Abstract
Breast cancer is the second leading cause of cancer deaths among women. Multiple microRNAs (miRNAs) have been reported to be associated with breast cancer progression or metastasis. The purpose of the current study was to identify plasma extracellular miRNAs associated with incident breast cancer. Levels of 166 plasma miRNA were measured using qRT-PCR in 2140 Framingham Heart Study female participants with a median follow up of 15.7 years. Prospective analyses of the associations of miRNAs with the occurrence of 56 new-onset breast cancer events were conducted using proportional hazards regression. The expression levels miR-134-5p (P=0.002) and miR-505-3p (P=0.005) were found to be positively associated with incident breast cancer after adjusting for age, body mass index, and cigarette smoking. These results highlight plasma miRNAs as potential biomarkers of breast cancer risk. Validation of these findings in larger and more diverse cohorts is warranted.
Keywords: Breast cancer, microRNA, extracellular RNA, biomarker, risk factors
Introduction
MicroRNAs (miRNAs) are small, non-coding RNAs comprised of 18-24 nucleotides that regulate gene expression by binding to complementary or near-complementary sequences in 3’-untranslated regions of messenger RNA (mRNA). In doing so, miRNAs can alter gene expression via mRNA transcript degradation or translational repression. It has been suggested that miRNAs regulate approximately two-thirds of human protein-coding genes [1,2]. Accordingly, miRNAs have been implicated as powerful regulators of a range of physiological and pathophysiological processes [1].
While miRNAs can be found inside the cell, miRNAs are also found extracellularly in circulating blood and other body fluids [1,3]. These miRNAs can be transported across cell membranes to mediate cell-to-cell communication [4]. Moreover, expression levels of extracellular miRNAs (ex-miRNAs) have been reported to be associated with a wide range of diseases, including cancer [5].
Ex-miRNAs play a role in numerous cancer-related processes, including cell proliferation, differentiation, and apoptosis [2]. Dysregulation of ex-miRNAs is often observed in various cancers due to altered miRNA biogenesis and perturbed transcription of miRNA-encoding genes. As a result, some ex-miRNAs become significantly overexpressed while the expression of others significantly decreases [6].
Given that ten percent of known human miRNAs can be detected in plasma, are stable, and can be measured by polymerase chain reaction (PCR), ex-miRNAs may be ideal candidates as cancer biomarkers [6]. Most studies of miRNAs and breast cancer have examined associations of miRNA expression in tumor tissue with prevalent breast cancer; there are few studies examining plasma miRNAs as predictors of new-onset breast cancer [7,8]. Therefore, in the present study, we investigated associations of plasma ex-miRNAs with incident breast cancer in a large population.
Sample collection
The Framingham Heart Study (FHS) is a population-based, observational cohort study established in 1948 to identify risk factors for the development of cardiovascular disease [9]. The study sample for the present investigation included female participants in the FHS Third Generation cohort (n=2,140 at Exam 1) in whom plasma miRNA levels were measured. A total of 24 participants with prevalent breast cancer were excluded, leaving 2,116 eligible participants. The FHS protocol was approved by the Boston Medical Center IRB; informed consent was provided by all participants.
Study participants attended the first examination in the years 2002-2005 and underwent a fasting blood draw. Plasma samples were immediately isolated (Applied Biosystems, catalog #A27828) and processed (ThermoFisher Flex Magnetic Particle Processor, 96DW) before being stored at -80°C until assay. The isolated miRNAs were converted to cDNA (Applied Biosystems, catalog #A28007) and synthesis and preamplification (Applied Biosystems, catalog #4484075) were performed. qRT-PCR (Applied Biosystems, catalog #A25576; BioMark HD, catalogue #BMKHD-BMKHD; Fluidigm, catalog #BMK-M-96.96) was used to quantitate miRNAs and data are reported as cycle threshold (Ct) values. We required that an ex-miRNA be measurable in at least 50% of participants, yielding 166 ex-miRNAs that were carried forward in the analysis. The methods for plasma miRNA isolation and measurement have been previously reported and are described in greater detail elsewhere [10]. Self-reported smoking history was obtained and cigarette smoking status (current/former/never) was defined according to previously published classifications [10].
Participants were prospectively followed through 2018 for the occurrence of incident breast cancer. Breast cancer events were identified by surveillance through routine examinations, health history updates, hospital admissions, or death records. Cancer cases were adjudicated and coded on the basis of tumor topology and morphology and graded by two independent physicians using International Classification of Diseases codes [11].
We used proportional hazards regression with plasma miRNA levels as the independent variable and incident breast cancer as the outcome. The covariates for the models were age, cigarette smoking status, and body mass index (BMI). All statistical analyses were conducted using SAS 9.4 (SAS Institute Inc. 2020. SAS/STAT® 15.2 User’s Guide. Cary, NC: SAS Institute Inc.) procedure PHREG.
Results and discussion
Among eligible participants (n=2116; mean age: 40±9 years), 1160 (55%) were never smokers, 640 (30%) were former smokers, and 315 (15%) were current smokers. During a median follow-up time of 15.7 years, 56 women developed new-onset breast cancer. The full table of clinical characteristics of the study participants can be found in Table 1.
Table 1.
Clinical characteristics of the study participants
| Clinical characteristic | Mean (standard deviation) |
|
| |
| Age, years | 40 (9) |
| Systolic blood pressure, mmHg | 113 (14) |
| Diastolic blood pressure, mmHg | 73 (9) |
| Triglycerides, mg/dL | 97 (62) |
| Total cholesterol, mg/dL | 185 (34) |
| High-density lipoprotein, mg/dL | 61 (16) |
| Fasting blood glucose, mg/dL | 92 (18) |
| BMI, kg/m2 | 26 (6) |
|
| |
| Dichotomous outcome | Number of participants (%) |
|
| |
| Never smoker | 1160 (54.8) |
| Former smoker | 640 (30.3) |
| Current smoker | 315 (14.9) |
| Type 2 diabetes mellitus | 46 (2.2) |
| Incident breast cancer | 56 (2.7) |
Among the 166 plasma ex-miRNAs included in analysis, two ex-miRNAs (miR-134-5p and miR-505-3p) were associated with incident breast cancer at P < 0.005 before adjusting for multiple testing (Table 2). The association results for all ex-miRNAs included in the prospective analyses are provided in Supplementary Table 1.
Table 2.
Ex-miRNAs associated with incident breast cancer in the FHS
| Ex-miRNA | N analyzed | Event | Estimate* | Standard Error | Chi Squared | Probability Chi Squared | Hazard Ratio | HR Lower Confidence Level | HR Upper Confidence Level |
|---|---|---|---|---|---|---|---|---|---|
| 134-5p | 1594 | 51 | -0.126 | 0.0413 | 9.296 | 2.30E-03 | 0.882 | 0.813 | 0.956 |
| 505-3p | 1805 | 49 | -0.163 | 0.0582 | 7.882 | 4.99E-03 | 0.849 | 0.758 | 0.952 |
Estimates are reported as Ct values such that a negative estimate indicates a higher Ct value (lower ex-miRNA expression).
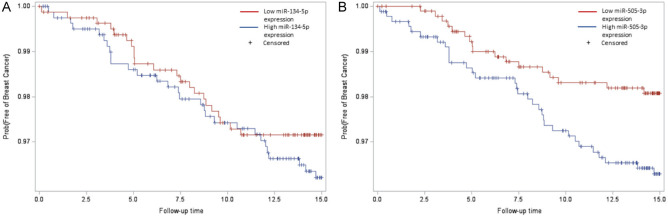
The Kaplan-Meier breast cancer-free survival plot for miR-134-5p (Figure 1A) indicates that there was a higher proportion of event-free survival for participants with low miR-134-5p expression (miR-134-5p ≥ median Ct-values) compared to participants with high miR-134-5p expression (miR-134-5p < median Ct-values). The Kaplan-Meier survival plot for miR-505-3p depicts analogous behavior, i.e., lower miR-505-3p expression levels associated with higher event-free survival (Figure 1B).
Figure 1.
Kaplan-Meier plots for ex-miRNAs. The x-axis “Follow-up time” indicates years to new breast cancer event, and the y-axis “Prob(Free of Breast Cancer)” indicates the proportion of participants with event-free survival. The variable ex-miRNA was defined as: if ex-miRNA ≥ median Ct-value, then low ex-miRNA expression (red); if ex-miRNA < median Ct-value, then high ex-miRNA expression (blue). A. Among the 1622 female participants with analyzed miR-134-5p levels, there were 51 new breast cancer cases (among 811 participants with miR-134-5p levels above the median, there were 22 new breast cancer cases; among 811 participants with miR-134-5p levels below the median, there were 29 new breast cancer cases). B. Among the 1843 female participants with analyzed miR-505-3p levels, there were 49 new breast cancer cases (among 923 participants with miR-505-3p levels above median, there were 18 new breast cancer cases; among 920 participants with miR-505-3p levels below median, there were 32 new breast cancer cases).
The purpose of our study was to examine associations of plasma ex-miRNAs with new-onset breast cancer in middle-aged women. To this end, we identified two plasma ex-miRNAs (miR-134-5p and miR-505-3p) whose expression levels were positively associated with incident breast cancer during a median follow-up of 15.7 years. Previous studies have enumerated a negative association between the expression levels of miR-134-5p and miR-505-3p and breast cancer progression in tumor samples or cell lines [12-16]. However, such studies examining miRNAs isolated from tumor tissue or cell lines demonstrate an association between cellular miRNAs and prevalent breast cancer. To our knowledge, this is the first study to demonstrate the association of extracellular miR-134-5p and miR-505-3p expression with incident breast cancer. These findings highlight the potential of ex-miRNAs to serve as biomarkers of breast cancer risk.
Discordance in the direction of effect of miRNAs on breast cancer has been observed between breast cancer tissue samples or cell lines and miRNAs derived from cell-free blood samples for other miRNAs, including miR-122, miR-145-5p, and miR-125b-5p [17-23]. For example, expression levels of miR-122 isolated from human breast cancer cells have been reported to be negatively associated with prevalent breast cancer, whereas expression levels of serum miR-122 have been found to be positively associated with prevalent breast cancer [22,23]. One proposed explanation for this apparent paradox may be that breast cancer cells secrete these miRNAs at higher rates than normal cells, decreasing cellular miRNA levels and increasing ex-miRNA levels [22]. The current study, however, is unable to provide a mechanism of differential expression or evidence of ex-miRNA communication with specific tissues or cell lines. Functional studies investigating the molecular targets and pathways of miR-134-5p and miR-505-3p are warranted.
The present study has several strengths, including a large sample size of middle-aged women with available plasma ex-miRNA and the availability of new-onset breast cancer outcomes during long-term follow-up. Yet, there are several limitations. Of the 166 plasma ex-miRNAs included in the analysis, only two ex-miRNAs were significantly associated with incident breast cancer after adjusting for covariates; these associations were not significant, however, after correcting for multiple testing. Statistical power was limited by the relatively small number of new-onset breast cancer cases in the study sample. Additionally, the study population consisted largely of participants of European ancestry. Future studies that include larger sample sizes and greater racial and ethnic diversity are warranted.
This population-based study reveals two plasma ex-miRNAs associated with incident breast cancer. Moreover, these findings motivate further investigation into the molecular targets and mechanistic pathways of the identified ex-miRNAs. This effort may help advance the utility of plasma ex-miRNAs as clinically useful biomarkers.
Acknowledgements
The Framingham Heart Study is funded by National Institutes of Health contracts N01-HC-25195, HHSN268201500001, and 75N92019D00031. The laboratory work for this investigation was funded by the Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD and was conducted at the Uniformed Services University of Health Sciences, Bethesda, MD. This manuscript does not necessarily reflect the opinions or views of the NHLBI, NIH or DHHS.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Scott H. Extracellular microRNAs as messengers in the central and peripheral nervous system. Neuronal Signal. 2017;1:NS20170112. doi: 10.1042/NS20170112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jansson MD, Lund AH. MicroRNA and cancer. Mol Oncol. 2012;6:590–610. doi: 10.1016/j.molonc.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Condrat CE, Thompson DC, Barbu MG, Bugnar OL, Boboc A, Cretoiu D, Suciu N, Cretoiu SM, Voinea SC. miRNAs as biomarkers in disease: latest findings regarding their role in diagnosis and prognosis. Cells. 2020;9:276. doi: 10.3390/cells9020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mori MA, Ludwig RG, Garcia-Martin R, Brandão BB, Kahn CR. Extracellular miRNAs: from biomarkers to mediators of physiology and disease. Cell Metab. 2019;30:656–673. doi: 10.1016/j.cmet.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ortiz-Quintero B. Extracellular microRNAs as intercellular mediators and noninvasive biomarkers of cancer. Cancers (Basel) 2020;12:3455. doi: 10.3390/cancers12113455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, Peng R, Wang J, Qin Z, Xue L. Circulating microRNAs as potential cancer biomarkers: the advantage and disadvantage. Clin Epigenetics. 2018;10:59. doi: 10.1186/s13148-018-0492-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Zou W, Wang Y, Liao Z, Li L, Zhai Y, Zhang L, Gu S, Zhao X. Plasma-based microRNA signatures in early diagnosis of breast cancer. Mol Genet Genomic Med. 2020;8:e1092. doi: 10.1002/mgg3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou R, Loke SY, Tang YC, Too HP, Zhou L, Lee ASG, Hartman M. Development and validation of a circulating microRNA panel for the early detection of breast cancer. Br J Cancer. 2022;126:472–481. doi: 10.1038/s41416-021-01593-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D’Agostino RB Sr, Fox CS, Larson MG, Murabito JM, O’Donnell CJ, Vasan RS, Wolf PA, Levy D. The third generation cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 10.Karlin H, Sooda M, Larson M, Rong J, Huan T, Mens MMJ, van Rooij FJA, Ikram MA, Courchesne P, Freedman JE, Joehanes R, Mueller GP, Kavousi M, Ghanbari M, Levy D. Plasma extracellular microRNAs associated with cardiovascular disease risk factors in middle-aged and older adults. J Am Heart Assoc. 2024;13:e033674. doi: 10.1161/JAHA.123.033674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu EE, Suthahar N, Paniagua SM, Wang D, Lau ES, Li SX, Jovani M, Takvorian KS, Kreger BE, Benjamin EJ, Meijers WC, Bakker SJL, Kieneker LM, Gruppen EG, van der Vegt B, de Bock GH, Gansevoort RT, Hussain SK, Hoffmann U, Splansky GL, Vasan RS, Larson MG, Levy D, Cheng S, de Boer RA, Ho JE. Association of cardiometabolic disease with cancer in the community. JACC CardioOncol. 2022;4:69–81. doi: 10.1016/j.jaccao.2022.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang C, Zhang G, Zhang Y, Zhang S, Li J, Liu Y. Exosome miR-134-5p restrains breast cancer progression via regulating PI3K/AKT pathway by targeting ARHGAP1. J Obstet Gynaecol Res. 2021;47:4037–4048. doi: 10.1111/jog.14983. [DOI] [PubMed] [Google Scholar]
- 13.Su X, Zhang L, Li H, Cheng P, Zhu Y, Liu Z, Zhao Y, Xu H, Li D, Gao H, Zhang T. MicroRNA-134 targets KRAS to suppress breast cancer cell proliferation, migration and invasion. Oncol Lett. 2017;13:1932–1938. doi: 10.3892/ol.2017.5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Liu H, Li M. Downregulation of miR-505 promotes cell proliferation, migration and invasion, and predicts poor prognosis in breast cancer. Oncol Lett. 2019;18:247–254. doi: 10.3892/ol.2019.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao P, Guan H, Dai Z, Ma Y, Zhao Y, Liu D. Long noncoding RNA DLX6-AS1 promotes breast cancer progression via miR-505-3p/RUNX2 axis. Eur J Pharmacol. 2019;865:172778. doi: 10.1016/j.ejphar.2019.172778. [DOI] [PubMed] [Google Scholar]
- 16.Pan JY, Zhang F, Sun CC, Li SJ, Li G, Gong FY, Bo T, He J, Hua RX, Hu WD, Yuan ZP, Wang X, He QQ, Li DJ. miR-134: a human cancer suppressor? Mol Ther Nucleic Acids. 2017;6:140–149. doi: 10.1016/j.omtn.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashirbekov Y, Abaildayev A, Omarbayeva N, Botbayev D, Belkozhayev A, Askandirova A, Neupokoyeva A, Utegenova G, Sharipov K, Aitkhozhina N. Combination of circulating miR-145-5p/miR-191-5p as biomarker for breast cancer detection. PeerJ. 2020;8:e10494. doi: 10.7717/peerj.10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jusoh AR, Mohan SV, Lu Ping T, Tengku Din TADAAB, Haron J, Romli RC, Jaafar H, Nafi SN, Tuan Salwani TI, Yahya MM. Plasma circulating mirnas profiling for identification of potential breast cancer early detection biomarkers. Asian Pac J Cancer Prev. 2021;22:1375–1381. doi: 10.31557/APJCP.2021.22.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang W, Zhang X, Tan W, Gao J, Pan L, Ye X, Chen L, Zheng W. miR-145-5p suppresses breast cancer progression by inhibiting SOX2. J Surg Res. 2019;236:278–287. doi: 10.1016/j.jss.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 20.Matamala N, Vargas MT, Gonzalez-Campora R, Minambres R, Arias JI, Menendez P, Andres-Leon E, Gomez-Lopez G, Yanowsky K, Calvete-Candenas J, Inglada-Perez L, Martinez-Delgado B, Benitez J. Tumor microRNA expression profiling identifies circulating microRNAs for early breast cancer detection. Clin Chem. 2015;61:1098–1106. doi: 10.1373/clinchem.2015.238691. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Wang Y, Fan H, Zhang Z, Li N. miR-125b-5p inhibits breast cancer cell proliferation, migration and invasion by targeting KIAA1522. Biochem Biophys Res Commun. 2018;504:277–282. doi: 10.1016/j.bbrc.2018.08.172. [DOI] [PubMed] [Google Scholar]
- 22.Saleh AA, Soliman SE, Habib MSE, Gohar SF, Abo-Zeid GS. Potential value of circulatory microRNA122 gene expression as a prognostic and metastatic prediction marker for breast cancer. Mol Biol Rep. 2019;46:2809–2818. doi: 10.1007/s11033-019-04727-5. [DOI] [PubMed] [Google Scholar]
- 23.Wang B, Wang H, Yang Z. MiR-122 inhibits cell proliferation and tumorigenesis of breast cancer by targeting IGF1R. PLoS One. 2012;7:e47053. doi: 10.1371/journal.pone.0047053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.