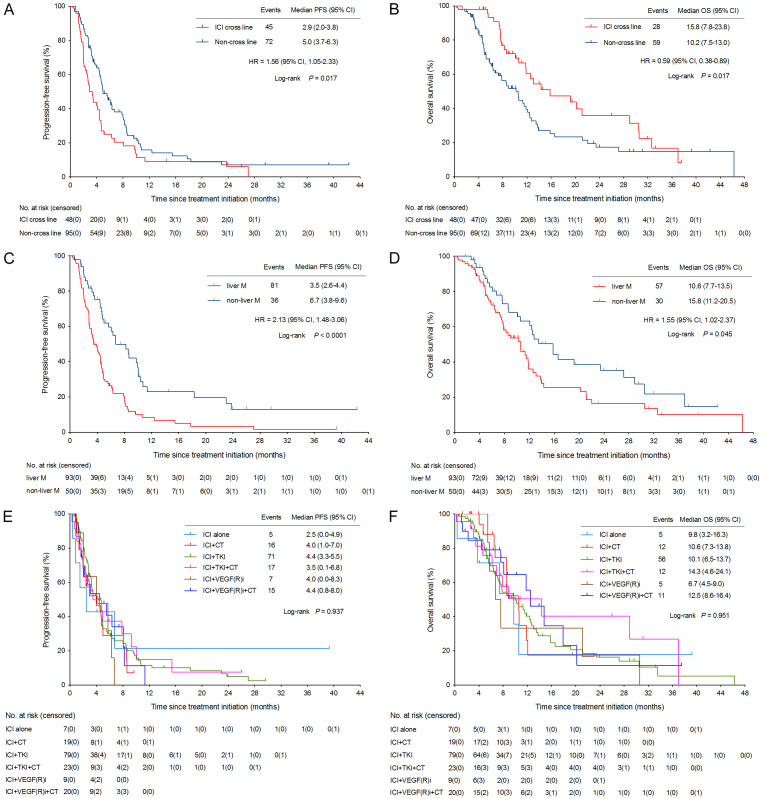
As of data cutoff on April 10, 2024, with a median follow-up of 23.1 months (95% CI, 11.8-34.4), the median PFS was 4.6 months (95% CI, 4.1-5.1) and median OS was 11.8 months (95% CI, 10.2-13.4) (Figure 3A, 3B). Median PFS (4.7 months vs 4.4 months; P = 0.562) and median OS (12.4 months vs 10.2 months; P = 0.054) in the 3L cohort were better than those in the ≥ 4L cohort, but significant differences were not found (Figure 3A, 3B). Interestingly, however, we observed a significant improvement in median OS in 48 patients who underwent ICI cross-line therapy compared to those who did not receive ICI cross-line therapy (15.8 months vs 10.2 months; HR = 0.59, 95% CI, 0.38-0.89; P = 0.017) (Figure 4B). But, the OS benefit here was independent of PFS, and patients in the cross-line cohort had relatively short PFS (Figure 4A).
Figure 3.
Kaplan-Meier curves of PFS and OS. CI, confidence interval; OS, overall survival; PFS, progression-free survival; 3L, third-line (therapy); ≥ 4L, fourth-line and above (therapy).
Figure 4.
Kaplan-Meier curves of PFS and OS in subgroup analysis. CI, confidence interval; HR, hazard ratio; CT, chemotherapy; ICI, immune checkpoint inhibitor; OS, overall survival; PFS, progression-free survival; TKI, tyrosine kinase inhibitor; VEGF(R)i, vascular endothelial growth factor (receptor) inhibitor.
The univariate and multivariate analyses of risk factors for PFS and OS are shown in Table 4. Patients with liver metastasis had worse median PFS (3.5 months vs 6.7 months; HR = 2.13, 95% CI, 1.48-3.06; P < 0.0001) and OS (10.6 months vs 15.8 months; HR = 1.55, 95% CI, 1.02-2.37; P = 0.045) than those without liver metastasis (Figure 4C, 4D). Multivariate analysis indicated that liver metastasis was an independent prognostic factors for PFS (HR = 2.35, 95% CI, 1.54-3.59; P = 0.000) and OS (HR = 1.77, 95% CI, 1.06-2.96; P = 0.030) in mCRC patients treated with later-line immunotherapy (Table 4).
Table 4.
Univariate and multivariate analyses of risk factors for PFS and OS
| PFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
|
|
|
|
|
|||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age (years) (>/≤ 60) | 0.68 (0.47-0.98) | 0.038 | 0.68 (0.46-1.01) | 0.056 | 1.04 (0.68-1.61) | 0.842 | ||
| Sex (male/female) | 0.62 (0.41-0.95) | 0.013 | 0.73 (0.47-1.13) | 0.157 | 0.76 (0.48-1.20) | 0.208 | ||
| ECOG PS (0-1/2) | 0.57 (0.25-1.31) | 0.086 | 0.77 (0.39-1.51) | 0.450 | 0.36 (0.13-0.99) | 0.001 | 0.41 (0.20-0.83) | 0.013 |
| Primary site (left/right) | 0.84 (0.53-1.31) | 0.404 | 0.65 (0.38-1.11) | 0.071 | 0.65 (0.39-1.10) | 0.111 | ||
| No. of metastatic organs (≥ 2/1) | 1.96 (1.33-2.89) | 0.003 | 1.96 (1.21-3.17) | 0.006 | 1.84 (1.15-2.94) | 0.026 | 1.52 (0.81-2.84) | 0.191 |
| Liver metastasis (yes/no) | 2.13 (1.48-3.06) | < 0.0001 | 2.35 (1.54-3.59) | 0.000 | 1.55 (1.02-2.37) | 0.045 | 1.77 (1.06-2.96) | 0.030 |
| Lung metastasis (yes/no) | 0.98 (0.67-1.42) | 0.900 | 0.80 (0.52-1.25) | 0.310 | ||||
| RAS/BRAF (MT/WT) | 1.51 (0.98-2.33) | 0.052 | 1.57 (0.98-2.50) | 0.061 | 1.57 (0.95-2.59) | 0.071 | 2.22 (1.26-3.94) | 0.006 |
| Prior surgery (yes/no) | 0.74 (0.44-1.24) | 0.195 | 0.55 (0.28-1.07) | 0.024 | 0.49 (0.27-0.89) | 0.019 | ||
| Prior radiotherapy (yes/no) | 0.80 (0.56-1.16) | 0.238 | 0.70 (0.46-1.06) | 0.090 | 0.68 (0.42-1.10) | 0.116 | ||
| Prior immunotherapy (yes/no) | 1.44 (0.80-2.60) | 0.156 | 0.70 (0.38-1.28) | 0.307 | ||||
| Prior EGFRi (yes/no) | 0.95 (0.60-1.50) | 0.824 | 1.03 (0.60-1.75) | 0.916 | ||||
| Prior VEGF(R)i (yes/no) | 1.55 (0.96-2.50) | 0.116 | 1.68 (1.01-2.81) | 0.076 | 1.86 (0.92-3.75) | 0.084 | ||
| Current line of ICI (3L/> 3L) | 0.90 (0.62-1.31) | 0.562 | 0.66 (0.42-1.04) | 0.054 | 0.61 (0.37-0.99) | 0.047 | ||
| ICI crossline (yes/no) | 1.56 (1.05-2.33) | 0.017 | 1.48 (1.00-2.20) | 0.051 | 0.59 (0.38-0.89) | 0.017 | 0.45 (0.27-0.75) | 0.002 |
The bold font represents statistical significance at P < 0.05. Abbreviations: 3L, third-line; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFRi, epidermal growth factor receptor inhibitor; HR, hazard ratio; ICI, immune checkpoint inhibitor; MT, mutated type; OS, overall survival; PFS, progression-free survival; VEGF(R)i, vascular endothelial growth factor (receptor) inhibitor; WT, wild type.
Patients who received ICI alone had the shortest median PFS of 2.5 months (95% CI, 0.0-4.9), while the combinations of ICI plus TKI and ICI plus anti-VEGF(R)/EGFR/HER2 mAb and chemotherapy both developed a median PFS of 4.4 months (Figure 4E). The median OS for patients received ICI plus TKI and chemotherapy (14.3 months; 95% CI, 4.6-24.1) was highest compared with those who received ICI plus chemotherapy (10.6 months; 95% CI, 7.3-13.8) or those who received ICI in combination with anti-VEGF(R)/EGFR/HER2 mAb and chemotherapy (12.5 months; 95% CI, 8.6-16.4), of course, not to mention ICI alone (9.8 months; 95% CI, 3.2-16.3) (Figure 4F). The survival curves for each treatment regimen (up to 48 months) are presented in Figure 4E, 4F.
Safety
All patients received at least one dose of ICI were included in the safety analysis. Most patients (93.9%) experienced at least one TRAE, but it was generally grade 1 or 2 (Table 5). The most common TRAEs were leukopenia (33.6%), hand and foot skin reaction (29.4%), thrombocytopenia (28.7%), and abnormal liver function (27.3%). Grade 3-4 TRAEs were reported in 57 (39.9%) patients, mainly including thrombocytopenia (9.8%), leukopenia (7.7%), neutropenia (6.3%), thyroid dysfunction (4.2%), and diarrhea (4.2%).
Table 5.
Safety
| Adverse events, No. (%) | Any | Grade ≥ 3 |
|---|---|---|
| All AEs | 134 (93.7) | 57 (39.9) |
| irAEs | 60 (42.0) | 19 (13.3) |
| Leukopenia | 48 (33.6) | 11 (7.7) |
| Neutropenia | 28 (19.6) | 9 (6.3) |
| Thrombocytopenia | 41 (28.7) | 14 (9.8) |
| Anemia | 33 (23.1) | 4 (2.8) |
| Nausea and/or vomiting | 26 (18.2) | 0 |
| Diarrhea | 23 (16.1) | 6 (4.2) |
| Abnormal liver test results | 39 (27.3) | 5 (3.5) |
| Ileus | 12 (8.4) | 3 (2.1) |
| Thromboembolic event | 5 (3.5) | 2 (1.4) |
| Neurotoxicity | 13 (9.1) | 0 |
| Proteinuria | 22 (15.4) | 5 (3.5) |
| Urine occult blood | 25 (17.5) | 3 (2.1) |
| Hypoalbuminemia | 24 (16.8) | 2 (1.4) |
| Hypertension | 27 (18.9) | 2 (1.4) |
| Hyperglycemia | 13 (9.1) | 3 (2.1) |
| Mucositis | 21 (14.7) | 1 (0.7) |
| Hand-foot skin reaction | 42 (29.4) | 5 (3.5) |
| RCCEP | 10 (7.0) | 3 (2.1) |
| Rash | 18 (12.6) | 1 (0.7) |
| Myasthenia gravis | 2 (1.4) | 0 |
| Immune myositis | 7 (4.9) | 0 |
| Abnormal cardiac function | 14 (9.8) | 2 (1.4) |
| Thyroid dysfunction | 37 (25.9) | 6 (4.2) |
| Pneumonitis | 20 (14.0) | 5 (3.5) |
Abbreviations: AEs, adverse events; irAEs, immune-related adverse events; RCCEP, reactive cutaneous capillary endothelial proliferation.
The incidence of irAEs of any grade was 42.0%. Most of the irAEs were graded 1-2. The incidence of grade 3-4 irAEs was 13.3%, mainly including rash, reactive cutaneous capillary endothelial proliferation, hypothyroidism, hypertension, hyperglycemia and abnormal liver function. All irAEs were controllable after appropriate symptomatic therapy. No treatment-related deaths occurred.
Discussion
For mCRC, compared with chemotherapy alone, the introduction of targeted agents has brought significant survival benefits to these population and rewritten clinical treatment guidelines. Recently, with the in-depth exploration of molecular biology, the impact of tumor molecular typing and tumor microenvironment (TME) on the survival and prognosis of cancer patients has attracted more and more attention. CRC is no longer regarded as a single unique disease, but presents complex molecular subtypes, each with distinct genetic and epigenetic features. Chromosomal instability, CpG island methylator phenotype, and MSI are the three main pathways of genomic instability that contribute to the development of CRC [16]. Among them, MSI is currently the biomarker commonly used to judge the immunotherapeutic response in mCRC patients.
The phase II KEYNOTE-016 trial showed that compared with 40% ORR in MSI-H mCRC patients, MSS mCRC patients had little response to immune-monotherapy, with an ORR of 0% and a median PFS and OS of only 2.2 months and 5.0 months, respectively [17]. This high response to ICI observed in dMMR/MSI-H mCRC patients has been reported to associated with the hypermutated phenotype resulting from a large number of DNA replication errors due to the impairment of any DNA mismatch repair proteins, including MLH1, PMS2, MSH2, and MSH6 [18]. Obviously, in addition to the high tumor mutational burden, the immune inflamed TME, including tumor infiltrating lymphocytes, immune memory cells and cytotoxic T lymphocytes, is also an important factor affecting immune response [19].
However, more than 95% of mCRC patients were MSS phenotype, with low neoantigen load in an immune-excluded or immune-desert TME. Novel combination strategies and screening of right populations have become the direction of exploration in the later-line treatment of mCRC. Increasing number of ICI-based approaches with chemotherapy, radiotherapy, targeted agents or vaccines under the rationale of overcoming immune resistance, prompting immune cycle, transforming the cold tumor into an immune-activated TME have been developed. In the REGONIVO trial, regorafenib combined with nivolumab reported an ORR of 33% and a median PFS of 7.9 month in 25 MSS mCRC patients after failure of standard treatment [10]. The LEAP-005 trial showed an ORR of 22% and a median PFS of 2.3 months of pembrolizumab plus lenvatinib in MSS mCRC patients [20]. Regorafenib combined with avelumab in the phase II REGOMUNE trial showed an ORR of 0%, with a median PFS of 3.6 months [11]. Tislelizumab plus fruquintinib and stereotatic body radiotherapy as a later-line therapy in MSS mCRC exhibited an ORR of 26% and median PFS of 8.5 months in the FRUIT trial [13]. The phase II RENMIN-215 trial conducted by our research group explored the efficacy of ICI combined with fruquintinib and fecal microbiota transplantation in the later-line treatment of MSS mCRC, achieving an ORR of 20% and a median PFS of 9.6 months [14]. Different from immuno-monotherapy, the ICI plus TKI model seems to have better clinical outcomes than the guideline-recommended third-line targeted agents for mCRC.
Based on this, this study reviewed 143 mCRC patients who received third-line or above immunotherapy in our cancer center in the past 5 years. With a median follow-up of 23.1 months, the median PFS was 4.6 months and median OS was 11.8 months, with an ORR of 12.5% and DCR of 79.6%. Our preliminary results showed that ICI combined with TKI is the combination strategy with the highest proportion in the later-line setting for mCRC in the real world. Patients who received ICI plus TKI obtained a median PFS of 4.4 months and median OS of 10.1 months. ICI in combination with TKI and chemotherapy, in particular, earned a median OS of 14.3 months for this population. Compared with ICI or TKI alone, the ICI+TKI±CT mode achieved a relatively longer survival in mCRC patients without compromising patients’ quality of life. Judging from the pre-clinical data, the treatment mode of ICI+TKI is also fully scientific. Anti-angiogenic agent transformed the immunosuppressive TME into immunosupportive one and enhanced anti-tumor immunity by normalizing the vascular system, increasing tumor perfusion and oxygenation, promoting chemokine release and effector T cells infiltration, reducing maturation and accumulation of myeloid suppressor cells [21]. On the other, the positive feedback mechanism between ICI-induced immune reprogramming and TKI-mediated tumor vascular normalization further promoted immune-mediated tumor clearance [22].
In addition, because ICI works slowly and has a long after-effect, it is clinically common for cancer patients to receive ICI rechallenge after interruption or discontinuation of immunotherapy or disease progression, that is, cross-line immunotherapy. In this study, 48 (33.6%) patients received cross-line immunotherapy. After comparison, we observed an improvement of 5.6 months in median OS with a 41% lower risk of death (HR = 0.59, 95% CI, 0.38-0.89; P = 0.017). This real-world clinical outcome may provide reliable data support for further clinical studies of immunotherapy rechallenge in patients with MSS CRC.
Previous studies have shown that CRC patients with liver metastasis often have poor immune response and survival prognosis [23]. Similar results were observed in this study. The 93 patients with liver metastases had worse median PFS (3.5 months vs 6.7 months; P < 0.0001) and OS (10.6 months vs 15.8 months; P = 0.045) than the 50 patients without liver metastases. Liver metastasis of CRC is the most significant cohort for mCRC. However, the TME of liver metastases appears to be immunosuppressive due to complex internal factors. Yu et al. found in the mouse model that liver metastases can recruit and siphon CD8+ T lymphocytes activated in the peripheral circulation and drive the apoptosis of antigen-specific Fas+CD8+ T cells in hepatocytes through interaction with FasL+CD11b+F4/80+ monocyte-derived macrophages, thus forming an immune-desert TME [24]. In addition to the decrease in the number of T cells in peripheral blood, the diversity and function of T cells in the liver metastases of mCRC patients are correspondingly reduced. Therefore, the rationale combination of liver metastases-directed radiotherapy, antiangiogenic agents or other cytotoxic drugs and ICI may be a worthwhile method to overcome immunotherapy resistance and stimulate effective immune response. On the other hand, more detailed molecular and genetic analyses will be included in future to reveal the molecular mechanisms of immunotherapy resistance in mCRC patients with liver metastasis.
In terms of safety, this study showed that the AEs of ICI-based therapy were consistent with the those of ICIs, antiangiogenic agents or chemotherapy. No new safety signals were reported. Most of the TRAEs were classified as grade 1-2, and the incidence of all grade 3-4 AEs was 39.9%. Patient survival benefits did not come at the expense of safety, and the TRAEs were manageable after symptomatic treatment.
The study has several limitations. Firstly, it was a single-center, retrospective study, which might introduce selection bias. Secondly, the limited sample size reduces the statistical power, especially in subgroup analysis. Thirdly, real-world findings may be influenced by routine clinical practice, such as comorbidities and so on. Finally, the PD-L1 combined positive score (CPS) and tumor mutation burden (TMB) of most patients in this study were unknown, so the two indicators could not be used to determine the right population for ICI treatment. Therefore, a multicenter, prospective and randomized controlled trial with a large sample should be performed to validate the current results with higher level of medical evidence.
In conclusion, ICI-based combination therapy, especially ICI plus TKI with or without chemotherapy showed a clinically meaningful survival improvement with a manageable safety profile for heavily pretreated patients with MSS mCRC.
Acknowledgements
This work was funded by National Natural Science Foundation of China (No. 82102954). We thank all patients and their families, and the investigators and staff involved with the study.
Disclosure of conflict of interest
None.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Wang S, Zheng R, Li J, Zeng H, Li L, Chen R, Sun K, Han B, Bray F, Wei W, He J. Global, regional, and national lifetime risks of developing and dying from gastrointestinal cancers in 185 countries: a population-based systematic analysis of GLOBOCAN. Lancet Gastroenterol Hepatol. 2024;9:229–237. doi: 10.1016/S2468-1253(23)00366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. 2024;4:47–53. doi: 10.1016/j.jncc.2024.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145–164. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 5.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouche O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 6.Dasari A, Lonardi S, Garcia-Carbonero R, Elez E, Yoshino T, Sobrero A, Yao J, Garcia-Alfonso P, Kocsis J, Cubillo Gracian A, Sartore-Bianchi A, Satoh T, Randrian V, Tomasek J, Chong G, Paulson AS, Masuishi T, Jones J, Csoszi T, Cremolini C, Ghiringhelli F, Shergill A, Hochster HS, Krauss J, Bassam A, Ducreux M, Elme A, Faugeras L, Kasper S, Van Cutsem E, Arnold D, Nanda S, Yang Z, Schelman WR, Kania M, Tabernero J, Eng C FRESCO-2 Study Investigators. Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO-2): an international, multicentre, randomised, double-blind, phase 3 study. Lancet. 2023;402:41–53. doi: 10.1016/S0140-6736(23)00772-9. [DOI] [PubMed] [Google Scholar]
- 7.Xu J, Kim TW, Shen L, Sriuranpong V, Pan H, Xu R, Guo W, Han SW, Liu T, Park YS, Shi C, Bai Y, Bi F, Ahn JB, Qin S, Li Q, Wu C, Ma D, Lin D, Li J. Results of a randomized, double-blind, placebo-controlled, phase III trial of trifluridine/tipiracil (TAS-102) monotherapy in asian patients with previously treated metastatic colorectal cancer: the TERRA study. J. Clin. Oncol. 2018;36:350–358. doi: 10.1200/JCO.2017.74.3245. [DOI] [PubMed] [Google Scholar]
- 8.Tabernero J, Prager GW, Fakih M, Ciardiello F, Van Cutsem E, Elez E, Cruz FM, Wyrwicz L, Stroyakovskiy D, Papai Z, Poureau PG, Liposits G, Cremolini C, Bondarenko I, Modest DP, Benhadji KA, Fougeray R, Leger C, Amellal N, Taieb J. Trifluridine/tipiracil plus bevacizumab for third-line treatment of refractory metastatic colorectal cancer: the phase 3 randomized SUNLIGHT study. J. Clin. Oncol. 2023;41:4. [Google Scholar]
- 9.Gryfe R, Kim H, Hsieh ET, Aronson MD, Holowaty EJ, Bull SB, Redston M, Gallinger S. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342:69–77. doi: 10.1056/NEJM200001133420201. [DOI] [PubMed] [Google Scholar]
- 10.Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, Hirano N, Wakabayashi M, Nomura S, Sato A, Kuwata T, Togashi Y, Nishikawa H, Shitara K. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603) J. Clin. Oncol. 2020;38:2053–2061. doi: 10.1200/JCO.19.03296. [DOI] [PubMed] [Google Scholar]
- 11.Cousin S, Cantarel C, Guegan JP, Gomez-Roca C, Metges JP, Adenis A, Pernot S, Bellera C, Kind M, Auzanneau C, Le Loarer F, Soubeyran I, Bessede A, Italiano A. Regorafenib-avelumab combination in patients with microsatellite stable colorectal cancer (REGOMUNE): a single-arm, open-label, phase II trial. Clin Cancer Res. 2021;27:2139–2147. doi: 10.1158/1078-0432.CCR-20-3416. [DOI] [PubMed] [Google Scholar]
- 12.Gou M, Yan H, E LT, Wang Z, Si H, Chen S, Pan Y, Fan R, Qian N, Dai G. Fruquintinib combination with sintilimab in refractory metastatic colorectal cancer patients in China. J. Clin. Oncol. 2020;38:4028. [Google Scholar]
- 13.Yuan X, Zhang M, Hou H. Fruquintinib combined with tislelizumab and SBRT as a later-line therapy in microsatellite stability (MSS) metastatic colorectal cancer (mCRC): results from the fruit trial. J. Clin. Oncol. 2023;41:150. [Google Scholar]
- 14.Zhao W, Lei J, Ke S, Chen Y, Xiao J, Tang Z, Wang L, Ren Y, Alnaggar M, Qiu H, Shi W, Yin L, Chen Y. Fecal microbiota transplantation plus tislelizumab and fruquintinib in refractory microsatellite stable metastatic colorectal cancer: an open-label, single-arm, phase II trial (RENMIN-215) EClinicalMedicine. 2023;66:102315. doi: 10.1016/j.eclinm.2023.102315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang F, Jin Y, Wang M, Luo HY, Fang WJ, Wang YN, Chen YX, Huang RJ, Guan WL, Li JB, Li YH, Wang FH, Hu XH, Zhang YQ, Qiu MZ, Liu LL, Wang ZX, Ren C, Wang DS, Zhang DS, Wang ZQ, Liao WT, Tian L, Zhao Q, Xu RH. Combined anti-PD-1, HDAC inhibitor and anti-VEGF for MSS/pMMR colorectal cancer: a randomized phase 2 trial. Nat Med. 2024;30:1035–1043. doi: 10.1038/s41591-024-02813-1. [DOI] [PubMed] [Google Scholar]
- 16.Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res. 2012;5:19–27. [PMC free article] [PubMed] [Google Scholar]
- 17.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li GM. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008;18:85–98. doi: 10.1038/cr.2007.115. [DOI] [PubMed] [Google Scholar]
- 19.Phillips SM, Banerjea A, Feakins R, Li SR, Bustin SA, Dorudi S. Tumour-infiltrating lymphocytes in colorectal cancer with microsatellite instability are activated and cytotoxic. Br J Surg. 2004;91:469–475. doi: 10.1002/bjs.4472. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Roca C, Yanez E, Im SA, Alvarez EC, Senellart H, Doherty M, García-Corbacho J, Lopez JS, Basu B, Maurice-Dror C, Gill SS, Ghori R, Kubiak P, Jin F, Norwood KG, Chung HC. LEAP-005: a phase II multicohort study of lenvatinib plus pembrolizumab in patients with previously treated selected solid tumors-results from the colorectal cancer cohort. J. Clin. Oncol. 2021;39:94. [Google Scholar]
- 21.Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73:2943–2948. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan KA, Kerbel RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol. 2018;15:310–324. doi: 10.1038/nrclinonc.2018.9. [DOI] [PubMed] [Google Scholar]
- 23.Wang C, Sandhu J, Ouyang C, Ye J, Lee PP, Fakih M. Clinical response to immunotherapy targeting programmed cell death receptor 1/programmed cell death ligand 1 in patients with treatment-resistant microsatellite stable colorectal cancer with and without liver metastases. JAMA Netw Open. 2021;4:e2118416. doi: 10.1001/jamanetworkopen.2021.18416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, Rizvi SM, Qin A, Waninger JJ, Lang X, Chopra Z, El Naqa I, Zhou J, Bian Y, Jiang L, Tezel A, Skvarce J, Achar RK, Sitto M, Rosen BS, Su F, Narayanan SP, Cao X, Wei S, Szeliga W, Vatan L, Mayo C, Morgan MA, Schonewolf CA, Cuneo K, Kryczek I, Ma VT, Lao CD, Lawrence TS, Ramnath N, Wen F, Chinnaiyan AM, Cieslik M, Alva A, Zou W. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27:152–164. doi: 10.1038/s41591-020-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]