Abstract
Objective: To identify key risk factors for postoperative pulmonary infections (PPIs) in lung cancer (LC), patients undergoing radical surgery and construct a multiparametric nomogram model to improve PPI risk prediction accuracy, guiding individualized interventions. Methods: A retrospective analysis was conducted on LC patients treated at Yidu Central Hospital of Weifang from March 2020 to May 2023. Among the 1,084 LC cases reviewed, patients were divided into an infected group (n = 131) and an uninfected group (n = 953) based on infection status. Key factors for PPIs were screened using machine learning techniques, including least absolute shrinkage and selection operator (LASSO) regression, Support Vector Machine (SVM), and Extreme Gradient Boosting (XGBoost). A nomogram prediction model was developed, and its stability and clinical utility were evaluated using calibration curves and decision curve analysis, with internal validation through random case selection. Results: Thirteen factors - including tumor stage, diabetes history, chronic obstructive pulmonary disease (COPD), operation duration, mechanical ventilation duration, age, C-reactive protein, procalcitonin, high-mobility group box 1, interleukin-6, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and systemic immune-inflammation index - were identified as significantly associated with PPIs. The nomogram model demonstrated high predictive accuracy in internal validation (C-index = 0.935), strong calibration, and substantial clinical benefit. For two randomly selected cases, the model predicted a 63% infection probability for the infected patient and a 32% probability for the uninfected patient, affirming the model’s predictive effectiveness. Conclusions: The multiparametric nomogram model developed in this study provides a reliable method for PPI risk prediction in LC patients, supporting clinical decision-making and improving postoperative management.
Keywords: Lung cancer, postoperative pulmonary infection, nomogram model, machine learning
Introduction
Worldwide, lung cancer (LC) ranks among the malignancies with the highest morbidity and mortality rates [1]. In China, LC incidence is also rising, particularly among male cancer patients, where it is the most common malignancy. LC is increasingly prevalent among female patients as well [2,3]. Characterized by high morbidity and mortality, LC has become a major malignant tumor threatening human health and life [4]. LC’s causes are multifactorial; aside from genetic predisposition, smoking is the primary risk factor. Tobacco-derived nitrosamines damage bronchial epithelial cells and activate proto-oncogenes, transforming normal lung cells into cancerous cells [5,6].
Current treatments for LC include surgery, radiotherapy, chemotherapy, immunotherapy, and traditional Chinese medicine therapies [7,8]. Surgical resection remains one of the most effective treatments for early- to mid-stage LC, with common procedures including wedge resection, segmentectomy, lobectomy, and pneumonectomy. Of these, lobectomy with comprehensive lymph node dissection is the standard radical surgery for LC [9]. Surgical approaches vary based on tumor characteristics and patient tolerance, encompassing extended and local resections. However, despite radical resection, the five-year survival rate post-surgery remains low [10]. Postoperative LC patients frequently experience symptoms such as respiratory restriction, weak cough, and difficulty expectorating due to significant surgical trauma, stimulation to the heart and lungs, and altered chest cavity dynamics [11]. Furthermore, LC patients, often older with compromised immunity and respiratory function, are highly susceptible to infection, making postoperative pulmonary infections (PPIs) a leading cause of mortality [12]. PPIs not only delay recovery and increase hospitalization costs but can also lead to respiratory failure in severe cases [13]. Therefore, identifying risk factors for PPIs after LC resection is essential to reducing their incidence and supporting targeted prevention and treatment.
In current clinical practice, predicting postoperative infections in LC remains challenging. This study aims to improve PPI risk prediction by developing a multiparametric nomogram model that incorporates inflammatory markers, biochemical indices, and clinical parameters. Using machine learning techniques for feature selection and model optimization, this model enhances predictive accuracy. Additionally, the visualized nomogram provides clinicians with intuitive, actionable decision support, facilitating personalized interventions, reducing postoperative infection rates, and enhancing postoperative quality of life in LC patients. This model demonstrates the potential of integrating biomarkers and machine learning in LC management and provides a scientific basis and methodology for future postoperative care in LC.
Materials and methods
Case acquisition
Patient data from LC cases treated at Yidu Central Hospital of Weifang from March 2020 to May 2023 were collected retrospectively. Inclusion criteria: (1) LC diagnosis confirmed by chest computed tomography, bronchoscopy, cytology, or histopathology; (2) Absence of preoperative pulmonary infections; (3) Newly diagnosed non-small cell LC, with TNM stage below IIIb [14].
Exclusion criteria: (1) Preoperative pulmonary infection or other infections; (2) Preoperative anti-infection treatments; (3) Incomplete clinical records, referrals, or cases with patient death. Given the study’s retrospective design, informed consent was waived with the approval of the Ethics Committee of Yidu Central Hospital of Weifang.
Definition of PPIs
A diagnosis of postoperative pulmonary infection (PPI) requires meeting four out of the following six criteria within one month after surgery: (1) Leukocyte count >15×10^9/L; (2) Body temperature >38°C; (3) Cough and expectoration; (4) Pulmonary rales on auscultation; (5) Infiltrative lung lesions on chest imaging; (6) Positive bacterial culture from deep sputum samples [15].
Case grouping
Of 1,571 cases initially screened based on the inclusion criteria, 487 were excluded based on the exclusion criteria, resulting in 1,084 cases eligible for the study. These patients were grouped by PPI status into an infected group (n = 131) and an uninfected group (n = 953).
Clinical data acquisition
Using outpatient review and electronic medical records, patient information was collected, including age, sex, body mass index (BMI), smoking history, affected lung side, pathological classification, tumor stage, diabetes history, hypertension history, chronic obstructive pulmonary disease (COPD), operation duration, mechanical ventilation time, C-reactive protein (CRP), procalcitonin (PCT), high-mobility group protein B1 (HMGB1), interleukin-6 (IL-6), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), and systemic immune-inflammation index (SII).
Machine learning models
Least absolute shrinkage and selection operator (LASSO) regression is a penalized regression method that forces some coefficients to zero, making it highly effective in cases where the number of predictors exceeds the sample size, reducing variables and improving model performance [16].
Support Vector Machine (SVM) is a boundary-based supervised learning algorithm effective for classification and regression. It constructs hyperplanes in high-dimensional space to maximize the margin between classes, making it ideal for binary classification tasks [17].
Extreme Gradient Boosting (XGBoost) is a decision-tree-based ensemble algorithm that improves model performance by optimizing the loss function through gradient boosting and introduces regularization to enhance generalization across datasets [18].
Outcome measures
(1) Identification of differences in baseline data between infected and uninfected groups. (2) Screening of significant PPI factors using LASSO regression, SVM, and XGBoost. (3) Development of a nomogram model based on identified factors, with stability and clinical utility assessed through calibration curves and decision curve analysis (DCA). (4) Evaluation of the model’s predictive accuracy in PPI risk using two randomly selected cases.
Statistical analysis
Data preprocessing and initial analysis were conducted using SPSS 26.0. Categorical data were expressed as percentages (%) and analyzed with chi-square tests. For continuous data, the Kolmogorov-Smirnov test assessed data distribution. Normally distributed measurement data were represented as Mean ± SD and analyzed with independent t-tests; non-normally distributed data were presented as median (P25, P75). Further analysis was performed using R (version 4.3.3), employing packages including rms (version 6.4.0), ResourceSelection (version 0.3-5), openxlsx, Matrix, XGBoost, rpart, data.table, ggplot2, kernlab, and pROC. Statistical significance was defined as P<0.05 for all analyses.
Results
Comparison of baseline data
Baseline comparisons showed statistically significant differences in tumor staging, diabetes history, COPD, operation time, duration of mechanical ventilation, age, CRP, PCT, HMGB1, IL-6, NLR, PLR, and SII between infected and uninfected groups (all P<0.05, Table 1). No significant differences were observed in other clinical data (all P>0.05).
Table 1.
Classification of baseline data
| Variables | Infected group (n = 131) | Uninfected group (n = 953) | χ2/t | P |
|---|---|---|---|---|
| Sex | ||||
| Male | 96 | 677 | 0.283 | 0.595 |
| Female | 35 | 276 | ||
| Smoking history | ||||
| With | 76 | 496 | 1.646 | 0.199 |
| Without | 55 | 457 | ||
| Affected side | ||||
| Left | 51 | 353 | 0.233 | 0.89 |
| Right | 67 | 496 | ||
| Bilateral | 13 | 104 | ||
| Pathological classification | ||||
| Adenocarcinoma | 103 | 772 | 0.42 | 0.517 |
| Squamous cell carcinoma | 28 | 181 | ||
| Tumor staging | ||||
| I+II | 46 | 486 | 11.624 | <0.001 |
| III | 85 | 467 | ||
| History of diabetes | ||||
| With | 31 | 133 | 8.454 | 0.004 |
| Without | 100 | 820 | ||
| History of hypertension | ||||
| With | 26 | 162 | 0.652 | 0.419 |
| Without | 105 | 791 | ||
| COPD | ||||
| With | 54 | 267 | 9.634 | 0.002 |
| Without | 77 | 686 | ||
| Operation time | ||||
| ≥3 h | 85 | 457 | 13.207 | <0.001 |
| <3 h | 46 | 496 | ||
| Duration of mechanical ventilation | ||||
| ≥6 h | 79 | 429 | 10.812 | 0.001 |
| <6 h | 52 | 524 | ||
| Age (years old) | 64.37±8.26 | 58.76±8.06 | 7.305 | <0.001 |
| BMI (kg/m2) | 23.12±1.95 | 23.03±2.01 | 0.492 | 0.623 |
| CRP (mg/L) | 61.51±19.12 | 51.36±14.64 | 5.843 | <0.001 |
| PCT (ng/mL) | 2.71 [2.33, 3.27] | 1.69 [1.16, 2.23] | 11.831 | <0.001 |
| HMGB1 (pg/mL) | 484.08±120.63 | 396.64±84.90 | 8.027 | <0.001 |
| IL-6 (pg/mL) | 12.66 [9.00, 16.38] | 9.37 [6.26, 12.57] | 6.656 | <0.001 |
| NLR | 3.39 [2.29, 4.46] | 2.75 [1.69, 3.68] | 4.666 | <0.001 |
| PLR | 131.92±61.12 | 108.15±41.55 | 4.316 | <0.001 |
| LMR | 5.67±1.77 | 5.51±1.78 | 1.008 | 0.315 |
| SII | 509.11 [335.30, 694.25] | 438.23 [299.97, 576.66] | 3.581 | <0.001 |
Note: COPD, chronic obstructive pulmonary disease; BMI, body mass index; CRP, C-reactive Protein; PCT, procalcitonin; HMGB1, high mobility group box 1; IL-6, interleukin-6; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; SII, systemic immune-inflammation index.
Classification of measurement data
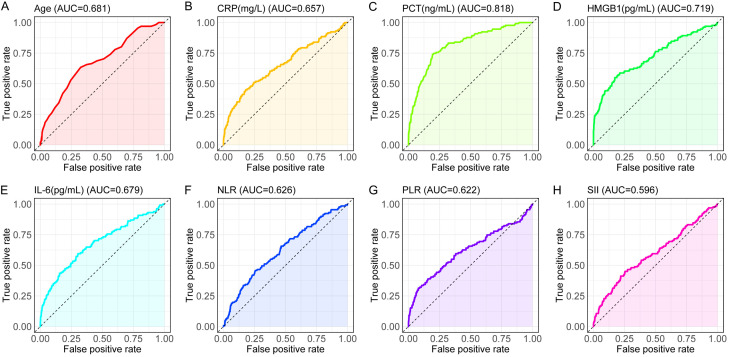
We employed logistic regression for analysis. Measurement data were adjusted to binary outcomes using receiver operating characteristic (ROC) curve cut-offs to standardize the analysis. ROC curves (Figure 1) were plotted, and specificity and sensitivity for each index in predicting PPIs were calculated (Table 2).
Figure 1.
ROC curves of measurement data. A: Age ROC curve, illustrating its discriminative power in prediction. B: CRP (mg/L) ROC curve, assessing its effectiveness in prediction. C: PCT (ng/mL) ROC curve, showing its predictive accuracy. D: HMGB1 (pg/mL) ROC curve, evaluating its performance in prediction. E: IL-6 (pg/mL) ROC curve, indicating its discriminative ability in prediction. F: NLR ROC curve, reflecting its predictive efficacy. G: PLR ROC curve, assessing its capacity for prediction. H: SII ROC curve, displaying its effectiveness in prediction. Note: CRP, C-reactive Protein; PCT, procalcitonin; HMGB1, high mobility group box 1; IL-6, interleukin-6; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index.
Table 2.
ROC curve parameters of measurement data
| Marker | AUC | 95% CI | Specificity | Sensitivity | Youden index | Cut off |
|---|---|---|---|---|---|---|
| Age | 0.681 | 0.632-0.731 | 67.47% | 63.36% | 30.83% | 62.5 |
| CRP | 0.657 | 0.602-0.713 | 82.06% | 45.04% | 27.09% | 64.495 |
| PCT | 0.818 | 0.779-0.858 | 80.59% | 74.05% | 54.63% | 2.375 |
| HMGB1 | 0.719 | 0.667-0.772 | 82.79% | 54.96% | 37.75% | 476.11 |
| IL-6 | 0.679 | 0.626-0.733 | 69.67% | 59.54% | 29.22% | 11.795 |
| NLR | 0.626 | 0.574-0.677 | 74.29% | 45.80% | 20.09% | 3.645 |
| PLR | 0.622 | 0.563-0.682 | 92.03% | 31.30% | 23.32% | 165.395 |
| SII | 0.596 | 0.540-0.652 | 74.61% | 45.04% | 19.64% | 572.67 |
Note: ROC, receiver operating characteristic; CRP, C-reactive Protein; PCT, procalcitonin; HMGB1, high mobility group box 1; IL-6, interleukin-6; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index.
Screening of characteristic factors of PPIs
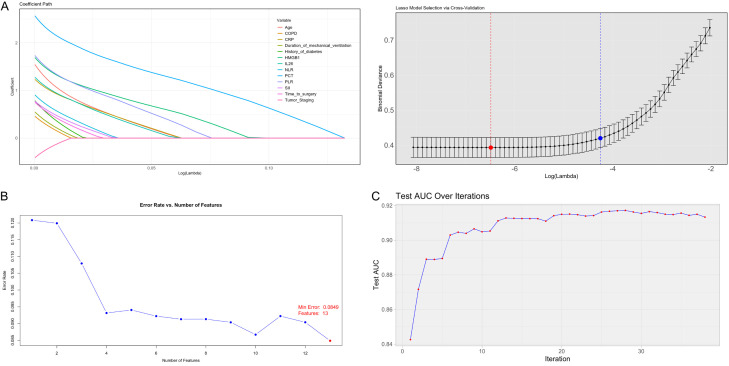
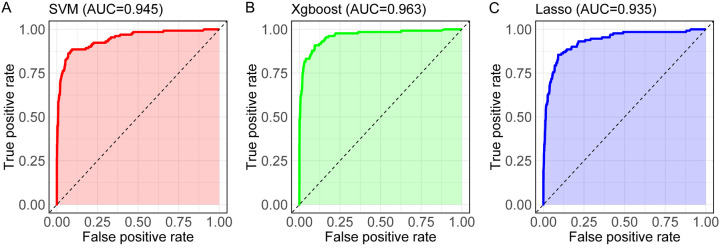
Data with significant baseline differences were included and assigned values (Table 3). LASSO-Logistic, XGBoost-Logistic, and SVM methods were applied to identify key feature variables, resulting in the selection of 13 significant factors (age, CRP, PCT, HMGB1, IL-6, NLR, PLR, SII, tumor staging, diabetes history, COPD, operation time, and duration of mechanical ventilation). LASSO regression analysis (Figure 2A) demonstrated the shrinkage of feature coefficients with increasing regularization (λ), using the minimum criterion and 1-SE rule to identify key predictors. SVM feature selection (Figure 2B) showed the error rate variation with feature inclusion, identifying optimal classification features. The XGBoost model’s iterative process (Figure 2C) used gradient boosting for enhanced feature selection. ROC curves for the models (SVM, XGBoost, and LASSO) revealed high discriminative accuracy, with AUC values exceeding 0.9: 0.945 for SVM, 0.963 for XGBoost, and 0.935 for LASSO, indicating model robustness and reliability of selected variables (Figure 3; Table 4).
Table 3.
Assignment table
| Variables | Assignment content |
|---|---|
| Age | <62.5 = 0, ≥62.5 = 1 |
| CRP | <64.495 = 0, ≥64.495 = 1 |
| PCT | <2.375 = 0, ≥2.375 = 1 |
| HMGB1 | <476.11 = 0, ≥476.11 = 1 |
| IL-6 | <11.795 = 0, ≥11.795 = 1 |
| NLR | <3.645 = 0, ≥3.645 = 1 |
| PLR | <165.395 = 0, ≥165.395 = 1 |
| SII | <572.67 = 0, ≥572.67 = 1 |
| Tumor staging | I+II = 1, III = 0 |
| History of diabetes | With = 0, without = 1 |
| COPD | With = 0, without = 1 |
| Operation time | ≥3 h = 1, <3 h = 0 |
| Duration of mechanical ventilation | ≥6 h = 1, <6 h = 0 |
| Infection | With = 1, without = 0 |
Note: COPD, chronic obstructive pulmonary disease; CRP, C-reactive Protein; PCT, procalcitonin; HMGB1, high mobility group box 1; IL-6, interleukin-6; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index.
Figure 2.
Screening of the characteristic factors of postoperative pulmonary infections using the 3 models. A: LASSO regression regularization and min and 1se. B: The variation of SVM error rate with the number of features. C: XGBoost optimal model iteration path diagram. Note: SVM, Support Vector Machine; XGBoost, Extreme Gradient Boosting; LASSO, Least Absolute Shrinkage and Selection Operator.
Figure 3.
ROC curves of the three machine learning models. A: SVM model ROC curve, demonstrating its classification capability. B: XGBoost model ROC curve, showing its predictive effectiveness. C: Lasso model ROC curve, illustrating its performance in classification. Note: SVM, Support Vector Machine; XGBoost, Extreme Gradient Boosting; LASSO, Least Absolute Shrinkage and Selection Operator.
Table 4.
Comparison of SVM, XGBoost, and LASSO model performance metrics
| Marker | SVM | XGBoost | LASSO |
|---|---|---|---|
| AUC | 0.945 | 0.963 | 0.935 |
| 95% CI | 0.922-0.968 | 0.944-0.982 | 0.910-0.960 |
| Specificity | 92.97% | 90.56% | 90.87% |
| Sensitivity | 86.26% | 90.84% | 85.50% |
| Youden index | 79.23% | 81.40% | 76.37% |
| Cut off | 0.122 | 0.177 | 0.169 |
| Accuracy | 92.16% | 90.59% | 90.22% |
| Precision | 86.26% | 90.84% | 85.50% |
| F1 Score | 72.67% | 70.00% | 67.88% |
Note: SVM, Support Vector Machine; XGBoost, Extreme Gradient Boosting; LASSO, Least Absolute Shrinkage and Selection Operator.
Construction of a nomogram prediction model for PPIs in LC
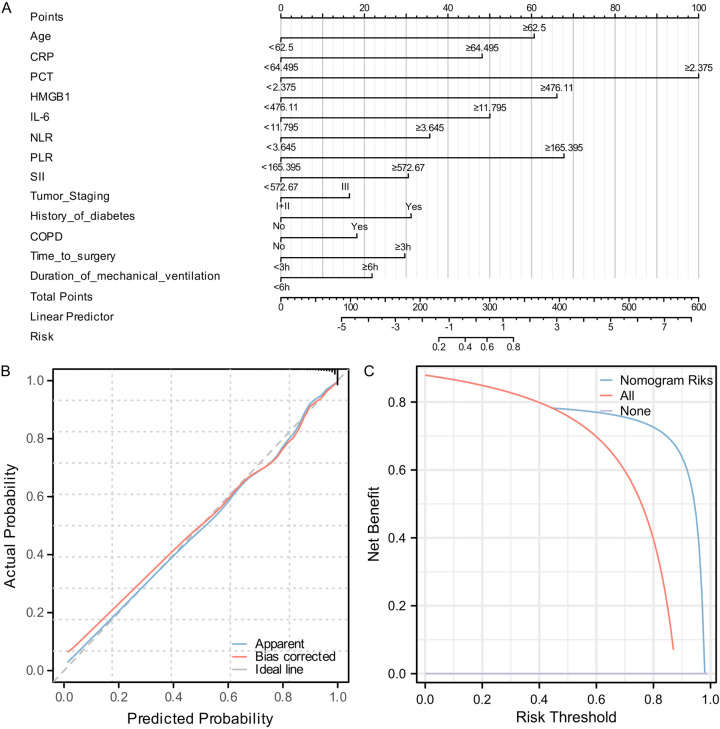
To enable clinical application, we visualized 13 selected features in a Nomogram model. PCT showed strong correlation with PPIs in LC patients (Figure 4A). Calibration curve analysis indicated significant overlap between Apparent and Bias-corrected predictions, closely aligning with the Ideal line, demonstrating good model fit and stability. The model’s C-index was 0.935 (0.910-0.960), and the goodness-of-fit test yielded a chi-square value of 11.276 (P = 0.186), suggesting no significant evidence to reject good model fit. DCA showed a benefit rate range of 0%-98%, with a peak benefit of 87.91% (Figure 4B, 4C).
Figure 4.
Construction of a nomogram prediction model for postoperative pulmonary infections in lung cancer. A: Nomogram prediction model for postoperative pulmonary infections in lung cancer. B: Stability evaluation of the Nomogram prediction model using calibration curves. C: Evaluation of the clinical benefit rate of the Nomogram prediction model using DCA. Note: CRP, C-reactive Protein; PCT, procalcitonin; HMGB1, high mobility group box 1; IL-6, interleukin-6; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index.
Internal validation of the model
An infected and an uninfected patient were randomly selected to evaluate the model’s predictive capability. The Nomogram predicted an infection incidence rate of 63% for the infected patient and 32% for the uninfected patient (Table 5).
Table 5.
Information on randomly sampled patients and calculation of incidence
| Type | Infected case | Score | Uninfected case | Score |
|---|---|---|---|---|
| Age | 84 | 61 | 55 | 0 |
| CRP | 78.34 | 47.5 | 80.03 | 47.5 |
| PCT | 2.48 | 100 | 1.59 | 0 |
| HMGB1 | 409.77 | 0 | 365.63 | 0 |
| IL-6 | 15.96 | 50 | 13.55 | 50 |
| NLR | 5.85 | 36 | 4.93 | 36 |
| PLR | 107.61 | 0 | 11.12 | 0 |
| SII | 733.65 | 31 | 274.1 | 0 |
| Tumor staging | I+II | 0 | I+II | 0 |
| History of diabetes | No | 0 | Yes | 31 |
| COPD | Yes | 28 | No | 0 |
| Operation time | ≥3 h | 30 | <3 h | 0 |
| Duration of mechanical ventilation | <6 h | 0 | ≥6 h | 22 |
| Total score | 383.5 | 186.5 | ||
| Incidence rate | 63% | 32% | ||
Note: COPD, chronic obstructive pulmonary disease; CRP, C-reactive Protein; PCT, procalcitonin; HMGB1, high mobility group box 1; IL-6, interleukin-6; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index.
Discussion
After LC surgery, patients may face a heightened risk of pulmonary infections, often caused by multiple factors that significantly impair respiratory function and elevate postoperative mortality risk [19]. LC predominantly affects middle-aged and elderly patients, whose physical functions, including immune system performance, naturally decline with age [20]. A range of factors can contribute to the development of pulmonary infections during surgery and the postoperative period, especially in older patients. Variations in infection rates between hospitals may be influenced by physicians’ understanding of infection risk factors, patient management approaches, and levels of medical care available [21]. PPIs not only extend recovery time but also increase hospitalization duration and financial costs. Therefore, surgeons must promptly identify and address the potential PPI risk factors that LC patients may encounter after surgery.
In this study, we analyzed 1,084 cases, finding that 131 patients experienced PPIs, with an incidence rate of 12%. In a study by Ding et al. [22], 27 of 244 patients developed PPIs, yielding an incidence rate of 11%. Similarly, Li et al. [23] examined 1,395 patients, reporting a surgical site infection incidence of 13.47% (188/1,395). These findings align closely with ours, suggesting that PPIs are a common concern for patients undergoing LC surgery. We further examined PPI risk factors in LC patients using three machine learning methods. Interestingly, all three methods consistently identified age, CRP, PCT, HMGB1, IL-6, NLR, PLR, SII, tumor staging, diabetes history, COPD, operation time, and duration of mechanical ventilation as independent risk factors for PPIs. For clarity, these factors can be classified into four categories: patient characteristics, disease characteristics, surgery-related factors, and markers of inflammation and infection.
Among patient characteristics, age is a critical factor influencing PPI risk in non-small cell LC surgery. Elderly patients typically exhibit diminished immune function, lower physiological reserves, and reduced respiratory muscle strength, all of which increase susceptibility to postoperative infections [24]. Additionally, older adults often have multiple chronic conditions, such as diabetes and hypertension, further compromising immune response and reducing the body’s ability to manage surgery-induced stress and infections effectively [25]. Furthermore, elderly patients experience slower recovery, with delayed restoration of pulmonary function compared to younger patients, increasing the likelihood of PPIs. Studies have shown that advanced age correlates with higher rates of postoperative complications, such as pneumonia and respiratory failure, significantly impacting recovery and survival outcomes [26]. For example, Zhou et al. [27] found that advanced age substantially increased the probability of postoperative pneumonia in LC patients. Simonsen et al. [28] reported that in super-elderly LC patients (≥80 years), the risk of postoperative pneumonia was 3.64 times higher than in patients under 80.
From the perspective of disease characteristics, tumor staging, diabetes history, and COPD are significant risk factors for postoperative infections in patients with non-small cell lung cancer (NSCLC). Advanced tumor stages often correlate with a higher disease burden and more complex surgeries, which increase postoperative infection risk. Diabetic patients have an elevated susceptibility to infections due to poor glycemic control, compromised immune function, and delayed wound healing [29]. COPD patients, with impaired pulmonary function and reduced respiratory clearance capacity, are prone to sputum retention and pulmonary infections [30]. Additionally, COPD often necessitates prolonged mechanical ventilation, further heightening infection risk. Studies show that pre-existing conditions like these increase the likelihood of PPIs and other complications, adversely affecting recovery and prognosis [31]. A meta-analysis by Zhang et al. [32] confirms that advanced age, diabetes, and lung disease are all significant risk factors for postoperative infections, validating our findings.
Regarding surgery-related characteristics, operation duration and mechanical ventilation time are key factors affecting infection risk after NSCLC surgery. Longer surgeries expose patients to an extended surgical environment and increase infection risk. Prolonged procedures also heighten physiological stress responses and may impair immune function [33]. Similarly, extended mechanical ventilation time increases the likelihood of airway compromise and bacterial invasion, raising the risk of ventilator-associated pneumonia and damaging airway mucosa, thus compromising local immunity [34]. Studies indicate that reducing surgery and ventilation times significantly lowers postoperative infection rates [35]. Clinical optimization of surgical and postoperative processes is, therefore, critical in preventing infection. Research by Zeng et al. [36] and Li et al. [23] supports the link between extended operation time and increased nosocomial infection risk in LC patients. Additionally, Wang et al. [37] identified surgical method and duration as independent risk factors for PPIs.
Inflammation and infection markers are also essential in predicting infection risk following NSCLC surgery [38]. Commonly used markers, including CRP, PCT, HMGB1, IL-6, NLR, PLR, and SII, reflect the body’s inflammatory and infection status. CRP and PCT are sensitive indicators; their elevated levels often signal bacterial infections post-surgery [39]. Higher levels of HMGB1 and IL-6, both proinflammatory cytokines, correlate with severe inflammatory reactions and increased infection risk [40]. Liu et al. [41] demonstrated that CRP and PCT are useful early predictors for PPIs in cervical cancer patients, and Suberviola’s team [42] found that rising PCT levels correlated with higher early infection rates in lung transplant patients. NLR, PLR, and SII provide a comprehensive assessment of immune and inflammatory responses, aiding in the evaluation of infection risk after surgery [43]. Utilizing these markers together can significantly enhance infection risk prediction accuracy [35,44], facilitating more individualized intervention strategies and reducing infection rates. For instance, NLR is a key factor in the hospital mortality prediction model for patients with pulmonary infection by Ren et al. [45]. Similarly, Yao et al. [46] reported that NLR, PLR, and SII levels reliably increase in pneumonia patients post-hip fracture surgery.
In the final stage of this study, we developed a multiparametric nomogram model to predict the risk of PPIs in patients with NSCLC. The model achieved a C-index of 0.935, indicating high predictive accuracy. Calibration curve analysis showed strong consistency between actual and predicted outcomes, further confirming the model’s stability. Additionally, DCA demonstrated a high clinical benefit rate across a threshold range of 0%-98%, with a maximum benefit rate of 87.91%. This model enables clinicians to assess a patient’s risk of pulmonary infections before or shortly after surgery, allowing for timely preventive and therapeutic interventions. For instance, in elderly patients or those with chronic conditions, early identification of elevated infection risk and implementation of rigorous infection control measures - such as enhanced preoperative and postoperative care, shortened operation time, and reduced mechanical ventilation duration - can effectively decrease postoperative infection rates. Moreover, the inclusion of inflammatory and infection markers within the model provides clinicians with valuable reference indicators, supporting more precise and individualized interventions.
While the nomogram model has demonstrated robust predictive performance in internal validation, further external validation and optimization are needed for broader clinical application. Future studies should consider incorporating multicenter data to confirm the model’s applicability and reliability across different populations. Additionally, advances in medical technology, including emerging biomarkers and big-data analysis techniques, may further enhance the model’s predictive accuracy and clinical value.
In summary, the multiparametric nomogram model developed in this study effectively predicts the risk of postoperative pulmonary infections in LC patients, providing a valuable tool for clinical decision-making and supporting improvements in postoperative management.
Acknowledgements
This study was supported by Scientific Research Project of Weifang Municipal Health Commission (No. WFWSJK-2022-027).
Disclosure of conflict of interest
None.
References
- 1.Adams SJ, Stone E, Baldwin DR, Vliegenthart R, Lee P, Fintelmann FJ. Lung cancer screening. Lancet. 2023;401:390–408. doi: 10.1016/S0140-6736(22)01694-4. [DOI] [PubMed] [Google Scholar]
- 2.Wolf AMD, Oeffinger KC, Shih TY, Walter LC, Church TR, Fontham ETH, Elkin EB, Etzioni RD, Guerra CE, Perkins RB, Kondo KK, Kratzer TB, Manassaram-Baptiste D, Dahut WL, Smith RA. Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA Cancer J Clin. 2024;74:50–81. doi: 10.3322/caac.21811. [DOI] [PubMed] [Google Scholar]
- 3.Felsinger R, Kunze U, Groman E. Gender differences in lung cancer epidemiology - do Austrian male lung cancer patients still die earlier in life? Front Public Health. 2023;11:1099165. doi: 10.3389/fpubh.2023.1099165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frega S, Dal Maso A, Ferro A, Bonanno L, Conte P, Pasello G. Heterogeneous tumor features and treatment outcome between males and females with lung cancer (LC): do gender and sex matter? Crit Rev Oncol Hematol. 2019;138:87–103. doi: 10.1016/j.critrevonc.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Miao S, Qiu H. The microbiome in the pathogenesis of lung cancer: the role of microbiome in lung cancer pathogenesis. APMIS. 2024;132:68–80. doi: 10.1111/apm.13359. [DOI] [PubMed] [Google Scholar]
- 6.Arnal-Estapé A, Foggetti G, Starrett JH, Nguyen DX, Politi K. Preclinical models for the study of lung cancer pathogenesis and therapy development. Cold Spring Harb Perspect Med. 2021;11:a037820. doi: 10.1101/cshperspect.a037820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD, Peters S ESMO Guidelines Committee. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 8.National Health Commission of The People’s Republic of China. National guidelines for diagnosis and treatment of lung cancer 2022 in China (English version) Chin J Cancer Res. 2022;34:176–206. doi: 10.21147/j.issn.1000-9604.2022.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng Y, Wang X, Li C, Chen X, Wu Y, Jin R, Li H. Margin quality analysis for wedge resection of lung cancer and construction of a predictive model. Transl Lung Cancer Res. 2024;13:540–551. doi: 10.21037/tlcr-24-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang JY, Mehran RJ, Feng L, Verma V, Liao Z, Welsh JW, Lin SH, O’Reilly MS, Jeter MD, Balter PA, McRae SE, Berry D, Heymach JV, Roth JA STARS Lung Cancer Trials Group. Stereotactic ablative radiotherapy for operable stage I non-small-cell lung cancer (revised STARS): long-term results of a single-arm, prospective trial with prespecified comparison to surgery. Lancet Oncol. 2021;22:1448–1457. doi: 10.1016/S1470-2045(21)00401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo J, Zhou R, Li L, Yao L, Zhang C. Postoperative lung infection in an immunocompromised older adult patient with lung cancer after oncological surgery: a case report. Ann Palliat Med. 2021;10:12894–12899. doi: 10.21037/apm-21-3381. [DOI] [PubMed] [Google Scholar]
- 12.Yamanashi K, Marumo S, Fukui M, Huang CL. Nontuberculous mycobacteria infection and prognosis after surgery of lung cancer: a retrospective study. Thorac Cardiovasc Surg. 2017;65:581–585. doi: 10.1055/s-0036-1584883. [DOI] [PubMed] [Google Scholar]
- 13.Yamamichi T, Ichinose J, Omura K, Hashimoto K, Matsuura Y, Nakao M, Okumura S, Ikeda N, Mun M. Impact of postoperative complications on the long-term outcome in lung cancer surgery. Surg Today. 2022;52:1254–1261. doi: 10.1007/s00595-022-02452-4. [DOI] [PubMed] [Google Scholar]
- 14.Feng SH, Yang ST. The new 8th TNM staging system of lung cancer and its potential imaging interpretation pitfalls and limitations with CT image demonstrations. Diagn Interv Radiol. 2019;25:270–279. doi: 10.5152/dir.2019.18458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long DR, Holmes EA, Goss CH, Singh PK, Waalkes A, Salipante SJ. Cell-free DNA detects pseudomonas aeruginosa lung infection in modulator-treated people with cystic fibrosis. Am J Respir Crit Care Med. 2023;208:944–947. doi: 10.1164/rccm.202305-0844LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo Y, Li L, Zheng K, Du J, Nie J, Wang Z, Hao Z. Development and validation of a survival prediction model for patients with advanced non-small cell lung cancer based on LASSO regression. Front Immunol. 2024;15:1431150. doi: 10.3389/fimmu.2024.1431150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang R, Xiong X, Wang H, Li W. Explainable machine learning model to prediction EGFR mutation in lung cancer. Front Oncol. 2022;12:924144. doi: 10.3389/fonc.2022.924144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan X, Du Y, Ma R, Teng N, Ou S, Zhao H, Li X. Construction of the XGBoost model for early lung cancer prediction based on metabolic indices. BMC Med Inform Decis Mak. 2023;23:107. doi: 10.1186/s12911-023-02171-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pettigrew MM, Tanner W, Harris AD. The lung microbiome and pneumonia. J Infect Dis. 2021;223(Suppl 2):S241–S245. doi: 10.1093/infdis/jiaa702. [DOI] [PubMed] [Google Scholar]
- 20.Singh A, Mazzola E, Xie Y, Marshall MB, Jaklitsch MT, Wilder FG. Lung cancer outcomes in the elderly: potential disparity in screening. Eur J Cardiothorac Surg. 2024;65:ezae080. doi: 10.1093/ejcts/ezae080. [DOI] [PubMed] [Google Scholar]
- 21.Abraham AS, Sasikumar NK, Rajan S, Abubaker R, Manoharan KS, Kumar L. Incidence and severity of postoperative complications in patients undergoing surgery following COVID-19 infection at a tertiary care center in South India. Anesth Essays Res. 2022;16:268–271. doi: 10.4103/aer.aer_134_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding Z, Wang X, Jiang S, Liu J. Risk factors for postoperative pulmonary infection in patients with non-small cell lung cancer: analysis based on regression models and construction of a nomogram prediction model. Am J Transl Res. 2023;15:3375–3384. [PMC free article] [PubMed] [Google Scholar]
- 23.Li S, Lin J, Ye L, Qiao F, Huang W, Peng Y, Huang J. Surgical site infection following open lobectomy in patients with lung cancer: a prospective study. J Evid Based Med. 2023;16:194–199. doi: 10.1111/jebm.12544. [DOI] [PubMed] [Google Scholar]
- 24.Zhou C, Jiang Y, Sun L, Li H, Liu X, Huang L. Secondary pulmonary infection and co-infection in elderly COVID-19 patients during the pandemics in a tertiary general hospital in Beijing, China. Front Microbiol. 2023;14:1280026. doi: 10.3389/fmicb.2023.1280026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang W, Wu Y. Refinement of specialized nursing intervention in elderly patients with diabetes complicated by pulmonary infection and the impact on the patient’s condition and prognosis. Altern Ther Health Med. 2024 AT10766. [PubMed] [Google Scholar]
- 26.Ahn J, Chang JS, Kim JW. Postoperative pneumonia and aspiration pneumonia following elderly hip fractures. J Nutr Health Aging. 2022;26:732–738. doi: 10.1007/s12603-022-1821-9. [DOI] [PubMed] [Google Scholar]
- 27.Zhou J, Wu D, Zheng Q, Wang T, Lin M, Lu T, Lin F. A clinical prediction model for postoperative pneumonia after lung cancer surgery. J Surg Res. 2023;284:62–69. doi: 10.1016/j.jss.2022.11.021. [DOI] [PubMed] [Google Scholar]
- 28.Simonsen DF, Søgaard M, Bozi I, Horsburgh CR, Thomsen RW. Risk factors for postoperative pneumonia after lung cancer surgery and impact of pneumonia on survival. Respir Med. 2015;109:1340–1346. doi: 10.1016/j.rmed.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Bian H, Liu M, Liu J, Dong M, Hong G, Agrafiotis AC, Patel AJ, Ding L, Wu J, Chen J. Seven preoperative factors have strong predictive value for postoperative pneumonia in patients undergoing thoracoscopic lung cancer surgery. Transl Lung Cancer Res. 2023;12:2193–2208. doi: 10.21037/tlcr-23-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy E, Rheault J, Pigeon MA, Ugalde PA, Racine C, Simard S, Chouinard G, Lippens A, Lacasse Y, Maltais F. Lung cancer resection and postoperative outcomes in COPD: a single-center experience. Chron Respir Dis. 2020;17:1479973120925430. doi: 10.1177/1479973120925430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patterson JT, Bohl DD, Basques BA, Arzeno AH, Grauer JN. Does preoperative pneumonia affect complications of geriatric hip fracture surgery? Am J Orthop (Belle Mead NJ) 2017;46:E177–E185. [PubMed] [Google Scholar]
- 32.Zhang J, Zhao T, Long S, Liu X, Yu H. Risk factors for postoperative infection in Chinese lung cancer patients: a meta-analysis. J Evid Based Med. 2017;10:255–262. doi: 10.1111/jebm.12276. [DOI] [PubMed] [Google Scholar]
- 33.Liu R, Li W, Li Y, Han Y, Ma M, Zhu W, Li M, Dai Q, Cao Y, Xu G, Liu X. Total time of operation is a risk factor of stroke-associated pneumonia in acute ischemic stroke patients with intra-arterial treatment. Medicine (Baltimore) 2016;95:e3958. doi: 10.1097/MD.0000000000003958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kózka M, Sega A, Wojnar-Gruszka K, Tarnawska A, Gniadek A. Risk factors of pneumonia associated with mechanical ventilation. Int J Environ Res Public Health. 2020;17:656. doi: 10.3390/ijerph17020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chu FL, Li C, Liu Y, Dong B, Qiu Y, Fan G. Peripheral blood parameters for predicting PICU admission and mechanical ventilation in pediatric inpatients with human parainfluenza virus-induced pneumonia. J Med Virol. 2023;95:e28752. doi: 10.1002/jmv.28752. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Cai XJ, Shi L, Li FY, Lin NM. Risk factors of postoperative nosocomial pneumonia in stage I-IIIa lung cancer patients. Asian Pac J Cancer Prev. 2014;15:3071–3074. doi: 10.7314/apjcp.2014.15.7.3071. [DOI] [PubMed] [Google Scholar]
- 37.Wang JY, Pang QY, Yang YJ, Feng YM, Xiang YY, An R, Liu HL. Development and validation of a nomogram for predicting postoperative pulmonary infection in patients undergoing lung surgery. J Cardiothorac Vasc Anesth. 2022;36:4393–4402. doi: 10.1053/j.jvca.2022.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Deretic V. Autophagy in inflammation, infection, and immunometabolism. Immunity. 2021;54:437–453. doi: 10.1016/j.immuni.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng S, Zhang W. Predictive values of sTREM-1, PCT and CRP for multiple trauma-induced acute respiratory distress syndrome complicated with pulmonary infection. Clin Lab. 2022;68 doi: 10.7754/Clin.Lab.2022.211258. [DOI] [PubMed] [Google Scholar]
- 40.Sivakorn C, Dechsanga J, Jamjumrus L, Boonnak K, Schultz MJ, Dondorp AM, Phumratanaprapin W, Ratanarat R, Naorungroj T, Wattanawinitchai P, Siripoon T, Duangdee C, Techarang T. High mobility group box 1 and interleukin 6 at intensive care unit admission as biomarkers in critically Ill COVID-19 patients. Am J Trop Med Hyg. 2021;105:73–80. doi: 10.4269/ajtmh.21-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y, Tian L, You J, Li Y. The predictive value of postoperative C-reactive protein (CRP), procalcitonin (PCT) and triggering receptor expressed on myeloid cells 1 (TREM-1) for the early detection of pulmonary infection following laparoscopic general anesthesia for cervical cancer treatment. Ann Palliat Med. 2021;10:4502–4508. doi: 10.21037/apm-21-554. [DOI] [PubMed] [Google Scholar]
- 42.Suberviola B, Rellan L, Riera J, Iranzo R, Garcia Campos A, Robles JC, Vicente R, Miñambres E, Santibanez M. Role of biomarkers in early infectious complications after lung transplantation. PLoS One. 2017;12:e0180202. doi: 10.1371/journal.pone.0180202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li D, Gu W, Xu H, Zhang Z, Zhao C, He C, Zhu X, Li Y. Inflammation in the peripheral blood system of Crohn’s disease. Clin Exp Med. 2023;23:2805–2812. doi: 10.1007/s10238-023-01030-3. [DOI] [PubMed] [Google Scholar]
- 44.Kuikel S, Pathak N, Poudel S, Thapa S, Bhattarai SL, Chaudhary G, Pandey KR. Neutrophil-lymphocyte ratio as a predictor of adverse outcome in patients with community-acquired pneumonia: a systematic review. Health Sci Rep. 2022;5:e630. doi: 10.1002/hsr2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ren Y, Zhang L, Xu F, Han D, Zheng S, Zhang F, Li L, Wang Z, Lyu J, Yin H. Risk factor analysis and nomogram for predicting in-hospital mortality in ICU patients with sepsis and lung infection. BMC Pulm Med. 2022;22:17. doi: 10.1186/s12890-021-01809-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao W, Wang W, Tang W, Lv Q, Ding W. Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic immune inflammation index (SII) to predict postoperative pneumonia in elderly hip fracture patients. J Orthop Surg Res. 2023;18:673. doi: 10.1186/s13018-023-04157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]