Abstract
Hepatocellular carcinoma (HCC), which originates from hepatocytes, accounts for the majority of primary liver cancers. Globally, HCC ranks among the most common cancers and is a leading cause of cancer-related deaths. Obesity, a growing health issue worldwide, is increasingly recognized as a critical risk factor for HCC, influenced by both epidemiological and clinical factors. Adipokines, secreted by adipocytes, have been shown to play pivotal roles in the tumor microenvironment, affecting cancer progression, metastasis, and resistance to therapies through various signaling mechanisms. Despite inconsistencies in certain findings, a substantial body of research supports the significant role of adipokines in HCC development. This review focuses on exploring newly identified adipokines and their mechanisms in HCC, with the goal of uncovering potential therapeutic targets for improved management and treatment strategies.
Keywords: Adipokines, hepatocellular carcinoma, tumorigenesis, prognosis
Introduction
Cancer continues to pose a significant global health challenge, with its incidence rate escalating worldwide [1,2]. By 2025, it is projected that over one million individuals will be diagnosed with cancer annually. Liver cancer stands as one of the predominant malignant tumors, primarily comprising two pathological histological types: hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (iCCA) [3]. There is increasing evidence that adipose tissue, inflammation, non-alcoholic fatty liver disease (NAFLD), and alcoholic fatty liver disease (AFLD) are closely linked to the risk of HCC occurrence [4-6]. Therefore, maintaining a healthy lifestyle and avoiding hepatocellular carcinoma risk factors are additional strategies for the prevention of HCC [7]. Currently, in blood-based surveillance tests, detection of elevated serum alpha-fetoprotein levels is usually used as an adjunct to liver ultrasonography, but its use as a surveillance test for HCC remains controversial because of its low sensitivity of 40-60% and specificity of 80-90% [8].
Adipocytes function as a crucial endocrine organ, secreting various functional adipokines, peptides, and non-coding RNAs. These act on adipose tissue itself or other distant tissues or organs via autocrine, paracrine, or endocrine mechanisms [9-11]. Adipokines exhibit diverse roles across various cancer types, often influencing cell proliferation, migration, invasion, and metastasis pathways in contradictory ways. This complexity necessitates further research into the role of adipokines in the tumor environment. In recent years, the number of identified adipokines has surged, including adiponectin, resistin, visfatin, apelin, retinol-binding protein-4, serum amyloid A, plasminogen activator inhibitor-1, angiotensinogen, vaspin, omentin, chemerin, and zinc-alpha2-glycoprotein. Pro-inflammatory cytokines produced by macrophage infiltration into white adipose tissue, such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), are also classified as adipokines [12].
It is widely recognized that metabolic disorders elevate the risk of cancer and its associated mortality [13]. Growing evidence indicates that these disorders can also instigate HCC and are linked to its progression [14]. In recent years, the correlation between metabolic syndrome (MS), a cluster of conditions encompassing insulin resistance, obesity, hypertension, and hyperlipidemia, and malignancies has gained significant attention [15]. A comprehensive review and meta-analysis of data on the prevention of future cancer post-bariatric surgery revealed a reduced incidence of subsequent HCC [16]. Several large-scale epidemiological investigations have demonstrated that individuals with overweight and obesity exhibit elevated risks of liver cancer compared to the general populace [17,18]. Furthermore, patients with obesity not only face an increased likelihood of developing HCC but also a heightened risk of liver cancer-related mortality [17,19].
Adipose tissue, with its inherent metabolic activity, secretes cytokines known as adipokines. These adipokines can either promote or inhibit hepatocarcinogenesis. They play a crucial role in regulating cell growth, proliferation, the cell cycle, angiogenesis, and tumor growth and metastasis [20]. For instance, substantial evidence suggests that leptin and adiponectin may exacerbate steatohepatitis in patients with viral hepatitis, thereby increasing the likelihood of HCC [21]. Adipokines are believed to facilitate cancer progression by enhancing growth, inflammation, migration, and anti-apoptotic mechanisms, which in turn promote cancer metastasis [22]. Recent studies have revealed that a deficiency in p62 in adipose tissue supports the nutritional supply of cancer cells by inhibiting energy utilization pathways in adipocytes [23]. The systemic and hepatic molecular mechanisms involved in obesity and NAFLD-induced hepatocarcinogenesis, as well as potential early markers of hepatocellular carcinoma, are currently under extensive investigation. Adiponectin and leptin are recognized adipokines that can influence the regulation of liver cancer. Adiponectin induces apoptosis and inhibits hepatic stellate cells, affects Kupffer cell survival, and promotes the transformation to M2 phenotype to release mediators that stimulate M1 macrophage apoptosis [24]. Leptin is reported to stimulate liver cancer cell proliferation and inhibit apoptosis [25]. Leptin induces autophagy through the p53/Foxo3A axis, thereby eliminating cancer cell apoptosis [26]. However, data on the role of certain adipokines in the pathogenesis of HCC in patients with viral hepatitis are contradictory. For example, visfatin is elevated in CHC and HCC patients, but increased visfatin concentration is also associated with reduced necroinflammatory activity in the liver [27,28]. On the basis of above, we explore and summarize the effects of novel adipokines on HCC in this review (Table 1).
Table 1.
Clinical studies concerning the changes of novel adipokines in HCC patients
| Adipokines | Subjects | Sample size | Change In HCC | Promoting(+)/inhibitory(-) effect of cancer | Prognosis | Study | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| HCC | Healthy controls | ||||||
| Metrnl/IL-41 | Human | 176 | 19 | Upregulated in HCC tissues | + | The serum IL-41 level was lower in patients with late recurrence (2 years after resection) than in patients with early recurrence and death | Yazhao Li et al., 2024 [30] |
| RBP4 | Human | 290 | 269 | Reduction in both liver tissues and serum samples | - | The serum RBP4 level exhibited a negative correlation with overall survival time | Fengjie Wan et al., 2024 [48] |
| Irisin | Human | 36 | 7 | Reduced irisin concentrations | + | Low serum irisin levels are associated with high CCI scores after hepatectomy in HCC patients | Monika Pazgan-Simon et al., 2020 [71] |
| Human | 69 | 20 | Decline of serum irisin | M Pazgan-Simon et al., 2020 [72] | |||
| Vaspin | Human | 36 | 7 | Higher concentrations of vaspin | + | Unkown | Monika Pazgan-Simon et al., 2020 [71] |
| Human | 37 | 20 | Serum vaspin were higher | Walaa Abdelhamed, 2023 [86] | |||
| LCN-2 | Human | 167 | 106 | Levels in HCC sera were higher | - | Blood NGAL levels predict the rate of survival, even in patients presenting with HCC | Lin Du, 2022 [151] |
| sFRP4 | Human | 142 | 33 | Serum sFRP-4 levels higher | - | Unkown | Cheng Xu, 2015 [122] |
| ApoM | Human | 36 | 64 | The plasma relative ApoM levels was higher | - | Multivariate COX proportional hazard analysis suggested that high serum AZGP1 level was associated with better survival of HCC patients | Jingting Jiang, 2011 [136] |
Metrnl/meteorin-β/IL-41
Meteorin-like (Metrnl), also known as meteorin-β or IL-41, is a novel secreted protein that shares homology with the neurotrophic factor metrnl, which is also referred to as cometin, subfatin, and interleukin 39. This nomenclature reflects its diverse functions, including neurotrophic action, adipokine activity, and potential roles as a cytokine [29]. Identified as a potential diagnostic marker for hepatocellular carcinoma (HCC), Metrnl exhibits marked upregulation in HCC tissues and high expression in the serum of patients with alpha-fetoprotein (AFP)-negative HCC, demonstrating its diagnostic value [30]. Consequently, Metrnl could serve as a novel serum marker for the diagnosis of AFP-negative HCC.
Immunohistochemistry analysis reveals that high tissue expression of Metrnl correlates with early recurrence, mortality, multiple metastases, and microvascular invasion post-HCC resection, suggesting that Metrnl could be a predictive factor for poor prognosis and malignant progression of HCC [30]. In terms of treatment, Metrnl may modulate the response of liver cancer patients to chemotherapy drugs. It has been shown to mitigate doxorubicin-induced cardiotoxicity without compromising its anticancer effects [31]. Du et al. demonstrated that Metrnl overexpression significantly inhibits the release of pro-inflammatory cytokines TNF and IL-1β, reduces chemokine-dependent inflammatory cell infiltration into the liver, and ameliorates acute hepatitis in mice [32]. In contrast, a study by Liu et al. found reduced serum Metrnl levels in adult non-alcoholic fatty liver disease (NAFLD) patients, suggesting that Metrnl may act as a protective factor in the pathogenesis of NAFLD [33].
Retinol binding protein 4 (RBP4)
RBP4 is a 21 kDa monomeric binding protein crucial for transporting retinol (vitamin A) in the blood, primarily secreted by the liver [34,35]. Research has identified RBP4 as a novel adipokine associated with insulin resistance, which may impact glucose homeostasis and induce inflammation in mice [36]. Further studies have linked RBP4 to various metabolic diseases, including type 2 diabetes, hepatic steatosis, and steatohepatitis [37-39], all of which are closely related to primary liver cancer. Additionally, RBP4 levels vary in certain tumors, such as head and neck cancer, ovarian cancer, colorectal cancer, breast cancer, and acute lymphoblastic leukemia [40-45]. Relevant research has demonstrated that serum RBP4 concentration in early HCV-infected patients is inversely proportional to disease severity; as liver fibrosis escalates, serum RBP4 concentration decreases [46,47].
Research indicates that RBP4 levels in both liver tissue and serum samples from HCC patients are significantly diminished [48,49]. Moritoshi et al. discovered a strong correlation between low RBP4 expression in HCC tissues and the incidence of HCC. They identified differentially expressed genes associated with chronic viral hepatitis in HCC through differential gene display analysis, revealing insufficient expression of the RBP4 gene in cancer tissues from 12 HCC patients [50]. Consequently, RBP4 holds significant potential as a biomarker due to its substantial diagnostic value, serving as a supplement to AFP in HCC diagnosis. Recently, Li M et al. employed online bioinformatics tools to analyze RBP4, revealing significant downregulation of RBP4 expression in both HCC and cholangiocarcinoma. This downregulation was found to be indicative of poor prognosis for HCC and was closely linked to immune cell infiltration within the tumor microenvironment [51]. Furthermore, studies have demonstrated a significant correlation between serum RBP4 levels in HCC patients and factors such as cirrhosis, tumor size, venous invasion, disease stage, and poor prognosis [15]. Therefore, it is anticipated that RBP4 could be integrated into a combined prognostic model as a prognostic biomarker, significantly enhancing the prognostic efficiency of HCC.
RBP4 expression has also been linked to immune cell infiltration, particularly during inflammation [52]. This protein can stimulate macrophages and CD4+ T cells via the TLR4 and JNK-dependent pathway, resulting in the production of cytokines such as TNF-α, IL-1β, and IL-6 [53,54]. Consequently, we propose that RBP4 may offer a protective effect against HCC through metabolic regulation and immune cell infiltration. Additionally, it may contribute to the development of precancerous liver lesions via exosome-mediated macrophage activation. Interestingly, RBP4 is inversely correlated with the severity of liver fibrosis. This correlation could be attributed to diminished RBP4 levels, which are involved in the overactivation of hepatic stellate cells and the accumulation of type I collagen in the liver, promoting the progression of liver fibrosis [47,55], potentially leading to cirrhosis or HCC.
Irisin
Irisin, the ectodomain of fibronectin type III domain-containing protein 5 (FNDC5) [56-58], was initially identified in skeletal muscle [59]. However, it has been demonstrated to be ubiquitous, with hepatocytes, Kupffer cells, and sinusoidal endothelial cells also capable of producing it, albeit in small quantities. The role of irisin in the liver remains unclear [60]. Studies have shown a significant decrease in irisin expression in subjects with steatohepatitis [61,62] and in mice models of liver injury induced by ischemia-reperfusion (I/R) [63,64]. Long-term exercise-induced irisin or supplementation with exogenous recombinant irisin (r-irisin) has been found to protect against non-alcoholic fatty liver disease (NAFLD) [65,66], hepatic glucose disorder [67,68], and I/R-induced liver injury [69]. These findings suggest a potential role for irisin in myokine-hepatokine crosstalk [70].
Studies have indicated that serum irisin levels in patients with HCC are diminished, particularly in those with advanced disease grades, and are also negatively correlated with the severity of liver dysfunction [71,72]. However, a study by Aydin et al. did not detect a significant change in irisin protein in HCC tissues [73]. In contrast, Melania et al. demonstrated that plasma irisin levels were reduced in HCC patients [74]. Furthermore, a recent study suggested that the liver could be a target tissue for irisin and may contribute to its metabolic clearance in conjunction with the kidney [75]. Given that irisin is primarily expressed in skeletal muscle, and that muscle loss progresses as liver disease severity increases, this could potentially explain the reduction in irisin concentration observed in patients with cirrhosis and HCC [76].
A study indicated that, among HCC patients undergoing hepatectomy, the AFP level in the group with low serum irisin was over 14 times higher than that in the group with high serum irisin [77]. Furthermore, a preoperatively low serum irisin level was significantly correlated with a high CCI score post-hepatectomy. Consequently, irisin could be utilized in conjunction with AFP for the diagnosis of HCC, and preoperative serum irisin levels could serve as a predictor of the overall risk of postoperative complications.
A recent study demonstrated that exercise-induced irisin competitively inhibits the binding of myeloid differentiation factor 2 (MD2) and Toll-like receptor 4 by forming a complex with MD2 in hepatocytes, thereby suppressing the inflammatory response [78]. The mRNA expression of SCD-1, NOTCH1, tumor necrosis factor-α, and interleukin-6 is elevated in the livers of HCC patients. Overexpression of FNDC5/irisin in HCC-liver tissues correlates with genes involved in adipogenesis, inflammation, and cancer mediators, suggesting that this hormone may have a protective effect on liver injury [74]. However, elevated levels of irisin may significantly increase cell proliferation, invasion, and migration ability by partially activating the PI3K/AKT pathway, indicating its protective role in hepatocellular carcinoma cells. This may promote liver cancer progression and reduce sensitivity to chemotherapy [79]. On the other hand, it has also been shown that irisin may improve insulin resistance, thereby reducing the risk of developing HCC in patients with viral hepatitis [80-82].
Vaspin
The novel adipokine vaspin, also known as Visceral Adipose Tissue-Derived Serine Protease Inhibitor, was initially identified in obese OLETF rats [83]. Primarily expressed in visceral adipose tissue, vaspin enhances glucose tolerance and insulin sensitivity while simultaneously reducing the production of proinflammatory cytokines. Research indicates that serum vaspin levels decrease in patients with non-advanced liver fibrosis due to chronic hepatitis C (CHC), and an increase in vaspin levels correlates with the progression of liver fibrosis [84]. The heightened expression of vaspin in patients with pronounced fibrosis may serve as a compensatory mechanism, offering protection against further liver injury and fibrosis progression. This hypothesis is supported by observations that vaspin can suppress the expression of leptin, TNF-α, and resistin [85]. However, in a study conducted by Monika et al., vaspin concentration was significantly elevated in HCC patients compared to healthy controls, yet it did not emerge as a significant predictor of HCC [71]. Furthermore, it has been determined that the notable increase in serum vaspin levels in HCC patients is associated with tumor staging, suggesting its potential as a biomarker for HCC [86].
Vaspin exhibits anti-apoptotic effects in various cell types, including ovarian cells, osteoblasts, macrophages, aortic endothelial cells, hepatocellular carcinoma cells, and cardiomyocytes. It has also been found to promote the proliferation of normal and cancerous ovarian cells, preadipocytes, hepatocellular carcinoma cells, and bone mesenchymal stem cells [87]. Several mechanisms have been proposed to elucidate how vaspin can facilitate tumor development, including insulin resistance, stimulation of growth in cells with malignant potential, and prevention of apoptosis through cellular pathways [88].
Lipocalin 2 (LCN-2)
Lipocalin-2 (LCN-2), also known as neutrophil gelatinase-associated lipocalin (NGAL), is a small, secreted protein and iron-binding glycoprotein that is significantly upregulated during the progression of severe cancers [89]. Multiple in vivo and in vitro studies have consistently reported elevated LCN-2 levels in the tissues and serum of hepatocellular carcinoma (HCC) patients compared to healthy individuals [90]. Moreover, LCN-2 is highly expressed in human HCC tissues and in the livers of various mouse models of HCC, triggered by factors such as inflammation or genotoxicity [91-93].
Recent findings suggest that blood LCN-2 levels serve as a reliable prognostic marker for survival in chronic liver disease complicated by HCC [94]. The upregulation of LCN-2 and its receptor, LCN2R (also known as solute carrier family 22 member 17, SLC22A17), in HCC has been proposed as a prognostic indicator for overall survival [95]. Additionally, studies have shown that LCN-2 is primarily produced by damaged tumor AFP-positive hepatocytes, inflammatory infiltration, and hepatic progenitor cells, highlighting its potential use alongside AFP in HCC diagnosis [96]. Notably, LCN-2 levels above 225 ng/ml offer superior diagnostic accuracy compared to AFP, especially in distinguishing HCC from cirrhosis [97].
LCN-2 has also been found to inhibit the proliferation, invasion, and metastasis of HCC, positioning it as a potential metastatic suppressor and therapeutic target [98-100]. Furthermore, its overexpression induces apoptosis in HCC cells through mitochondrial activity, further reinforcing its therapeutic potential [99]. LCN-2 has been reported to negatively regulate the epithelial-mesenchymal transition (EMT) in HCC cells, suggesting its role in suppressing metastasis warrants further investigation [101].
Comprehensive studies have confirmed the crucial role of LCN-2 in HCC progression, with its expression validated in clinical samples, including patient serum and tumor tissues. Targeting LCN-2 therapeutically has shown strong antitumor effects, such as inhibiting angiogenesis, enhancing sensitivity to sorafenib, and promoting natural killer cell cytotoxicity [102]. In terms of signaling pathways, LCN-2 regulates EMT at least partially via the EGF (or TGF-β1)/LCN2/Twist1 axis [98]. Additionally, LCN-2-induced cell migration has been linked to activation of the Met/FAK cascade [103]. The apoptotic characteristics induced by LCN-2 include the cleavage of caspase-9, -8, -3, and PARP proteins, along with a reduction in mitochondrial membrane potential (MMP). It also downregulates Bcl-2 and upregulates Bax expression, contributing to apoptosis. Importantly, treatment with a neutralizing antibody significantly diminished LCN-2-induced apoptosis, suggesting that LCN-2 overexpression could effectively induce apoptosis in HCC cells, making it a promising therapeutic strategy [99].
In summary, LCN-2 plays a pivotal role in the pathogenesis and progression of HCC. Its elevated expression in HCC tissues and serum, along with its multifaceted role in inhibiting metastasis, promoting apoptosis, and influencing key signaling pathways, underscores its potential as both a diagnostic and therapeutic target in HCC. Further research is essential to fully explore LCN-2’s clinical applications and therapeutic implications.
Neuregulin 4 (NRG4)
Neuregulin 4 (NRG4), a member of the epidermal growth factor (EGF) family, binds to and activates the receptor tyrosine kinase of Erb-B2 Receptor Tyrosine Kinase 4 (ErbB4) [104]. This secreted factor, originating from adipose tissue, influences liver function, thereby maintaining metabolic health in mice [105-107]. A reduction in adipose NRG4 expression and plasma levels correlates with human obesity, insulin resistance, and non-alcoholic fatty liver disease (NAFLD) [107-110]. Furthermore, NRG4 plays a significant role in the initiation and progression of various cancers, including prostate cancer, breast cancer, and gastrointestinal malignant lymphoma [111-113].
Woo Sun Rou et al. demonstrated that Erb-B2 Receptor Tyrosine Kinase 2 (ERBB2) and NRG4 serve as independent prognostic indicators for tumor recurrence. Moreover, the combined assessment of serum levels of ERBB2, NRG4, and mitogen-inducible gene 6 (MIG6) offers a more accurate prediction of mortality in HCC patients compared to AFP [114]. Downregulation of NRG4 expression has been observed in HCC [115]. Emerging evidence suggests a novel role for NRG4 in regulating the liver immune microenvironment and the development of NASH-associated HCC. Several RNA sequencing and single-cell transcriptomic studies have revealed that NRG4 deficiency promotes the induction of NASH-associated macrophages (NAMs) and exacerbates intrahepatic CD8+ T cell exhaustion following diet-induced NASH in mice. Conversely, transgenic or recombinant adeno-associated virus (AAV)-mediated overexpression of NRG4 inhibits NAM marker expression and genes associated with T cell exhaustion. Thus, NRG4 may function as a hormone checkpoint to suppress tumor-prone immune features [115].
Previous research has shown that the cellular receptor ErbB4 for NRG4 is present on macrophages and mediates NRG4’s effects on macrophage survival and function [116,117]. Interestingly, liver-specific ErbB4 knockout mice exhibit increased susceptibility to liver injury and diethylnitrosamine (DEN)-induced HCC [118]. The expression of ERBB4 is frequently diminished in cancers and cancer cell lines [119], further supporting a potential tumor-suppressive role for NRG4-ERBB4 signaling.
In conclusion, the ability of NRG4 to influence multiple aspects of liver biology, including hepatic lipid metabolism, hepatocyte injury, and the immune microenvironment, strongly suggests that this hormone pathway may serve as an attractive target for therapeutic intervention in NASH-associated HCC.
Secreted frizzled-related protein 4 (sFRP4)
sFRP4, a member of the secreted frizzled-related protein (SFRP) family, contains a cysteine-rich domain that resembles the putative Wnt binding site of coiled-coil proteins [120]. Several studies have identified sFRP4 as a tumor suppressor in various cancers [121]. Xu et al. reported that sFRP4 levels were significantly upregulated in the serum of HCC patients, and combining sFRP4 with AFP enhanced the diagnostic accuracy for HCC [122].
In approximately 30% of HCC patients, the Wnt/β-catenin signaling pathway is overactivated [123]. sFRP4 can regulate this pathway by directly binding to Wnt ligands, thereby preventing their interaction with Wnt receptors [124,125]. Overexpression of sFRP4 increases glycogen synthase kinase-3β (GSK-3β) expression and decreases β-catenin levels, indicating that sFRP4 inhibits the Wnt/β-catenin signaling cascade [126]. As a result, sFRP4 suppresses the malignant behavior of HCC cells by blocking this pathway, suggesting that it plays a negative regulatory role in HCC carcinogenesis. Therefore, sFRP4 holds potential as a therapeutic target for HCC treatment.
Apolipoprotein M (ApoM)
The majority of ApoM is found in high-density lipoprotein (HDL), with smaller amounts present in low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), and chylomicrons (CM) [127]. Only about 5% of HDL particles and less than 2% of LDL particles contain ApoM, with a significant correlation observed between ApoM levels and HDL concentrations [128,129]. Research has shown that ApoM can be modulated by various inflammatory factors, such as platelet-activating factor (PAF) and lipopolysaccharides [130-134].
Through suppression subtractive hybridization and cDNA microarray analysis, elevated ApoM expression was observed in HCC samples [135]. However, further studies revealed that HCC tissues have a reduced capacity to synthesize ApoM compared to adjacent non-tumor tissues, suggesting that the increased ApoM levels primarily originate from the surrounding non-tumorous areas [136]. Additionally, plasma ApoM levels in HCC patients were found to be higher than those in healthy individuals but lower than those in patients with chronic hepatitis or cirrhosis. These variations may be linked to immune system abnormalities or other underlying factors [137].
Bai et al. demonstrated that deletion of the ApoM gene in mice exposed to N-nitrosodiethylamine accelerated liver cancer development, highlighting ApoM’s antitumor role in HCC progression [138]. Xu et al. found that PAF significantly increased ApoM mRNA levels in HepG2 cell cultures, suggesting that the elevated ApoM levels in HCC patients may be mediated through PAF-induced inflammatory responses [139]. Furthermore, inhibiting ApoM gene expression selectively regulated phosphofructokinase liver type (PFKL) via the transcription factor SREBF1, enhancing PFKL promoter activity. This likely explains the significant increase in proliferation, migration, and invasion observed in hepatoma cells with ApoM knockdown [140]. Reduced ApoM expression has also been shown to promote hepatic lipid accumulation by inhibiting autophagy in hepatocytes [141]. Overexpression of ApoM was found to downregulate MUC1 (a gene associated with ferroptosis suppression) by upregulating miR-4489, which disrupted the GSH-GPX4 antiferroptotic mechanism, thereby inhibiting hepatoma cell progression [142].
Zinc-alpha2-glycoprotein (AZGP1)
AZGP1, a 42 kDa soluble secreted protein, exhibits structural homology and similar amino acid sequences to proteins of the major histocompatibility complex class I family [143]. Previously identified as an anti-inflammatory adipokine with the ability to inhibit tumor development [12], AZGP1 is also linked to cancer cachexia due to its high amino acid sequence homology with lipid mobilization factors derived from tumors [144]. In mouse models, where AZGP1 is produced, it stimulates lipolysis in adipocytes, resulting in cachexia [145].
AZGP1 has been identified as a suppressor of HCC cell invasion, functioning by obstructing TGF-β-mediated epithelial-mesenchymal transition (EMT) [146]. This study revealed that the transcription factor Ikaros and histone deacetylation modulate AZGP1 expression in HCC. Additionally, a correlation was observed between the downregulation of AZGP1 in HCC serum samples and patient prognosis. Furthermore, AZGP1 was found to inhibit cell migration and invasion by regulating the PTEN/Akt and CD44s pathways in HCC [147]. Research conducted by Bingyi Lin et al. indicated that the suppression of LINC00844 expression significantly contributes to the pathogenesis and pathophysiology of HCC by promoting the AZGP1-mediated TGFβ1-ERK pathway, leading to HCC recurrence and adverse survival outcomes [148]. Similarly, Ming-Yi Xu et al. discovered that a deficiency in AZGP1 can trigger TGFβ1-ERK2 signaling-induced EMT, disrupt energy metabolism, decrease cell proliferation and induce apoptosis, activate survival signals, and promote invasion [149].
Conclusion
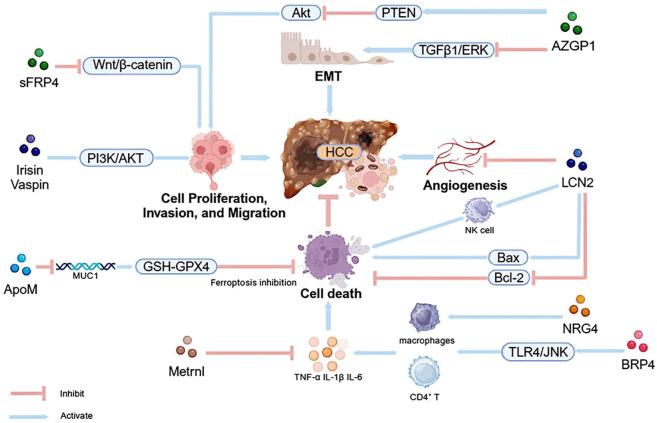
Compared to AFP, certain novel adipokines demonstrate enhanced sensitivity and specificity in predicting the onset of HCC, offering significant potential for clinical application. However, current research on these adipokines in relation to HCC is limited, and the mechanisms of most of these factors remain unclear. Existing studies suggest that these adipokines primarily influence HCC progression by modulating mechanisms related to growth, inflammation, migration, and apoptosis, as well as through pathways such as insulin resistance, which can either promote or protect liver cancer cells (Figure 1). The prognosis for HCC patients remains poor due to the tumor’s high propensity for metastasis and its poor response to drug treatments [150]. Therefore, identifying metastatic factors and elucidating the molecular mechanisms related to metastatic progression have become critical challenges. A deeper exploration of the mechanistic pathways involving adipokines could aid in the development of novel therapeutic drugs, providing promising targets for HCC treatment.
Figure 1.
The pathways through which novel adipokines affect HCC. Novel adipokines exert their influence on hepatocellular carcinoma (HCC) development primarily by dysregulating key signaling cascades, including the Wnt/β-catenin, PI3K/AKT, PTEN/AKT, and TGFβ1/ERK pathways. These cascades, in turn, modulate cellular processes such as proliferation, invasion, migration, epithelial-mesenchymal transition (EMT), angiogenesis, and apoptosis, often through the interplay with immune signaling pathways.
Acknowledgements
This work was supported by the Basic Medical Research Fund of Naval Medical University (2023QN034, 2023QN035). The authors would like to thank all the guest editors and anonymous reviewers for their constructive comments.
Disclosure of conflict of interest
None.
References
- 1.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 4.Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23:8263–8276. doi: 10.3748/wjg.v23.i47.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golabi P, Paik J, Reddy R, Bugianesi E, Trimble G, Younossi ZM. Prevalence and long-term outcomes of non-alcoholic fatty liver disease among elderly individuals from the United States. BMC Gastroenterol. 2019;19:56. doi: 10.1186/s12876-019-0972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osna NA, Donohue TM Jr, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38:147–161. [PMC free article] [PubMed] [Google Scholar]
- 7.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang TS, Wu YC, Tung SY, Wei KL, Hsieh YY, Huang HC, Chen WM, Shen CH, Lu CH, Wu CS, Tsai YH, Huang YH. Alpha-fetoprotein measurement benefits hepatocellular carcinoma surveillance in patients with cirrhosis. Am J Gastroenterol. 2015;110:836–844. doi: 10.1038/ajg.2015.100. quiz 845. [DOI] [PubMed] [Google Scholar]
- 9.Xie L, Wang H, Hu J, Liu Z, Hu F. The role of novel adipokines and adipose-derived extracellular vesicles (ADEVs): connections and interactions in liver diseases. Biochem Pharmacol. 2024;222:116104. doi: 10.1016/j.bcp.2024.116104. [DOI] [PubMed] [Google Scholar]
- 10.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Fasshauer M, Blüher M. Adipokines in health and disease. Trends Pharmacol Sci. 2015;36:461–470. doi: 10.1016/j.tips.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Leal Vde O, Mafra D. Adipokines in obesity. Clin Chim Acta. 2013;419:87–94. doi: 10.1016/j.cca.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Jiang J, Zheng Q, Zhu W, Chen X, Lu H, Chen D, Zhang H, Shao M, Zhou L, Zheng S. Alterations in glycolytic/cholesterogenic gene expression in hepatocellular carcinoma. Aging (Albany NY) 2020;12:10300–10316. doi: 10.18632/aging.103254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajesh Y, Sarkar D. Molecular mechanisms regulating obesity-associated hepatocellular carcinoma. Cancers (Basel) 2020;12:1290. doi: 10.3390/cancers12051290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang DD, Zhao YM, Wang L, Ren G, Wang F, Xia ZG, Wang XL, Zhang T, Pan Q, Dai Z, Chen JP, Dai HY, Zhang W, He HW, Zhou JM, Tang GY, Zhou J, Fan J, Tang ZY. Preoperative serum retinol-binding protein 4 is associated with the prognosis of patients with hepatocellular carcinoma after curative resection. J Cancer Res Clin Oncol. 2011;137:651–658. doi: 10.1007/s00432-010-0927-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson RB, Lathigara D, Kaushal D. Systematic review and meta-analysis of the impact of bariatric surgery on future cancer risk. Int J Mol Sci. 2023;24:6192. doi: 10.3390/ijms24076192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 18.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565–574. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 19.Utsunomiya T, Okamoto M, Kameyama T, Matsuyama A, Yamamoto M, Fujiwara M, Mori M, Aimitsu S, Ishida T. Impact of obesity on the surgical outcome following repeat hepatic resection in Japanese patients with recurrent hepatocellular carcinoma. World J Gastroenterol. 2008;14:1553–1558. doi: 10.3748/wjg.14.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Bai E, Zhang Y, Jia Z, He S, Fu J. Role of nampt and visceral adiposity in esophagogastric junction adenocarcinoma. J Immunol Res. 2017;2017:3970605. doi: 10.1155/2017/3970605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duan XF, Tang P, Li Q, Yu ZT. Obesity, adipokines and hepatocellular carcinoma. Int J Cancer. 2013;133:1776–1783. doi: 10.1002/ijc.28105. [DOI] [PubMed] [Google Scholar]
- 22.Booth A, Magnuson A, Fouts J, Foster M. Adipose tissue, obesity and adipokines: role in cancer promotion. Horm Mol Biol Clin Investig. 2015;21:57–74. doi: 10.1515/hmbci-2014-0037. [DOI] [PubMed] [Google Scholar]
- 23.Huang J, Duran A, Reina-Campos M, Valencia T, Castilla EA, Müller TD, Tschöp MH, Moscat J, Diaz-Meco MT. Adipocyte p62/SQSTM1 suppresses tumorigenesis through opposite regulations of metabolism in adipose tissue and tumor. Cancer Cell. 2018;33:770–784. e6. doi: 10.1016/j.ccell.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandal P, Pratt BT, Barnes M, Mcmullen MR, Nagy LE. Molecular mechanism for adiponectin-dependent M2 macrophage polarization: link between the metabolic and innate immune activity of full-length adiponectin. J Biol Chem. 2011;286:13460–13469. doi: 10.1074/jbc.M110.204644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mistry T, Digby JE, Desai KM, Randeva HS. Leptin and adiponectin interact in the regulation of prostate cancer cell growth via modulation of p53 and bcl-2 expression. BJU Int. 2008;101:1317–1322. doi: 10.1111/j.1464-410X.2008.07512.x. [DOI] [PubMed] [Google Scholar]
- 26.Nepal S, Kim MJ, Hong JT, Kim SH, Sohn DH, Lee SH, Song K, Choi DY, Lee ES, Park PH. Autophagy induction by leptin contributes to suppression of apoptosis in cancer cells and xenograft model: involvement of p53/FoxO3a axis. Oncotarget. 2015;6:7166–7181. doi: 10.18632/oncotarget.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai IT, Wang CP, Yu TH, Lu YC, Lin CW, Lu LF, Wu CC, Chung FM, Lee YJ, Hung WC, Hsu CC. Circulating visfatin level is associated with hepatocellular carcinoma in chronic hepatitis B or C virus infection. Cytokine. 2017;90:54–59. doi: 10.1016/j.cyto.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Kukla M, Zwirska-Korczala K, Gabriel A, Waluga M, Warakomska I, Berdowska A, Rybus-Kalinowska B, Kalinowski M, Janczewska-Kazek E, Wozniak-Grygiel E, Kryczka W. Visfatin serum levels in chronic hepatitis C patients. J Viral Hepat. 2010;17:254–260. doi: 10.1111/j.1365-2893.2009.01174.x. [DOI] [PubMed] [Google Scholar]
- 29.Zheng SL, Li ZY, Song J, Liu JM, Miao CY. Metrnl: a secreted protein with new emerging functions. Acta Pharmacol Sin. 2016;37:571–579. doi: 10.1038/aps.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Wang H, Ren D, Li J, Mu Z, Li C, He Y, Zhang J, Fan R, Yin J, Su J, He Y, Yao B. Interleukin-41: a novel serum marker for the diagnosis of alpha-fetoprotein-negative hepatocellular carcinoma. Front Oncol. 2024;14:1408584. doi: 10.3389/fonc.2024.1408584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu C, Zhang X, Song P, Yuan YP, Kong CY, Wu HM, Xu SC, Ma ZG, Tang QZ. Meteorin-like protein attenuates doxorubicin-induced cardiotoxicity via activating cAMP/PKA/SIRT1 pathway. Redox Biol. 2020;37:101747. doi: 10.1016/j.redox.2020.101747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du YN, Teng JM, Zhou TH, Du BY, Cai W. Meteorin-like protein overexpression ameliorates fulminant hepatitis in mice by inhibiting chemokine-dependent immune cell infiltration. Acta Pharmacol Sin. 2023;44:1404–1415. doi: 10.1038/s41401-022-01049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu M, Gao X, Tian Y, Li H, Yin Z, Han L, Zhang L. Serum metrnl is decreased in metabolic dysfunction-associated fatty liver disease: a case-control study. Diabetes Metab Syndr Obes. 2024;17:533–543. doi: 10.2147/DMSO.S447127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blaner WS. Retinol-binding protein: the serum transport protein for vitamin A. Endocr Rev. 1989;10:308–316. doi: 10.1210/edrv-10-3-308. [DOI] [PubMed] [Google Scholar]
- 35.van Dam RM, Hu FB. Lipocalins and insulin resistance: etiological role of retinol-binding protein 4 and lipocalin-2? Clin Chem. 2007;53:5–7. doi: 10.1373/clinchem.2006.080432. [DOI] [PubMed] [Google Scholar]
- 36.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Sun L, Lin X, Yuan JM, Koh WP, Pan A. Retinol binding protein 4 and risk of type 2 diabetes in Singapore Chinese men and women: a nested case-control study. Nutr Metab (Lond) 2019;16:3. doi: 10.1186/s12986-018-0329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaker O, El-Shehaby A, Zakaria A, Mostafa N, Talaat S, Katsiki N, Mikhailidis DP. Plasma visfatin and retinol binding protein-4 levels in patients with type 2 diabetes mellitus and their relationship to adiposity and fatty liver. Clin Biochem. 2011;44:1457–1463. doi: 10.1016/j.clinbiochem.2011.08.1148. [DOI] [PubMed] [Google Scholar]
- 39.Nderitu P, Bosco C, Garmo H, Holmberg L, Malmstrom H, Hammar N, Walldius G, Jungner I, Ross P, Van Hemelrijck M. The association between individual metabolic syndrome components, primary liver cancer and cirrhosis: a study in the Swedish AMORIS cohort. Int J Cancer. 2017;141:1148–1160. doi: 10.1002/ijc.30818. [DOI] [PubMed] [Google Scholar]
- 40.Kwak JY, Ma TZ, Yoo MJ, Choi BH, Kim HG, Kim SR, Yim CY, Kwak YG. The comparative analysis of serum proteomes for the discovery of biomarkers for acute myeloid leukemia. Exp Hematol. 2004;32:836–842. doi: 10.1016/j.exphem.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Noy N, Li L, Abola MV, Berger NA. Is retinol binding protein 4 a link between adiposity and cancer? Horm Mol Biol Clin Investig. 2015;23:39–46. doi: 10.1515/hmbci-2015-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Wang Y, Zhang Z. Adipokine RBP4 drives ovarian cancer cell migration. J Ovarian Res. 2018;11:29. doi: 10.1186/s13048-018-0397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fei W, Chen L, Chen J, Shi Q, Zhang L, Liu S, Li L, Zheng L, Hu X. RBP4 and THBS2 are serum biomarkers for diagnosis of colorectal cancer. Oncotarget. 2017;8:92254–92264. doi: 10.18632/oncotarget.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karunanithi S, Levi L, Devecchio J, Karagkounis G, Reizes O, Lathia JD, Kalady MF, Noy N. RBP4-STRA6 pathway drives cancer stem cell maintenance and mediates high-fat diet-induced colon carcinogenesis. Stem Cell Reports. 2017;9:438–450. doi: 10.1016/j.stemcr.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen KC, Hsueh WT, Ou CY, Huang CC, Lee WT, Fang SY, Tsai ST, Huang JS, Wong TY, Wu JL, Yen CJ, Wu YH, Lin FC, Yang MW, Chang JY, Liao HC, Wu SY, Hsiao JR, Lin CL, Wang YH, Weng YL, Yang HC, Chen YS, Chang JS. Alcohol drinking obliterates the inverse association between serum retinol and risk of head and neck cancer. Medicine (Baltimore) 2015;94:e1064. doi: 10.1097/MD.0000000000001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kataria Y, Deaton RJ, Enk E, Jin M, Petrauskaite M, Dong L, Goldenberg JR, Cotler SJ, Jensen DM, van Breemen RB, Gann PH. Retinoid and carotenoid status in serum and liver among patients at high-risk for liver cancer. BMC Gastroenterol. 2016;16:30. doi: 10.1186/s12876-016-0432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fayed HM, Mahmoud HS, Elaiw Mohamed Ali A. The utility of retinol-binding protein 4 in predicting liver fibrosis in chronic hepatitis C patients in response to direct-acting antivirals. Clin Exp Gastroenterol. 2020;13:53–63. doi: 10.2147/CEG.S229689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wan F, Zhu Y, Wu F, Huang X, Chen Y, Zhou Y, Li H, Liang L, Qin L, Wang Q, He M. Retinol-binding protein 4 as a promising serum biomarker for the diagnosis and prognosis of hepatocellular Carcinoma. Transl Oncol. 2024;45:101979. doi: 10.1016/j.tranon.2024.101979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li H, He X, Wen S, Yang L, Chen Q, Li Y, Huang S, Huang X, Wan F, He M. Optimised expression and purification of RBP4 and preparation of anti-RBP4 monoclonal antibody. FEBS Open Bio. 2022;12:430–442. doi: 10.1002/2211-5463.13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kinoshita M, Miyata M. Underexpression of mRNA in human hepatocellular carcinoma focusing on eight loci. Hepatology. 2002;36:433–438. doi: 10.1053/jhep.2002.34851. [DOI] [PubMed] [Google Scholar]
- 51.Li M, Wang Z, Zhu L, Shui Y, Zhang S, Guo W. Down-regulation of RBP4 indicates a poor prognosis and correlates with immune cell infiltration in hepatocellular carcinoma. Biosci Rep. 2021;41:BSR20210328. doi: 10.1042/BSR20210328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yao-Borengasser A, Varma V, Bodles AM, Rasouli N, Phanavanh B, Lee MJ, Starks T, Kern LM, Spencer HJ 3rd, Rashidi AA, Mcgehee RE Jr, Fried SK, Kern PA. Retinol binding protein 4 expression in humans: relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin Endocrinol Metab. 2007;92:2590–2597. doi: 10.1210/jc.2006-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee SA, Yuen JJ, Jiang H, Kahn BB, Blaner WS. Adipocyte-specific overexpression of retinol-binding protein 4 causes hepatic steatosis in mice. Hepatology. 2016;64:1534–1546. doi: 10.1002/hep.28659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moraes-Vieira PM, Yore MM, Dwyer PM, Syed I, Aryal P, Kahn BB. RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metab. 2014;19:512–526. doi: 10.1016/j.cmet.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chayanupatkul M, Honsawek S, Chongsrisawat V, Vimolket L, Poovorawan Y. Serum retinol binding protein 4 and clinical outcome in postoperative biliary atresia. Hepatol Int. 2011;5:906–912. doi: 10.1007/s12072-011-9262-2. [DOI] [PubMed] [Google Scholar]
- 56.Arias-Loste MT, Ranchal I, Romero-Gomez M, Crespo J. Irisin, a link among fatty liver disease, physical inactivity and insulin resistance. Int J Mol Sci. 2014;15:23163–23178. doi: 10.3390/ijms151223163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mo L, Shen J, Liu Q, Zhang Y, Kuang J, Pu S, Cheng S, Zou M, Jiang W, Jiang C, Qu A, He J. Irisin is regulated by CAR in liver and is a mediator of hepatic glucose and lipid metabolism. Mol Endocrinol. 2016;30:533–542. doi: 10.1210/me.2015-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dong B, Saha PK, Huang W, Chen W, Abu-Elheiga LA, Wakil SJ, Stevens RD, Ilkayeva O, Newgard CB, Chan L, Moore DD. Activation of nuclear receptor CAR ameliorates diabetes and fatty liver disease. Proc Natl Acad Sci U S A. 2009;106:18831–18836. doi: 10.1073/pnas.0909731106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, Mantzoros CS. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. MRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61:1725–1738. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Komolka K, Albrecht E, Schering L, Brenmoehl J, Hoeflich A, Maak S. Locus characterization and gene expression of bovine FNDC5: is the myokine irisin relevant in cattle? PLoS One. 2014;9:e88060. doi: 10.1371/journal.pone.0088060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shanaki M, Moradi N, Emamgholipour S, Fadaei R, Poustchi H. Lower circulating irisin is associated with nonalcoholic fatty liver disease and type 2 diabetes. Diabetes Metab Syndr. 2017;11(Suppl 1):S467–S472. doi: 10.1016/j.dsx.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 62.Zhang HJ, Zhang XF, Ma ZM, Pan LL, Chen Z, Han HW, Han CK, Zhuang XJ, Lu Y, Li XJ, Yang SY, Li XY. Irisin is inversely associated with intrahepatic triglyceride contents in obese adults. J Hepatol. 2013;59:557–562. doi: 10.1016/j.jhep.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 63.Bi J, Zhang J, Ren Y, Du Z, Li Q, Wang Y, Wei S, Yang L, Zhang J, Liu C, Lv Y, Wu R. Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative stress. Redox Biol. 2019;20:296–306. doi: 10.1016/j.redox.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fan X, Du J, Wang MH, Li JM, Yang B, Chen Y, Dai JC, Zhang C, Zhou J. Irisin contributes to the hepatoprotection of dexmedetomidine during intestinal ischemia/reperfusion. Oxid Med Cell Longev. 2019;2019:7857082. doi: 10.1155/2019/7857082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Canivet CM, Bonnafous S, Rousseau D, Leclere PS, Lacas-Gervais S, Patouraux S, Sans A, Luci C, Bailly-Maitre B, Iannelli A, Tran A, Anty R, Gual P. Hepatic FNDC5 is a potential local protective factor against non-alcoholic fatty liver. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165705. doi: 10.1016/j.bbadis.2020.165705. [DOI] [PubMed] [Google Scholar]
- 66.Polyzos SA, Kountouras J, Anastasilakis AD, Geladari EV, Mantzoros CS. Irisin in patients with nonalcoholic fatty liver disease. Metabolism. 2014;63:207–217. doi: 10.1016/j.metabol.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 67.Liu TY, Shi CX, Gao R, Sun HJ, Xiong XQ, Ding L, Chen Q, Li YH, Wang JJ, Kang YM, Zhu GQ. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin Sci (Lond) 2015;129:839–850. doi: 10.1042/CS20150009. [DOI] [PubMed] [Google Scholar]
- 68.Xin C, Liu J, Zhang J, Zhu D, Wang H, Xiong L, Lee Y, Ye J, Lian K, Xu C, Zhang L, Wang Q, Liu Y, Tao L. Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int J Obes (Lond) 2016;40:443–451. doi: 10.1038/ijo.2015.199. [DOI] [PubMed] [Google Scholar]
- 69.Bi J, Yang L, Wang T, Zhang J, Li T, Ren Y, Wang M, Chen X, Lv Y, Wu R. Irisin improves autophagy of aged hepatocytes via increasing telomerase activity in liver injury. Oxid Med Cell Longev. 2020;2020:6946037. doi: 10.1155/2020/6946037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pinto AP, Ropelle ER, Quadrilatero J, da Silva ASR. Physical exercise and liver autophagy: potential roles of IL-6 and irisin. Exerc Sport Sci Rev. 2022;50:89–96. doi: 10.1249/JES.0000000000000278. [DOI] [PubMed] [Google Scholar]
- 71.Pazgan-Simon M, Zuwala-Jagiello J, Kukla M, Grzebyk E, Simon K. Serum concentrations of selected adipokines in virus-related liver cirrhosis and hepatocellular carcinoma. Clin Exp Hepatol. 2020;6:235–242. doi: 10.5114/ceh.2020.99517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pazgan-Simon M, Zuwala-Jagiello J, Menzyk T, Bator M, Derra A, Lekstan A, Grzebyk E, Simon K, Kukla M. Serum betatrophin and irisin levels in hepatocellular carcinoma. J Physiol Pharmacol. 2020;71 doi: 10.26402/jpp.2020.1.11. [DOI] [PubMed] [Google Scholar]
- 73.Aydin S, Kuloglu T, Ozercan MR, Albayrak S, Aydin S, Bakal U, Yilmaz M, Kalayci M, Yardim M, Sarac M, Kazez A, Kocdor H, Kanat B, Ozercan IH, Gonen M, Bilgen M, Balgetir F. Irisin immunohistochemistry in gastrointestinal system cancers. Biotech Histochem. 2016;91:242–250. doi: 10.3109/10520295.2015.1136988. [DOI] [PubMed] [Google Scholar]
- 74.Gaggini M, Cabiati M, Del Turco S, Navarra T, De Simone P, Filipponi F, Del RY S, Gastaldelli A, Basta G. Increased FNDC5/Irisin expression in human hepatocellular carcinoma. Peptides. 2017;88:62–66. doi: 10.1016/j.peptides.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 75.Lv J, Pan Y, Li X, Cheng D, Ju H, Tian J, Shi H, Zhang Y. Study on the distribution and elimination of the new hormone irisin in vivo: new discoveries regarding irisin. Horm Metab Res. 2015;47:591–595. doi: 10.1055/s-0035-1547261. [DOI] [PubMed] [Google Scholar]
- 76.Dasarathy S. Cause and management of muscle wasting in chronic liver disease. Curr Opin Gastroenterol. 2016;32:159–165. doi: 10.1097/MOG.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang J, Ke M, Ren Y, Bi J, Du Z, Zhang M, Wang Y, Zhang L, Wu Z, Lv Y, Wu R. Serum irisin predicts posthepatectomy complications in patients with hepatocellular carcinoma. Dis Markers. 2019;2019:9850191. doi: 10.1155/2019/9850191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu W, Sahar NE, Javaid HMA, Pak ES, Liang G, Wang Y, Ha H, Huh JY. Exercise-induced irisin decreases inflammation and improves NAFLD by competitive binding with MD2. Cells. 2021;10:3306. doi: 10.3390/cells10123306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi G, Tang N, Qiu J, Zhang D, Huang F, Cheng Y, Ding K, Li W, Zhang P, Tan X. Irisin stimulates cell proliferation and invasion by targeting the PI3K/AKT pathway in human hepatocellular carcinoma. Biochem Biophys Res Commun. 2017;493:585–591. doi: 10.1016/j.bbrc.2017.08.148. [DOI] [PubMed] [Google Scholar]
- 80.Polyzos SA, Anastasilakis AD, Efstathiadou ZA, Makras P, Perakakis N, Kountouras J, Mantzoros CS. Irisin in metabolic diseases. Endocrine. 2018;59:260–274. doi: 10.1007/s12020-017-1476-1. [DOI] [PubMed] [Google Scholar]
- 81.Kloting N, Berndt J, Kralisch S, Kovacs P, Fasshauer M, Schon MR, Stumvoll M, Bluher M. Vaspin gene expression in human adipose tissue: association with obesity and type 2 diabetes. Biochem Biophys Res Commun. 2006;339:430–436. doi: 10.1016/j.bbrc.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 82.Wada J. Vaspin: a novel serpin with insulin-sensitizing effects. Expert Opin Investig Drugs. 2008;17:327–333. doi: 10.1517/13543784.17.3.327. [DOI] [PubMed] [Google Scholar]
- 83.Hida K, Wada J, Eguchi J, Zhang H, Baba M, Seida A, Hashimoto I, Okada T, Yasuhara A, Nakatsuka A, Shikata K, Hourai S, Futami J, Watanabe E, Matsuki Y, Hiramatsu R, Akagi S, Makino H, Kanwar YS. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci U S A. 2005;102:10610–10615. doi: 10.1073/pnas.0504703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kukla M, Waluga M, Sawczyn T, Berdowska A, Kajor M, Boryczka G, Stygar D, Gabriel A, Zwirska-Korczala K, Hartleb M. Serum vaspin may be a good indicator of fibrosis in chronic hepatitis C and is not altered by antiviral therapy. Pol J Pathol. 2012;63:213–220. doi: 10.5114/pjp.2012.32767. [DOI] [PubMed] [Google Scholar]
- 85.Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Mol Med. 2008;14:741–751. doi: 10.2119/2008-00058.Rabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abdelhamed W, Morsy KH, Hefny HM, Abudeif A. The role of serum visfatin and vaspin in hepatocellular carcinoma in hepatitis C-related liver cirrhosis. Clin Exp Hepatol. 2023;9:210–220. doi: 10.5114/ceh.2023.130499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pich K, Respekta N, Dawid M, Mlyczynska E, Kurowska P, Rak A. New insights into cell apoptosis and proliferation: the potential role of vaspin. J Physiol Pharmacol. 2021;72 doi: 10.26402/jpp.2021.6.02. [DOI] [PubMed] [Google Scholar]
- 88.Fazeli MS, Dashti H, Akbarzadeh S, Assadi M, Aminian A, Keramati MR, Nabipour I. Circulating levels of novel adipocytokines in patients with colorectal cancer. Cytokine. 2013;62:81–85. doi: 10.1016/j.cyto.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 89.Jaberi SA, Cohen A, D’Souza C, Abdulrazzaq YM, Ojha S, Bastaki S, Adeghate EA. Lipocalin-2: structure, function, distribution and role in metabolic disorders. Biomed Pharmacother. 2021;142:112002. doi: 10.1016/j.biopha.2021.112002. [DOI] [PubMed] [Google Scholar]
- 90.Schröder S, Asimakopoulou A, Weiskirchen R. Lipocalin 2 as a potential diagnostic and/or prognostic biomarker in prostate, lung and liver cancer. Clin Oncol. 2018;1:1–14. [Google Scholar]
- 91.Bettermann K, Vucur M, Haybaeck J, Koppe C, Janssen J, Heymann F, Weber A, Weiskirchen R, Liedtke C, Gassler N, Muller M, de Vos R, Wolf MJ, Boege Y, Seleznik GM, Zeller N, Erny D, Fuchs T, Zoller S, Cairo S, Buendia MA, Prinz M, Akira S, Tacke F, Heikenwalder M, Trautwein C, Luedde T. TAK1 suppresses a NEMO-dependent but NF-kappaB-independent pathway to liver cancer. Cancer Cell. 2010;17:481–496. doi: 10.1016/j.ccr.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 92.Vucur M, Reisinger F, Gautheron J, Janssen J, Roderburg C, Cardenas DV, Kreggenwinkel K, Koppe C, Hammerich L, Hakem R, Unger K, Weber A, Gassler N, Luedde M, Frey N, Neumann UP, Tacke F, Trautwein C, Heikenwalder M, Luedde T. RIP3 inhibits inflammatory hepatocarcinogenesis but promotes cholestasis by controlling caspase-8- and JNK-dependent compensatory cell proliferation. Cell Rep. 2013;4:776–790. doi: 10.1016/j.celrep.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 93.Wang T, Zhang KH, Hu PP, Wan QS, Han FL, Zhou JM, Huang DQ, Lv NH. Combination of dual serum fluorescence, AFP and hepatic function tests is valuable to identify HCC in AFP-elevated liver diseases. Oncotarget. 2017;8:97758–97768. doi: 10.18632/oncotarget.22050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yoshikawa K, Iwasa M, Eguchi A, Kojima S, Yoshizawa N, Tempaku M, Sugimoto R, Yamamoto N, Sugimoto K, Kobayashi Y, Hasegawa H, Takei Y. Neutrophil gelatinase-associated lipocalin level is a prognostic factor for survival in rat and human chronic liver diseases. Hepatol Commun. 2017;1:946–956. doi: 10.1002/hep4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang H, Tang XY, Liu M, Li X. Targeting alpha-fetoprotein represses the proliferation of hepatoma cells via regulation of the cell cycle. Clin Chim Acta. 2008;394:81–88. doi: 10.1016/j.cca.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 96.Asimakopoulou A, Vucur M, Luedde T, Schneiders S, Kalampoka S, Weiss TS, Weiskirchen R. Perilipin 5 and lipocalin 2 expression in hepatocellular carcinoma. Cancers (Basel) 2019;11:385. doi: 10.3390/cancers11030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barsoum I, Elgohary MN, Bassiony MAA. Lipocalin-2: a novel diagnostic marker for hepatocellular carcinoma. Cancer Biomark. 2020;28:523–528. doi: 10.3233/CBM-190084. [DOI] [PubMed] [Google Scholar]
- 98.Wang YP, Yu GR, Lee MJ, Lee SY, Chu IS, Leem SH, Kim DG. Lipocalin-2 negatively modulates the epithelial-to-mesenchymal transition in hepatocellular carcinoma through the epidermal growth factor (TGF-beta1)/Lcn2/Twist1 pathway. Hepatology. 2013;58:1349–1361. doi: 10.1002/hep.26467. [DOI] [PubMed] [Google Scholar]
- 99.Chien MH, Ying TH, Yang SF, Yu JK, Hsu CW, Hsieh SC, Hsieh YH. Lipocalin-2 induces apoptosis in human hepatocellular carcinoma cells through activation of mitochondria pathways. Cell Biochem Biophys. 2012;64:177–186. doi: 10.1007/s12013-012-9370-1. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Y, Fan Y, Mei Z. NGAL and NGALR overexpression in human hepatocellular carcinoma toward a molecular prognostic classification. Cancer Epidemiol. 2012;36:e294–e299. doi: 10.1016/j.canep.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 101.Lu Y, Zhu M, Li W, Lin B, Dong X, Chen Y, Xie X, Guo J, Li M. Alpha fetoprotein plays a critical role in promoting metastasis of hepatocellular carcinoma cells. J Cell Mol Med. 2016;20:549–558. doi: 10.1111/jcmm.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shen P, Jia Y, Zhou W, Zheng W, Wu Y, Qu S, Du S, Wang S, Shi H, Sun J, Han X. A biomimetic liver cancer on-a-chip reveals a critical role of LIPOCALIN-2 in promoting hepatocellular carcinoma progression. Acta Pharm Sin B. 2023;13:4621–4637. doi: 10.1016/j.apsb.2023.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chung IH, Chen CY, Lin YH, Chi HC, Huang YH, Tai PJ, Liao CJ, Tsai CY, Lin SL, Wu MH, Chen CY, Lin KH. Thyroid hormone-mediated regulation of lipocalin 2 through the Met/FAK pathway in liver cancer. Oncotarget. 2015;6:15050–15064. doi: 10.18632/oncotarget.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen M, Zhu J, Luo H, Mu W, Guo L. The journey towards physiology and pathology: tracing the path of neuregulin 4. Genes Dis. 2023;11:687–700. doi: 10.1016/j.gendis.2023.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen Z, Wang GX, Ma SL, Jung DY, Ha H, Altamimi T, Zhao XY, Guo L, Zhang P, Hu CR, Cheng JX, Lopaschuk GD, Kim JK, Lin JD. Nrg4 promotes fuel oxidation and a healthy adipokine profile to ameliorate diet-induced metabolic disorders. Mol Metab. 2017;6:863–872. doi: 10.1016/j.molmet.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guo L, Zhang P, Chen Z, Xia H, Li S, Zhang Y, Kobberup S, Zou W, Lin JD. Hepatic neuregulin 4 signaling defines an endocrine checkpoint for steatosis-to-NASH progression. J Clin Invest. 2017;127:4449–4461. doi: 10.1172/JCI96324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang GX, Zhao XY, Meng ZX, Kern M, Dietrich A, Chen Z, Cozacov Z, Zhou D, Okunade AL, Su X, Li S, Bluher M, Lin JD. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med. 2014;20:1436–1443. doi: 10.1038/nm.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dai YN, Zhu JZ, Fang ZY, Zhao DJ, Wan XY, Zhu HT, Yu CH, Li YM. A case-control study: association between serum neuregulin 4 level and non-alcoholic fatty liver disease. Metabolism. 2015;64:1667–1673. doi: 10.1016/j.metabol.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 109.Wang R, Yang F, Qing L, Huang R, Liu Q, Li X. Decreased serum neuregulin 4 levels associated with non-alcoholic fatty liver disease in children with obesity. Clin Obes. 2019;9:e12289. doi: 10.1111/cob.12289. [DOI] [PubMed] [Google Scholar]
- 110.Li Y, Jin L, Jiang F, Yan J, Lu Y, Yang Q, Zhang Y, Zhang H, Yu H, Zhang Y, He Z, Zhang R, Yang J, Hu C. Mutations of NRG4 contribute to the pathogenesis of nonalcoholic fatty liver disease and related metabolic disorders. Diabetes. 2021;70:2213–2224. doi: 10.2337/db21-0064. [DOI] [PubMed] [Google Scholar]
- 111.Ng YK, Lee JY, Supko KM, Khan A, Torres SM, Berwick M, Ho J, Kirkwood JM, Siegfried JM, Stabile LP. Pan-erbB inhibition potentiates BRAF inhibitors for melanoma treatment. Melanoma Res. 2014;24:207–218. doi: 10.1097/CMR.0000000000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hayes NV, Blackburn E, Smart LV, Boyle MM, Russell GA, Frost TM, Morgan BJ, Baines AJ, Gullick WJ. Identification and characterization of novel spliced variants of neuregulin 4 in prostate cancer. Clin Cancer Res. 2007;13:3147–3155. doi: 10.1158/1078-0432.CCR-06-2237. [DOI] [PubMed] [Google Scholar]
- 113.Marshall C, Blackburn E, Clark M, Humphreys S, Gullick WJ. Neuregulins 1-4 are expressed in the cytoplasm or nuclei of ductal carcinoma (in situ) of the human breast. Breast Cancer Res Treat. 2006;96:163–168. doi: 10.1007/s10549-005-9073-z. [DOI] [PubMed] [Google Scholar]
- 114.Rou WS, Eun HS, Choung S, Jeon HJ, Joo JS, Kang SH, Lee ES, Kim SH, Kwon IS, Ku BJ, Lee BS. Prognostic value of erythroblastic leukemia viral oncogene homolog 2 and neuregulin 4 in hepatocellular carcinoma. Cancers (Basel) 2023;15:2634. doi: 10.3390/cancers15092634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang P, Chen Z, Kuang H, Liu T, Zhu J, Zhou L, Wang Q, Xiong X, Meng Z, Qiu X, Jacks R, Liu L, Li S, Lumeng CN, Li Q, Zhou X, Lin JD. Neuregulin 4 suppresses NASH-HCC development by restraining tumor-prone liver microenvironment. Cell Metab. 2022;34:1359–1376. doi: 10.1016/j.cmet.2022.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schumacher MA, Dennis IC, Liu CY, Robinson C, Shang J, Bernard JK, Washington MK, Polk DB, Frey MR. NRG4-ErbB4 signaling represses proinflammatory macrophage activity. Am J Physiol Gastrointest Liver Physiol. 2021;320:G990–G1001. doi: 10.1152/ajpgi.00296.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schumacher MA, Hedl M, Abraham C, Bernard JK, Lozano PR, Hsieh JJ, Almohazey D, Bucar EB, Punit S, Dempsey PJ, Frey MR. ErbB4 signaling stimulates pro-inflammatory macrophage apoptosis and limits colonic inflammation. Cell Death Dis. 2017;8:e2622. doi: 10.1038/cddis.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu Y, Song L, Ni H, Sun L, Jiao W, Chen L, Zhou Q, Shen T, Cui H, Gao T, Li J. ERBB4 acts as a suppressor in the development of hepatocellular carcinoma. Carcinogenesis. 2017;38:465–473. doi: 10.1093/carcin/bgx017. [DOI] [PubMed] [Google Scholar]
- 119.Segers VFM, Dugaucquier L, Feyen E, Shakeri H, De Keulenaer GW. The role of ErbB4 in cancer. Cell Oncol (Dordr) 2020;43:335–352. doi: 10.1007/s13402-020-00499-4. [DOI] [PubMed] [Google Scholar]
- 120.Agostino M, Pohl SO, Dharmarajan A. Structure-based prediction of Wnt binding affinities for Frizzled-type cysteine-rich domains. J Biol Chem. 2017;292:11218–11229. doi: 10.1074/jbc.M117.786269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pawar NM, Rao P. Secreted frizzled related protein 4 (sFRP4) update: a brief review. Cell Signal. 2018;45:63–70. doi: 10.1016/j.cellsig.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 122.Xu C, Zeng XH, Wang L, Tao SQ, Wu QX, Zhu P, Deng GH, Wang YM. SFRP-4, a potential novel serum marker for chronic hepatitis B-related hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2015;14:164–170. doi: 10.1016/s1499-3872(15)60352-6. [DOI] [PubMed] [Google Scholar]
- 123.Song J, Xie C, Jiang L, Wu G, Zhu J, Zhang S, Tang M, Song L, Li J. Transcription factor AP-4 promotes tumorigenic capability and activates the Wnt/beta-catenin pathway in hepatocellular carcinoma. Theranostics. 2018;8:3571–3583. doi: 10.7150/thno.25194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Feng Han Q, Zhao W, Bentel J, Shearwood AM, Zeps N, Joseph D, Iacopetta B, Dharmarajan A. Expression of sFRP-4 and beta-catenin in human colorectal carcinoma. Cancer Lett. 2006;231:129–137. doi: 10.1016/j.canlet.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 125.Deshmukh A, Arfuso F, Newsholme P, Dharmarajan A. Regulation of cancer stem cell metabolism by secreted frizzled-related protein 4 (sFRP4) Cancers (Basel) 2018;10:40. doi: 10.3390/cancers10020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wu Q, Xu C, Zeng X, Zhang Z, Yang B, Rao Z. Tumor suppressor role of sFRP-4 in hepatocellular carcinoma via the Wnt/beta-catenin signaling pathway. Mol Med Rep. 2021;23:336. doi: 10.3892/mmr.2021.11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Huang LZ, Gao JL, Pu C, Zhang PH, Wang LZ, Feng G, Zhang Y. Apolipoprotein M: research progress, regulation and metabolic functions (Review) Mol Med Rep. 2015;12:1617–1624. doi: 10.3892/mmr.2015.3658. [DOI] [PubMed] [Google Scholar]
- 128.Axler O, Ahnström J, Dahlbäck B. An ELISA for apolipoprotein M reveals a strong correlation to total cholesterol in human plasma. J Lipid Res. 2007;48:1772–1780. doi: 10.1194/jlr.M700113-JLR200. [DOI] [PubMed] [Google Scholar]
- 129.Christoffersen C, Nielsen LB, Axler O, Andersson A, Johnsen AH, Dahlbäck B. Isolation and characterization of human apolipoprotein M-containing lipoproteins. J Lipid Res. 2006;47:1833–1843. doi: 10.1194/jlr.M600055-JLR200. [DOI] [PubMed] [Google Scholar]
- 130.Ruiz M, Frej C, Holmér A, Guo LJ, Tran S, Dahlbäck B. High-density lipoprotein-associated apolipoprotein M limits endothelial inflammation by delivering sphingosine-1-phosphate to the sphingosine-1-phosphate receptor 1. Arterioscler Thromb Vasc Biol. 2017;37:118–129. doi: 10.1161/ATVBAHA.116.308435. [DOI] [PubMed] [Google Scholar]
- 131.Feingold KR, Shigenaga JK, Chui LG, Moser A, Khovidhunkit W, Grunfeld C. Infection and inflammation decrease apolipoprotein M expression. Atherosclerosis. 2008;199:19–26. doi: 10.1016/j.atherosclerosis.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 132.Zhang X, Zhu Z, Luo G, Zheng L, Nilsson-Ehle P, Xu N. Liver X receptor agonist downregulates hepatic apoM expression in vivo and in vitro. Biochem Biophys Res Commun. 2008;371:114–117. doi: 10.1016/j.bbrc.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 133.Mosialou I, Zannis VI, Kardassis D. Regulation of human apolipoprotein M gene expression by orphan and ligand-dependent nuclear receptors. J Biol Chem. 2010;285:30719–30730. doi: 10.1074/jbc.M110.131771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mosialou I, Krasagakis K, Kardassis D. Opposite regulation of the human apolipoprotein M gene by hepatocyte nuclear factor 1 and Jun transcription factors. J Biol Chem. 2011;286:17259–17269. doi: 10.1074/jbc.M110.200659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu Y, Zhu X, Zhu J, Liao S, Tang Q, Liu K, Guan X, Zhang J, Feng Z. Identification of differential expression of genes in hepatocellular carcinoma by suppression subtractive hybridization combined cDNA microarray. Oncol Rep. 2007;18:943–951. [PubMed] [Google Scholar]
- 136.Jiang J, Wu C, Luo G, Zheng L, Chen L, Zhang X, Xu N. Expression of apolipoprotein M in human hepatocellular carcinoma tissues. Acta Histochem. 2011;113:53–57. doi: 10.1016/j.acthis.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 137.Jiang J, Zhang X, Wu C, Qin X, Luo G, Deng H, Lu M, Xu B, Li M, Ji M, Xu N. Increased plasma apoM levels in the patients suffered from hepatocellular carcinoma and other chronic liver diseases. Lipids Health Dis. 2008;7:25. doi: 10.1186/1476-511X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bai Y, Pei W, Zhang X, Zheng H, Hua C, Min J, Hu L, Du S, Gong Z, Gao J, Zhang Y. ApoM is an important potential protective factor in the pathogenesis of primary liver cancer. J Cancer. 2021;12:4661–4671. doi: 10.7150/jca.53115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xu N, Zhang XY, Dong X, Ekström U, Ye Q, Nilsson-Ehle P. Effects of platelet-activating factor, tumor necrosis factor, and Interleukin-1α on the expression of apolipoprotein M in HepG2 cells. Biochem Biophys Res Commun. 2002;292:944–950. doi: 10.1006/bbrc.2002.6755. [DOI] [PubMed] [Google Scholar]
- 140.Zhang X, Bai Y, Zhu W, Lv X, Pei W. ApoM regulates PFKL through the transcription factor SREBF1 to inhibit the proliferation, migration and metastasis of liver cancer cells. Oncol Lett. 2022;24:210. doi: 10.3892/ol.2022.13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang X, Zhang P, Gao J, Huang Q. Autophagy dysregulation caused by ApoM deficiency plays an important role in liver lipid metabolic disorder. Biochem Biophys Res Commun. 2018;495:2643–2648. doi: 10.1016/j.bbrc.2017.12.148. [DOI] [PubMed] [Google Scholar]
- 142.Liu M, Hu M, Liu R, Wang L, Wang J, Wang Y, Zhang R, Wang H, Liu M, Zhang Y, Wang L, Pei W, Zhang Y. Unveiling the role of APOM gene in liver cancer: investigating the impact of hsa-miR-4489/MUC1-mediated ferroptosis on the advancement of hepatocellular carcinoma cells. Gene. 2024;925:148591. doi: 10.1016/j.gene.2024.148591. [DOI] [PubMed] [Google Scholar]
- 143.Burgi W, Schmid K. Preparation and properties of Zn-alpha 2-glycoprotein of normal human plasma. J Biol Chem. 1961;236:1066–1074. [PubMed] [Google Scholar]
- 144.Russell ST, Tisdale MJ. The role of glucocorticoids in the induction of zinc-alpha2-glycoprotein expression in adipose tissue in cancer cachexia. Br J Cancer. 2005;92:876–881. doi: 10.1038/sj.bjc.6602404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bing C, Bao Y, Jenkins J, Sanders P, Manieri M, Cinti S, Tisdale MJ, Trayhurn P. Zinc-alpha2-glycoprotein, a lipid mobilizing factor, is expressed in adipocytes and is up-regulated in mice with cancer cachexia. Proc Natl Acad Sci U S A. 2004;101:2500–2505. doi: 10.1073/pnas.0308647100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huang Y, Li LZ, Zhang CZ, Yi C, Liu LL, Zhou X, Xie GB, Cai MY, Li Y, Yun JP. Decreased expression of zinc-alpha2-glycoprotein in hepatocellular carcinoma associates with poor prognosis. J Transl Med. 2012;10:106. doi: 10.1186/1479-5876-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tian H, Ge C, Zhao F, Zhu M, Zhang L, Huo Q, Li H, Chen T, Xie H, Cui Y, Yao M, Li J. Downregulation of AZGP1 by Ikaros and histone deacetylase promotes tumor progression through the PTEN/Akt and CD44s pathways in hepatocellular carcinoma. Carcinogenesis. 2017;38:207–217. doi: 10.1093/carcin/bgw125. [DOI] [PubMed] [Google Scholar]
- 148.Lin B, He H, Zhang Q, Zhang J, Xu L, Zhou L, Zheng S, Wu L. Long non-coding RNA00844 inhibits MAPK signaling to suppress the progression of hepatocellular carcinoma by targeting AZGP1. Ann Transl Med. 2020;8:1365. doi: 10.21037/atm-20-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xu MY, Chen R, Yu JX, Liu T, Qu Y, Lu LG. AZGP1 suppresses epithelial-to-mesenchymal transition and hepatic carcinogenesis by blocking TGFbeta1-ERK2 pathways. Cancer Lett. 2016;374:241–249. doi: 10.1016/j.canlet.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 150.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 151.Du L, Wang M, Li H, Li N, Wang F. Identification of CCL20 and LCN2 as efficient serological tools for detection of hepatocellular carcinoma. Dis Markers. 2022;2022:7758735. doi: 10.1155/2022/7758735. [DOI] [PMC free article] [PubMed] [Google Scholar]