Abstract
Urothelial carcinoma (UC) predominantly arises in the bladder, but upper tract urothelial carcinomas (UTUCs) comprise 5-10% of cases. Patients with end-stage renal disease (ESRD) are at increased risk for UC, and erythropoiesis-stimulating agents (ESAs) are frequently used to manage anemia in ESRD. However, ESA use in cancer patients raises concerns about tumor progression and survival outcomes. This study aimed to assess the impact of ESA use on tumor recurrence, cancer-specific survival (CSS), and overall survival (OS) in patients with ESRD and early-stage UC. We analyzed data from the Chang-Gung Research Database (CGRD) in Taiwan, including 850 patients with ESRD and non-muscle-invasive bladder cancer (NMIBC) and 492 patients with ESRD and localized UTUC. The ESA group was compared to a non-ESA cohort, and inverse probability of treatment weighting (IPTW) was applied to minimize selection bias. Kaplan-Meier curves and log-rank tests were used to evaluate bladder recurrence-free survival, CSS, and OS. In NMIBC patients, ESA use did not significantly affect bladder recurrence-free survival, CSS, or OS. Similarly, ESA use in localized UTUC patients did not increase the risk of bladder recurrence or negatively impact CSS and OS. However, UTUC patients treated with ESA demonstrated a significantly increased risk of contralateral recurrence (P < 0.001). The use of ESA in patients with ESRD and early-stage UC appears safe regarding bladder recurrence, CSS, and OS, but clinicians should remain vigilant for contralateral recurrence in localized UTUC. These findings provide valuable insights into the complex management of anemia in patients with concurrent ESRD and UC, emphasizing the need for tailored clinical monitoring in this high-risk population.
Keywords: Erythropoiesis-stimulating agent, end-stage renal disease, urothelial carcinoma, recurrence risk, survival
Introduction
Urothelial carcinoma (UC) is a malignancy of the urinary tract lining epithelium. Approximately 90%-95% of UC cases arise in the urinary bladder. The remaining 5%-10% of cases are upper tract urothelial carcinomas (UTUCs), which refer to malignancies that originate from the renal calyceal system to the distal ureter [1-3]. In Taiwan, UC is commonly observed in patients with end-stage renal disease (ESRD) [4,5]. Reported risk factors include analgesic abuse [6], groundwater containing arsenic [7], ingestion of Aristolochia-based herbal medicines [8], and compromised immune status [9].
In addition to urothelial malignancies, anemia is an important complication in patients with ESRD. Many studies have shown that anemia adversely affects the survival and quality of life (QOL) of patients with ESRD or cancer [10-12]. Anemia is also associated with a diminished response to chemotherapy or radiotherapy in patients with malignancies [13]. Reduced levels of erythropoietin (Epo), an essential hormone that regulates red blood cell (RBC) formation, may be one of the possible mechanisms leading to anemia [14,15]. The introduction of erythropoiesis-stimulating agents (ESAs) has reduced the need for blood transfusion and improved QOL [11,16,17]. However, some studies have demonstrated that ESA use is associated with decreased survival in patients with chronic kidney disease (CKD) and malignancies [18], especially during the active cancer phase [19,20], and with an increased risk of cancer progression or recurrence [21,22].
In clinical practice, physicians face the dilemma of using ESA in patients with ESRD and concurrent cancers. Without ESA treatment, patients may experience fatigue, reliance on transfusion therapy [23], transfusion-related iron overload, or infections [16]. However, ESA use has raised concerns about the risk of cancer progression or recurrence. Guidelines specifically addressing the use of ESA in patients with simultaneous ESRD and cancer are still lacking [24,25]. Several studies have demonstrated that ESA treatment increases the risk of thrombotic vascular adverse events [26,27], and is associated with increased mortality in certain cancers, such as head and neck or breast cancers [20,28-30]. Nevertheless, there is a notable gap in the literature regarding the impact of ESA use in patients with ESRD and UC.
Given the lack of evidence and the need for guidance in clinical practice, we analyzed data from the multi-medical institutional Chang-Gung Research Database (CGRD) in Taiwan. This study aimed to investigate the effects of ESA on tumor control and recurrence in patients with ESRD and early-stage UC, providing valuable insights into the nuanced interplay between ESA treatment and cancer outcomes in this specific patient population.
Materials and methods
Data source: the CGRD
The CGRD is a de-identified database updated annually with medical records from Chang Gung Memorial Hospital (CGMH). CGMH, comprising two medical centers and five local hospitals, is the largest healthcare delivery system in Taiwan, providing approximately 10%-12% of the healthcare services under the Taiwan National Health Insurance (NHI) program [31]. The Taiwan NHI is a compulsory, single-payer health insurance system that covers over 99% of the population [32]. The CGRD contains detailed diagnoses, prescriptions, and laboratory test results from both inpatient and outpatient settings.
Study population
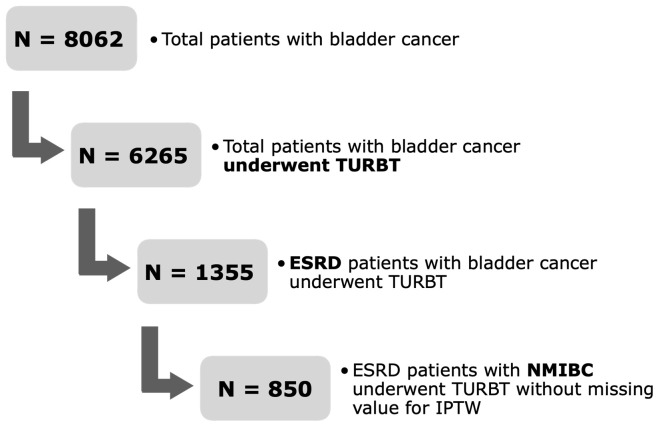
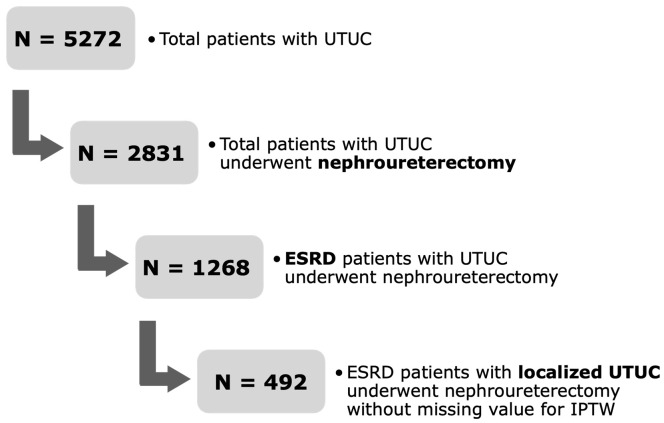
Between January 2005 and June 2022, 6265 patients with bladder cancer who underwent transurethral resection of bladder tumors (TURBT) and 2831 patients with UTUC who underwent radical nephroureterectomy (RNU) were enrolled in this study. We excluded patients with muscle-invasive or metastatic bladder cancer, advanced UTUC, or those who did not meet the diagnostic criteria for ESRD. A total of 850 patients with ESRD and non-muscle-invasive bladder cancer (NMIBC) who underwent TURBT and 492 patients with ESRD and localized UTUC who underwent RNU were included. Computed tomography (CT) was performed preoperatively in all patients to assess the presence of concurrent lymph nodes or distant metastases. This study was approved by the Institutional Review Board of the Chang Gung Memorial Medical Center (IRB number: 202201112B0).
Follow-up protocol and definition of oncological event
All UC specimens were histologically confirmed by genitourinary pathologists. Tumors were staged according to the American Joint Committee on Cancer tumor-node-metastasis (TNM) classification system. The follow-up protocol included postoperative cystoscopy every 3 months and annual abdominal CT to assess the lymph node status and tumor recurrence. Bone scans, chest CT, and magnetic resonance imaging (MRI) were performed when clinically indicated. Metastasis was defined as local failure at the operative site, distant sites, or regional lymph nodes. Bladder and contralateral recurrences were considered separately in recurrence-free survival analysis. The cause of death was determined through chart reviews or examination of death certificates. Cancer-specific death was defined as death due to concurrent UC metastasis or progressive disease.
Early stage of NMIBC
Abdominal imaging, including upper urinary tract imaging, was performed prior to TURBT. All treatment protocols followed the National Comprehensive Cancer Network (NCCN) guidelines. Because the management of NMIBC differs from that of MIBC, we only recruited patients with NMIBC for this study. Data on recurrence and progression of bladder cancer requiring cystectomy, metachronous UTUC treated with RNU, cancer-specific survival (CSS), and overall survival (OS) were recorded.
Early stage of localized UTUC
RNU with bladder cuff excision was performed for the upper urinary tract tumor as the standard treatment modality [33]. Cystoscopy and abdominal imaging were conducted preoperatively. All treatment protocols adhered to the NCCN guidelines. We only recruited patients with localized UTUC with pathological stages Ta, Tis, T1, or T2. Data on bladder recurrence, bladder cancer requiring cystectomy, metachronous contralateral UTUC requiring RNU, CSS, and OS were recorded.
ESRD and ESA use
The diagnosis of ESRD was confirmed through chart review or evidence of persistent dialysis. Types of ESAs included in the ESA group were epoetin alfa, epoetin beta, and darbepoetin alfa. Patients who had never used ESAs were classified as the non-ESA group.
Statistics
Descriptive statistical analysis results of continuous variables are reported as means and standard deviations, while categorical variables are summarized as n (%). To address systematic differences between the ESA and non-ESA groups, we applied inverse probability of treatment weighting (IPTW). The propensity score was calculated using logistic regression to model ESA use during the baseline period, based on age at the index date, body mass index (BMI), Charlson Comorbidity Index (CCI) score, sex, and T stage.
The algorithm combines weighted estimates from several parametric and nonparametric prediction models based on their predictive accuracy, creating an overall propensity score estimate to enhance the robustness of the analysis. Post-weighting balance of covariates between the treatment groups was assessed using standardized mean differences (SMD), with an imbalance defined as an SMD > 0.1.
Kaplan-Meier curves and log-rank tests were used to compare OS, CSS, and recurrence-free survival rates of bladder recurrence (BR) or contralateral recurrence (CR) between the ESA and non-ESA groups. All statistical tests were two-tailed and conducted at a 5% significance level using R version 3.6.3.
Results
Use of ESA in patients with ESRD and NMIBC did not affect BR, CSS, or OS
A flowchart of the patient selection process is shown in Figure 1. Table 1 illustrates the association between ESA use and clinical as well as pathological characteristics before and after IPTW matching. Of the 850 patients, 477 (56.1%) were in the non-ESA group and 373 (43.9%) in the ESA group. The mean follow-up periods for the non-ESA and ESA groups were 58.99 ± 43.98 months and 55.70 ± 40.72 months, respectively. Age, CCI scores, sex, and T stage were balanced using IPTW (SMD < 0.1 for all variables after IPTW matching).
Figure 1.
Flow chart for patient selection: non-muscle-invasive bladder cancer (NMIBC).
Table 1.
Clinical characteristics of patients before and after inverse probability of treatment weighting (IPTW) in NMIBC
| Variables | Study population before IPTW (n = 850) | After IPTW match | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Non-ESA (N = 477) | ESA (N = 373) | Standardized difference | Non-ESA (N = 849.6) | ESA (N = 846.4) | Standardized difference | |
| Follow up (mean ± SD) | 58.99 ± 43.98 | 55.70 ± 40.72 | 0.078 | 60.85 ± 45.05 | 54.35 ± 40.53 | 0.152 |
| Age (mean ± SD) | 70.78 ± 11.56 | 65.92 ± 11.31 | 0.425 | 68.64 ± 11.95 | 68.43 ± 11.19 | 0.018 |
| CCI scores (mean ± SD) | 7.67 ± 3.36 | 8.23 ± 3.24 | 0.169 | 7.93 ± 3.44 | 7.98 ± 3.06 | 0.015 |
| Gender-Male (%) | 347.0 (72.7) | 188.0 (50.4) | 0.472 | 538.1 (63.3) | 533.0 (63.0) | 0.007 |
| T stage (%) | 0.082 | 0.003 | ||||
| 1 | 302.0 (63.3) | 240.0 (64.3) | 543.6 (64.0) | 540.3 (63.8) | ||
| A | 165.0 (34.6) | 129.0 (34.6) | 291.6 (34.3) | 291.6 (34.4) | ||
| IS | 10.0 (2.1) | 4.0 (1.1) | 14.5 (1.7) | 14.6 (1.7) | ||
| Hb (mean ± SD)* | 12.03 ± 2.14 | 10.71 ± 1.80 | 0.664 | 12.00 ± 2.13 | 10.76 ± 1.79 | 0.629 |
| BR (%)* | 168.0 (35.2) | 142.0 (38.1) | 0.059 | 300.3 (35.3) | 311.6 (36.8) | 0.031 |
| BR times | 0.166 | 0.217 | ||||
| 0 | 254.0 (53.2) | 191.0 (51.2) | 454.5 (53.5) | 446.3 (52.7) | ||
| 1 | 67.0 (25.1) | 56.0 (24.5) | 185.4 (21.8) | 218.7 (25.8) | ||
| 2 | 25.0 (9.4) | 26.0 (11.4) | 85.7 (10.1) | 109.8 (13) | ||
| 3 | 32.0 (12.0) | 17.0 (7.4) | 124 (14.6) | 71.7 (8.5) | ||
| Cystectomy (%)* | 53.0 (11.1) | 54.0 (14.5) | 0.101 | 109.9 (12.9) | 107.3 (12.7) | 0.008 |
| Nephroureterectomy (%)* | 30.0 (6.3) | 63.0 (16.9) | 0.336 | 63.8 (7.5) | 124.1 (14.7) | 0.229 |
| CSS (%)* | 49.0 (10.3) | 40.0 (10.7) | 0.015 | 89.2 (10.5) | 89.5 (10.6) | 0.003 |
| OS (%)* | 91.0 (19.1) | 78.0 (20.9) | 0.046 | 155.7 (18.3) | 176.9 (20.9) | 0.065 |
IPTW, inverse probability of treatment weighting; NMIBC, non-muscle-invasive bladder cancer; ESA, Erythropoiesis-stimulating agents; SD, standard deviation; BMI, body mass index; CCI, Charlson Comorbidity Index; Hgb, hemoglobin; BR, bladder recurrence; CSS, Cancer specific survival; OS, Overall survival.
Observations endpoint without IPTW-adjusted.
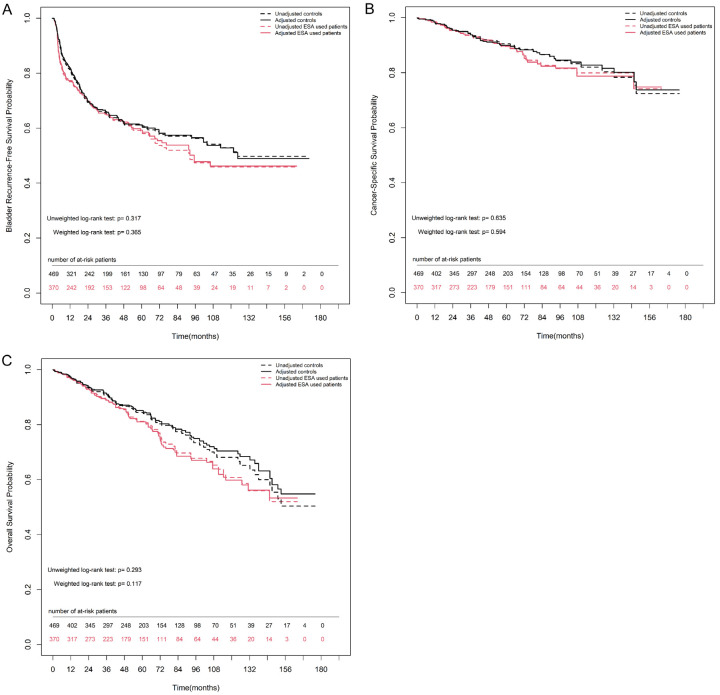
The overall 2-, 5-, and 10-year bladder recurrence-free survival estimates in the non-ESA group were 98.8% (97.8%-99.9%), 97.8% (96.2%-99.3%), and 93.6% (89.6%-97.7%), respectively. Similarly, the overall 2-, 5-, and 10-year bladder recurrence-free survival estimates in the ESA group were 96.1% (94.0%-98.2%), 92.6% (89.4%-95.9%), and 85.9% (80.0%-92.2%), respectively. ESA use did not increase the risk of progression to cystectomy. Figure 2A shows no significant difference in bladder recurrence-free survival rates between the groups, either after IPTW matching (weighted log-rank test, P = 0.365) or before IPTW matching (unweighted log-rank test, P = 0.317).
Figure 2.
Kaplan-Meier estimates for survival rate between non-ESA and ESA group either after or before inverse probability of treatment weighting (IPTW) match in patients with ESRD and NMIBC. A. Bladder recurrence-free survival. B. Cancer-specific survival. C. Overall survival. Numbers along x axis are the numbers of patients remaining in the risk set at each time point.
The overall CSS estimates at 2, 5, and 10 years in the non-ESA group were 95.6% (93.6%-97.6%), 90.5% (87.4%-93.7%), and 82.1% (76.7%-87.8%), respectively. The corresponding estimates for the ESA group at 2, 5, and 10 years were 95.4% (93.1%-97.7%), 89.6% (85.9%-93.4%), and 79.9% (73.5%-86.8%), respectively. Figure 2B shows no significant difference in CSS between the groups, either after IPTW matching (weighted log-rank test, P = 0.594) or before IPTW matching (unweighted log-rank test, P = 0.635).
The overall OS estimates at 2, 5, and 10 years in the non-ESA group were 93.4% (91.0%-95.8%), 84.4% (80.7%-88.3%), and 68.1% (61.7%-75.1%), respectively. The corresponding estimates at 2, 5, and 10 years for the ESA group were 93.0% (90.3%-95.8%), 81.2% (76.5%-86.1%), and 60.8% (52.8%-69.9%), respectively. Figure 2C shows no significant difference in OS between the groups, either after IPTW matching (weighted log-rank test, P = 0.117) or before IPTW matching (unweighted log-rank test, P = 0.293).
Use of ESA in patients with ESRD and localized UTUC did not affect BR, CSS, or OS, except for CR
A flowchart of the patient selection process is shown in Figure 3. Table 2 illustrates the association between ESA use and clinical as well as pathological characteristics before and after IPTW matching. Of the 492 patients, 249 (50.6%) were in the non-ESA group, and 243 (49.4%) were in the ESA group. The mean follow-up periods for the non-ESA group and ESA groups were 69.44 ± 43.06 months and 74.39 ± 44.72 months, respectively. Age, CCI scores, sex, and T stage were balanced using IPTW (SMD < 0.1 for all the above variables after the IPTW matching).
Figure 3.
Flow chart for patient selection: localized upper urinary tract urothelial carcinoma (UTUC).
Table 2.
Clinical characteristics of patients before and after inverse probability of treatment weighting (IPTW) in localized UTUC
| Variables | Study population before IPTW (n = 492) | After IPTW match | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Non-ESA (N = 249) | ESA (N = 243) | Standardized difference | Non-ESA (N = 495.6) | ESA (N = 488.2) | Standardized difference | |
| Follow up (mean ± SD) | 69.44 ± 43.06 | 74.39 ± 44.72 | 0.113 | 70.20 ± 42.90 | 73.23 ± 44.41 | 0.069 |
| Age (mean ± SD) | 69.16 ± 10.58 | 66.11 ± 10.63 | 0.287 | 67.53 ± 10.87 | 67.49 ± 10.39 | 0.004 |
| CCI scores (mean ± SD) | 7.20 ± 3.65 | 7.99 ± 3.38 | 0.224 | 7.66 ± 3.75 | 7.70 ± 3.20 | 0.011 |
| Gender-Male (%) | 127.0 (51.0) | 79.0 (32.5) | 0.382 | 204.2 (41.2) | 201.8 (41.3) | 0.003 |
| T stage (%) | 0.138 | 0.017 | ||||
| 1 | 121.0 (48.6) | 126.0 (51.9) | 249.7 (50.4) | 242.9 (49.8) | ||
| 2 | 82.0 (32.9) | 68.0 (28.0) | 150.1 (30.3) | 151.3 (31.0) | ||
| A | 43.0 (17.3) | 43.0 (17.7) | 85.8 (17.3) | 84.8 (17.4) | ||
| IS | 3.0 (1.2) | 6.0 (2.5) | 9.9 (2.0) | 9.2 (1.9) | ||
| Hb (mean ± SD)* | 11.78 ± 1.83 | 10.18 ± 1.67 | 0.909 | 11.72 (1.76) | 10.20 (1.68) | 0.882 |
| BR (%)* | 83.0 (33.3) | 72.0 (29.6) | 0.080 | 169.2 (34.1) | 143.0 (29.3) | 0.105 |
| CR (%)* | 6.0 (2.4) | 37.0 (15.2) | 0.464 | 18.2 (3.7) | 69.1 (14.1) | 0.374 |
| Cystectomy (%)* | 10.0 (4.0) | 48.0 (19.8) | 0.501 | 21.9 (4.4) | 93.1 (19.1) | 0.467 |
| Nephroureterectomy (%)* | 249.0 (100.0) | 243.0 (100.0) | < 0.001 | 495.6 (100.0) | 488.2 (100.0) | < 0.001 |
| CSS (%)* | 27.0 (10.8) | 18.0 (7.4) | 0.120 | 54.0 (10.9) | 37.0 (7.6) | 0.115 |
| OS (%)* | 46.0 (18.5) | 51.0 (21.0) | 0.063 | 87.1 (17.6) | 108.6 (22.3) | 0.118 |
IPTW, inverse probability of treatment weighting; UTUC, upper urinary tract urothelial carcinoma; ESA, Erythropoiesis-stimulating agents; SD, standard deviation; BMI, body mass index; CCI, Charlson Comorbidity Index; Hgb, hemoglobin; BR, bladder recurrence; CR, contralateral recurrence; CSS, Cancer specific survival; OS, Overall survival.
Observations endpoint without IPTW-adjusted.
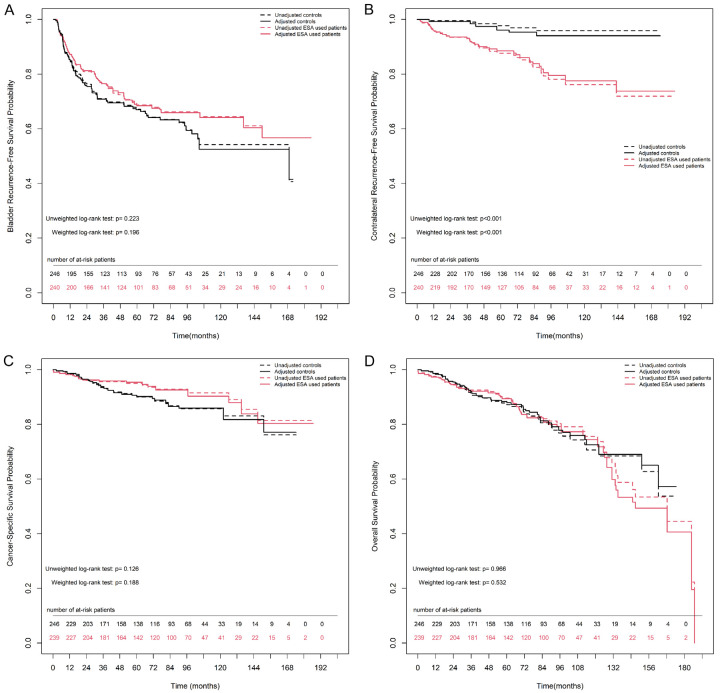
For UTUC, bladder and contralateral recurrences were considered separately to analyze the recurrence-free survival rate. The overall 2-, 5-, and 10-year bladder recurrence-free survival estimates in the non-ESA group were 76.7% (71.4%-82.4%), 67.2% (61.1%-73.9%), and 54.2% (45.8%-64.2%), respectively. Similarly, the overall 2-, 5-, and 10-year bladder recurrence-free survival estimates in the ESA group were 80.9% (76.0%-86.2%), 68.7% (62.6%-75.4%), and 64.5% (57.4%-72.3%), respectively. Figure 4A shows no significant difference in bladder recurrence-free survival rates between the groups, either after IPTW matching (weighted log-rank test, P = 0.196) or before IPTW matching (unweighted log-rank test, P = 0.223).
Figure 4.
Kaplan-Meier estimates for survival rate between non-ESA and ESA group either after or before inverse probability of treatment weighting (IPTW) match in patients with ESRD and localized UTUC. A. Bladder recurrence-free survival. B. Contralateral recurrence-free survival. C. Cancer-specific survival. D. Overall survival. Numbers along x axis are the numbers of patients remaining in the risk set at each time point.
The overall 2-, 5-, and 10-year contralateral recurrence-free survival estimates in the non-ESA group were 99.6% (98.8%-100.0%), 97.7% (95.5%-100.0%), and 95.9% (92.6%-99.3%), respectively. Notably, the overall 2-, 5-, and 10-year estimates for contralateral recurrence-free survival in the ESA group were 93.4% (90.3%-96.7%), 87.6% (83.1%-92.4%), and 76.1% (68.7%-84.3%), respectively. Figure 4B shows a significant difference in contralateral recurrence-free survival rates between the groups, either after IPTW matching (weighted log-rank test, P < 0.001) or before IPTW matching (unweighted log-rank test, P < 0.001).
The overall CSS estimates at 2, 5, and 10 years in the non-ESA group were 96.5% (94.2%-98.9%), 90.6% (86.7%-94.8%), and 85.7% (80.5%-91.3%), respectively. The overall estimates at 2, 5, and 10 years in the ESA group were 96.1% (93.6%-98.6%), 95.0% (92.1%-97.9%), and 91.5% (87.1%-96.1%), respectively. Figure 4C shows no significant difference in CSS between the groups, either after IPTW matching (weighted log-rank test, P = 0.188) or before IPTW matching (unweighted log-rank test, P = 0.126).
The overall OS estimates at 2, 5, and 10 years in the non-ESA group were 95.2% (92.5%-98.0%), 87.8% (83.4%-92.5%), and 70.6% (62.5%-79.8%), respectively. The overall estimates at 2, 5, and 10 years in the ESA group were 94.4% (91.5%-97.4%), 89.5% (85.4%-93.8%), and 75.5% (68.1%-83.8%), respectively. Figure 4D shows no significant difference in OS between the groups, either after IPTW matching (weighted log-rank test, P = 0.532) or before IPTW matching (unweighted log-rank test, P = 0.966).
Discussion
In our study, we found that the use of ESAs did not increase the risk of bladder recurrence and had no significant impact on CSS and OS in patients with ESRD, both in NMIBC and localized UTUC. Among patients with NMIBC, there was no increase in the risk of cancer progression to cystectomy. However, the use of ESAs in patients with ESRD and localized UTUC carries an increased risk of contralateral recurrence. Early clinical trials revealed that the use of ESA was associated with an increased risk of cancer progression or recurrence [20,21]. These early trials of ESA use in cancer patients indeed adopted large ESA doses to attain high hemoglobin (Hgb) targets. In addition, early studies suggested that the effect of large doses of ESAs on survival varied according to the type or stage of malignancy [20,29,30,34]. In a nested case-control study of 4574 dialysis patients whose Hgb was maintained at levels recommended by the KDIGO anemia guidelines, only a higher dose of ESA (> 70 g/week) was associated with a higher risk of cancer [35]. In Taiwan, the upper limit of ESA prescription set by the NHI program was 100 mcg/month. Therefore, the patients enrolled in this study were not exposed to the high ESA doses reported in the literature. The lower dose of ESA used in our study might partly explain why the risk of cancer recurrence did not increase.
At the molecular level, the erythropoietin receptor (EpoR) has been reported in many normal cells and tissues, as well as in various types of tumor cells [15,36]. One of the potential mechanisms for cancer progression associated with ESA use in malignancy [37] is the binding of ESAs to EpoR in tumor cells [38]. Higher EpoR expression has been linked to poorer overall survival [39]. The activation of EpoR is associated with the stimulation of tumor proliferation [40,41], decreased apoptosis [42], and increased resistance to cancer therapy [23,43]. According to Belda-Iniesta et al. [44], EpoR expression tends to be positive in advanced bladder tumors. This may explain why the use of ESAs did not increase the risk of bladder recurrence, CSS, or OS in the early stages of bladder cancer in our study.
In the context of localized UTUC, our findings were consistent with those observed in NMIBC, indicating that the use of ESAs does not increase the risk of bladder recurrence, CSS, or OS. However, in contrast to patients with early-stage bladder cancer, those with localized UTUC have an elevated risk of contralateral recurrence. Unlike bladder cancer, the expression of the EpoR in UTUC is not well documented, and the role of EpoR in UTUC remains largely unknown. The reported risk factors for contralateral recurrence in patients with initial unilateral UTUC receiving RNU include multiple tumors, advanced CKD, and an elevated WBC count [45]. Our results highlight ESA as a risk factor for the contralateral recurrence of localized UTUC in patients with ESRD. Based on our findings, clinicians should be alert when monitoring the contralateral urinary tract. This vigilance is crucial to prevent any recurrence that may arise.
Early clinical trials in cancer patients demonstrated that higher target Hgb levels were associated with increased tumor progression and higher mortality [20,21]. Regulatory authorities in the United States and Europe have suggested that ESAs should be limited to those patients with cancer when Hgb level is ≤ 10 g/dL and avoided when Hgb level of > 12 g/dL [37,46]. Among most malignancies, current trials using optimal ESA doses for CKD have shown no increase in the risk of cancer progression [13]. The mean Hgb level in the ESA group was approximately 10 g/dL, which was lower than that in the non-ESA group. The lack of normalization of Hgb levels may be associated with good OS in our patients.
Our study has several limitations. First, it was a retrospective study. Second, only Asian individuals were included, which may limit the generalizability of our findings, as UC behavior can vary across ethnicities [3]. Third, some studies have suggested a dose-dependent relationship between ESAs and patient outcomes. The lack of precise ESA dosing in our study may have introduced some errors.
In conclusion, our findings provide reassurance regarding the use of ESAs in patients with ESRD and early-stage UC when appropriately administered. However, clinicians should remain vigilant regarding the contralateral recurrence of localized UTUC.
Acknowledgements
We thank Chin Pei Medical Foundation for research material support. We thank the Genomics & Proteomics Core Laboratory, Department of Medical Research, Kaohsiung Chang Gung Memorial Hospital for technical supports. We appreciate the Center for Shockwave Medicine and Tissue Engineering, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan and Chang Gung Medical Foundation Kaohsiung Chang Gung Memorial Hospital Biobank and Tissue Bank Core Lab (CLRPG8L0083) for their excellent technical support. We thank Chih Yun Lin, Hsin Yi Chien, and the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital for statistics work.
Disclosure of conflict of interest
None.
References
- 1.Green DA, Rink M, Xylinas E, Matin SF, Stenzl A, Roupret M, Karakiewicz PI, Scherr DS, Shariat SF. Urothelial carcinoma of the bladder and the upper tract: disparate twins. J Urol. 2013;189:1214–1221. doi: 10.1016/j.juro.2012.05.079. [DOI] [PubMed] [Google Scholar]
- 2.Huang CC, Liu HY, Hsu TW, Lee WC. Updates on the pivotal roles of mitochondria in urothelial carcinoma. Biomedicines. 2022;10:2453. doi: 10.3390/biomedicines10102453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu HY, Chen YT, Huang SC, Wang HJ, Cheng YT, Kang CH, Lee WC, Su YL, Huang CC, Chang YL, Chuang YC, Luo HL, Chiang PH. The prognostic impact of tumor architecture for upper urinary tract urothelial carcinoma: a propensity score-weighted analysis. Front Oncol. 2021;11:613696. doi: 10.3389/fonc.2021.613696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang CH, Yang CM, Yang AH. Renal diagnosis of chronic hemodialysis patients with urinary tract transitional cell carcinoma in Taiwan. Cancer. 2007;109:1487–1492. doi: 10.1002/cncr.22557. [DOI] [PubMed] [Google Scholar]
- 5.Wang TY, Hu CJ, Kuo CW, Chen Y, Lin JL, Yang CW, Yen TH. High incidence and recurrence of transitional cell carcinoma in Taiwanese patients with end-stage renal disease. Nephrology (Carlton) 2011;16:225–231. doi: 10.1111/j.1440-1797.2010.01366.x. [DOI] [PubMed] [Google Scholar]
- 6.Gonwa TA, Corbett WT, Schey HM, Buckalew VM Jr. Analgesic-associated nephropathy and transitional cell carcinoma of the urinary tract. Ann Intern Med. 1980;93:249–252. doi: 10.7326/0003-4819-93-2-249. [DOI] [PubMed] [Google Scholar]
- 7.Chiou HY, Chiou ST, Hsu YH, Chou YL, Tseng CH, Wei ML, Chen CJ. Incidence of transitional cell carcinoma and arsenic in drinking water: a follow-up study of 8,102 residents in an arseniasis-endemic area in northeastern Taiwan. Am J Epidemiol. 2001;153:411–418. doi: 10.1093/aje/153.5.411. [DOI] [PubMed] [Google Scholar]
- 8.Nortier JL, Martinez MC, Schmeiser HH, Arlt VM, Bieler CA, Petein M, Depierreux MF, De Pauw L, Abramowicz D, Vereerstraeten P, Vanherweghem JL. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi) N Engl J Med. 2000;342:1686–1692. doi: 10.1056/NEJM200006083422301. [DOI] [PubMed] [Google Scholar]
- 9.Hortlund M, Arroyo Muhr LS, Storm H, Engholm G, Dillner J, Bzhalava D. Cancer risks after solid organ transplantation and after long-term dialysis. Int J Cancer. 2017;140:1091–1101. doi: 10.1002/ijc.30531. [DOI] [PubMed] [Google Scholar]
- 10.KDOQI; National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis. 2006;47(Suppl 3):S11–145. doi: 10.1053/j.ajkd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23:1631–1634. doi: 10.1681/ASN.2011111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan FA, Shukla AN, Joshi SC. Anaemia and cancer treatment: a conceptual change. Singapore Med J. 2008;49:759–764. [PubMed] [Google Scholar]
- 13.Choi MJ, Yee J. Erythropoiesis-stimulating agents and cancer: myth or truth. Adv Chronic Kidney Dis. 2019;26:221–224. doi: 10.1053/j.ackd.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Kalyani P, Jamil K. A study on biochemical facet of anemia in cancers: a strong link between erythropoietin and tumor necrosis factor alpha in anemic cancer patients. Indian J Cancer. 2015;52:127–132. doi: 10.4103/0019-509X.175579. [DOI] [PubMed] [Google Scholar]
- 15.Udupa KB. Functional significance of erythropoietin receptor on tumor cells. World J Gastroenterol. 2006;12:7460–7462. doi: 10.3748/wjg.v12.i46.7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winearls CG, Oliver DO, Pippard MJ, Reid C, Downing MR, Cotes PM. Effect of human erythropoietin derived from recombinant DNA on the anaemia of patients maintained by chronic haemodialysis. Lancet. 1986;2:1175–1178. doi: 10.1016/s0140-6736(86)92192-6. [DOI] [PubMed] [Google Scholar]
- 17.Chung EY, Palmer SC, Saglimbene VM, Craig JC, Tonelli M, Strippoli GF. Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: a network meta-analysis. Cochrane Database Syst Rev. 2023;2:CD010590. doi: 10.1002/14651858.CD010590.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 19.Tonia T, Mettler A, Robert N, Schwarzer G, Seidenfeld J, Weingart O, Hyde C, Engert A, Bohlius J. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev. 2012;12:CD003407. doi: 10.1002/14651858.CD003407.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, Zwahlen M, Clarke M, Weingart O, Kluge S, Piper M, Rades D, Steensma DP, Djulbegovic B, Fey MF, Ray-Coquard I, Machtay M, Moebus V, Thomas G, Untch M, Schumacher M, Egger M, Engert A. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet. 2009;373:1532–1542. doi: 10.1016/S0140-6736(09)60502-X. [DOI] [PubMed] [Google Scholar]
- 21.Leyland-Jones B, Semiglazov V, Pawlicki M, Pienkowski T, Tjulandin S, Manikhas G, Makhson A, Roth A, Dodwell D, Baselga J, Biakhov M, Valuckas K, Voznyi E, Liu X, Vercammen E. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J. Clin. Oncol. 2005;23:5960–5972. doi: 10.1200/JCO.2005.06.150. [DOI] [PubMed] [Google Scholar]
- 22.Henke M, Laszig R, Rübe C, Schäfer U, Haase KD, Schilcher B, Mose S, Beer KT, Burger U, Dougherty C, Frommhold H. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003;362:1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- 23.Debeljak N, Solar P, Sytkowski AJ. Erythropoietin and cancer: the unintended consequences of anemia correction. Front Immunol. 2014;5:563. doi: 10.3389/fimmu.2014.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bohlius J, Bohlke K, Castelli R, Djulbegovic B, Lustberg MB, Martino M, Mountzios G, Peswani N, Porter L, Tanaka TN, Trifirò G, Yang H, Lazo-Langner A. Management of cancer-associated anemia with erythropoiesis-stimulating agents: ASCO/ASH clinical practice guideline update. J. Clin. Oncol. 2019;37:1336–1351. doi: 10.1200/JCO.18.02142. [DOI] [PubMed] [Google Scholar]
- 25.Summary of recommendation statements. Kidney Int Suppl (2011) 2012;2:283–287. doi: 10.1038/kisup.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong Z, Xu Z, Duan Y, Sun X, Qi B. The effect of erythropoiesis‑stimulating agents on lung cancer patients: a meta‑analysis. Clin Exp Med. 2024;24:150. doi: 10.1007/s10238-024-01391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bozzini C, Busti F, Marchi G, Vianello A, Cerchione C, Martinelli G, Girelli D. Anemia in patients receiving anticancer treatments: focus on novel therapeutic approaches. Front Oncol. 2024;14:1380358. doi: 10.3389/fonc.2024.1380358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hedley BD, Allan AL, Xenocostas A. The role of erythropoietin and erythropoiesis-stimulating agents in tumor progression. Clin Cancer Res. 2011;17:6373–6380. doi: 10.1158/1078-0432.CCR-10-2577. [DOI] [PubMed] [Google Scholar]
- 29.Aapro M, Moebus V, Nitz U, O’Shaughnessy J, Pronzato P, Untch M, Tomita D, Bohac C, Leyland-Jones B. Safety and efficacy outcomes with erythropoiesis-stimulating agents in patients with breast cancer: a meta-analysis. Ann Oncol. 2015;26:688–695. doi: 10.1093/annonc/mdu579. [DOI] [PubMed] [Google Scholar]
- 30.Leyland-Jones B BEST Investigators and Study Group. Breast cancer trial with erythropoietin terminated unexpectedly. Lancet Oncol. 2003;4:459–460. doi: 10.1016/s1470-2045(03)01163-x. [DOI] [PubMed] [Google Scholar]
- 31.Shao SC, Chan YY, Kao Yang YH, Lin SJ, Hung MJ, Chien RN, Lai CC, Lai EC. The Chang Gung research database-a multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol Drug Saf. 2019;28:593–600. doi: 10.1002/pds.4713. [DOI] [PubMed] [Google Scholar]
- 32.Cheng TM. Taiwan’s new national health insurance program: genesis and experience so far. Health Aff (Millwood) 2003;22:61–76. doi: 10.1377/hlthaff.22.3.61. [DOI] [PubMed] [Google Scholar]
- 33.Luo HL, Chen TS, Wu WJ. The cancer behavior and current treatment strategy for upper urinary tract cancer. Urological Science. 2022;33:161–169. [Google Scholar]
- 34.Vansteenkiste J, Glaspy J, Henry D, Ludwig H, Pirker R, Tomita D, Collins H, Crawford J. Benefits and risks of using erythropoiesis-stimulating agents (ESAs) in lung cancer patients: study-level and patient-level meta-analyses. Lung Cancer. 2012;76:478–485. doi: 10.1016/j.lungcan.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 35.Rene E, Lazrak HH, Laurin LP, Elftouh N, Vallee M, Lafrance JP. Association of erythropoiesis-stimulating agents and the incidence risk of cancer diagnosis among chronic dialysis patients: a nested case-control study. Nephrol Dial Transplant. 2017;32:1047–1052. doi: 10.1093/ndt/gfw268. [DOI] [PubMed] [Google Scholar]
- 36.Acs G, Acs P, Beckwith SM, Pitts RL, Clements E, Wong K, Verma A. Erythropoietin and erythropoietin receptor expression in human cancer. Cancer Res. 2001;61:3561–3565. [PubMed] [Google Scholar]
- 37.Thavarajah S, Choi MJ. The use of erythropoiesis-stimulating agents in patients with CKD and cancer: a clinical approach. Am J Kidney Dis. 2019;74:667–674. doi: 10.1053/j.ajkd.2019.04.022. [DOI] [PubMed] [Google Scholar]
- 38.Elliott S, Sinclair AM. The effect of erythropoietin on normal and neoplastic cells. Biologics. 2012;6:163–189. doi: 10.2147/BTT.S32281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Zhu Y, Wang S, Feng YC, Li H. Erythropoietin receptor is a risk factor for prognosis: a potential biomarker in lung adenocarcinoma. Pathol Res Pract. 2023;251:154891. doi: 10.1016/j.prp.2023.154891. [DOI] [PubMed] [Google Scholar]
- 40.Ghezzi P, Mengozzi M. Activities of erythropoietin on tumors: an immunological perspective. Eur J Immunol. 2007;37:1427–1430. doi: 10.1002/eji.200737401. [DOI] [PubMed] [Google Scholar]
- 41.Lee AS, Kim DH, Lee JE, Jung YJ, Kang KP, Lee S, Park SK, Kwak JY, Lee SY, Lim ST, Sung MJ, Yoon SR, Kim W. Erythropoietin induces lymph node lymphangiogenesis and lymph node tumor metastasis. Cancer Res. 2011;71:4506–4517. doi: 10.1158/0008-5472.CAN-10-3787. [DOI] [PubMed] [Google Scholar]
- 42.Hazzan AD, Shah HH, Hong S, Sakhiya V, Wanchoo R, Fishbane S. Treatment with erythropoiesis-stimulating agents in chronic kidney disease patients with cancer. Kidney Int. 2014;86:34–39. doi: 10.1038/ki.2013.528. [DOI] [PubMed] [Google Scholar]
- 43.Glaspy J. Current status of use of erythropoietic agents in cancer patients. Semin Thromb Hemost. 2014;40:306–312. doi: 10.1055/s-0034-1370768. [DOI] [PubMed] [Google Scholar]
- 44.Belda-Iniesta C, Perona R, Carpeño Jde C, Cejas P, Casado E, Manguan-García C, Ibanez de Caceres I, Sanchez-Perez I, Andreu FB, Ferreira JA, Aguilera A, de la Peña J, Perez-Sánchez E, Madero R, Feliu J, Sereno M, González-Barón M. Human recombinant erythropoietin does not promote cancer growth in presence of functional receptors expressed in cancer cells. Cancer Biol Ther. 2007;6:1600–1605. doi: 10.4161/cbt.6.10.4726. [DOI] [PubMed] [Google Scholar]
- 45.Chien TM, Lee HY, Singla N, Margulis V, Lotan Y, Woldu SL, Huang CN, Li CC, Ke HL, Li WM, Li CY, Huang AM, Yang SF, Tu HP, Wu WJ, Yeh HC. Prognostic factors for contralateral recurrence of upper tract urothelial carcinoma after nephroureterectomy: a large multiregional study. Cancers (Basel) 2021;13:5935. doi: 10.3390/cancers13235935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujisaka Y, Sugiyama T, Saito H, Nagase S, Kudoh S, Endo M, Sakai H, Ohashi Y, Saijo N. Randomised, phase III trial of epoetin-beta to treat chemotherapy-induced anaemia according to the EU regulation. Br J Cancer. 2011;105:1267–1272. doi: 10.1038/bjc.2011.395. [DOI] [PMC free article] [PubMed] [Google Scholar]