Abstract
This study sought to identify the relationship between ADP-ribosylation factor GTPase-activating protein (ASAP1) expression and clinical outcomes in extrahepatic cholangiocarcinoma (EHCC) patients. Quantitative real-time PCR (qRT-PCR), Western blotting, and immunohistochemistry were used to analyze the expression of ASAP1 in cholangiocarcinoma (CC) tissue samples and cell lines. The survival rate and clinicopathological characteristics of CC patients were also examined. Cell Counting Kit-8 (CCK-8) and 5-ethynyl-2’-deoxyuridine (EdU) assays were used to detect cell proliferation. Flow cytometry was used to assess the cell cycle distribution. Both in vitro and in vivo experiments showed that ASAP1 knockdown decreased cell proliferation, inhibited cell cycle progression, and increased apoptosis. ASAP1 regulates Wnt/β-catenin pathway activity in CC, promoting cell migration, and invasion in culture; and promotes tumor development in vivo. ASAP1 plays a key role in EHCC tumor development and could serve as a potential therapeutic target for EHCC.
Keywords: Cholangiocarcinoma, ASAP1, cell proliferation, Wnt/β-catenin
Introduction
Cholangiocarcinoma (CC) refers to malignant tumors arising from the epithelial lining of the bile duct system and is classified into extrahepatic cholangiocarcinoma (EHCC) and intrahepatic cholangiocarcinoma (IHCC) based on its location. CC is a biliary tract disease that poses a significant threat to human health, despite its low incidence of 3% in gastrointestinal malignancies. The incidence rate in Western countries ranges from 0.3-6 cases per 100,000 people, while the incidence rate is notably higher in Asian countries, especially for IHCC [1]. Early symptoms of CC are typically nonspecific, leading to a low rate of early diagnosis. Most patients are diagnosed at an advanced stage, resulting in poor prognosis, with a 5-year survival rate of only 7-20%. Surgical resection remains the only possible cure, but it is viable in only 20-30% of cases [2,3]. Even for those who undergo surgery, resistance to postoperative chemoradiotherapy and tumor recurrence often contribute to poor prognosis and high mortality rates. For patients ineligible for radical surgery, palliative chemotherapy, radiotherapy, or percutaneous liver puncture biliary drainage can relieve symptoms like jaundice and improve quality of life [4], though these methods cannot eliminate tumor cells from the source. By the time CC is diagnosed, most tumors have already metastasized locally or distantly, rendering radical resection unfeasible. Gemcitabine- and cisplatin-based systemic chemotherapy has shown effectiveness in patients with unresectable CC. Target therapies and immunotherapy have been proposed as second- and third-line treatment. However, the median overall survival for patients undergoing systemic treatment rarely exceeds one year [5]. Therefore, to improve treatment outcomes, it is critical to understand the molecular mechanisms driving CC development, establish effective diagnostic tools, enhance early detection rates, and identify new therapeutic targets for more effective interventions.
ASAP1, also known as AMAP1, DDEF1, DEF1 or Centaurin β4, is a multidomain protein encoded by genes located on the long arm of chromosomal 8 (24.1-24.2). It binds to phosphoinositol phosphatide through its Pleckstrin homology domain [6]. ASAP1 also contains an SH3 domain and an SH3-binding precursor motif, along with ankyrin repeats, allowing it to interact with ligand proteins, such as C-SRC, Crk, CD2AP/CMS, CIN85, CrkL, POB1, and Pyk2. At its N-terminus, ASAP1 has a BAR domain, which enables ASAP1 to bend the membrane, a critical function for EGFR transport [7]. Subcellular analysis revealed that ASAP1 is located in adhesive plaques, plasma membrane folds, and around the nucleus [8-10]. According to a previous report, the expression of ASAP1 is also significantly increased in uveal melanomas, hepatocellular carcinoma, glioblastoma, thyroid cancer, colorectal cancer, gastric cancer and prostate cancer [11-17]. Moreover, previous studies have demonstrated that ASAP1 is involved in regulating cell movement and invasion [16-20].
ASAP1 may promote colorectal cancer metastasis by enhancing cell viability and aggressiveness, with its expression closely related to the aggressiveness of both colorectal cancer and prostate cancer cells [17,21]. Overexpression of ASAP1 has also been shown to promote cell migration in response to PDGF and IGF-1, with cell motility relying on the ARF-GAP activity of ASAP1 [22]. Mislocation of ASAP1 or siRNA-mediated downregulation of ASAP1 expression inhibits cell migration and disrupts epidermal growth factor (EGF) - dependent chemotaxis [20]. Numerous studies have shown that ASAP1 regulates key cell structures involved in cell viability and invasion, such as adhesion plaques and membrane folds [6,18]. ASAP1 interacts with proteins like C-SRC, SLK, Hck, Corn and betaPIX, which are dynamic adhesion structures that mainly exist in motor cells, facilitating tissue invasion and matrix reconstruction. These interactions have also been shown to play a role in the regulation of invasive pseudopodia formation [23,24]. ASAP1 overexpression inhibits cell proliferation and membrane folding induced by growth factors such as platelet-derived growth factor (PFGF). Mislocation of ASAP1 or siRNA-mediated knockdown also inhibits cell proliferation [20]. These observations may reflect the complex and multiple activities of ASAP1, and further studies are needed to understand its potential molecular biological mechanisms in tumor development. In summary, ASAP1 is an important regulator of tumor invasiveness and metastasis in a variety of tumor types. ASAP1, a multidomain adapter protein, influences tumor development through multiple pathways. Future research will likely focus on discovering new ways to interfere with the function of ASAP1 in metastatic cancer cells to treat tumors.
The fundamental traits of malignant tumors - metastasis and invasion - are directly linked to the poor prognosis of cancer patients. E-cadherin, N-cadherin, vimentin, and the matrix metalloproteinases (MMP2 and MMP9) exhibit aberrant expression during cancer cell invasion and metastasis. Tumor development involves a number of signaling pathways, one of which is the Wnt/β-catenin signaling pathway. This pathway is ubiquitous across many species and essential for tumor development, cell proliferation, and differentiation as well as for the invasion and migration of different tumors [25-28]. The role of β-catenin, a component of the Wnt signaling pathway, in CC remains to be further clarified. β-catenin is not only involved in intercellular cadherin interactions but also plays a critical role in both the cytoplasm and nucleus. Under normal conditions, β-catenin is maintained at low levels in the cytoplasm and nucleus through phosphorylation and proteasome degradation. However, studies have shown that approximately 15% of CCs exhibit abnormal nuclear localization of Wnt/β-catenin pathway proteins, along with decreased expression of β-catenin on the cell membrane. While Wnt activation is evident in CC, its exact mechanism remains unclear [25,29,30].
Materials and methods
Clinical specimen collection and case information
Sixteen surgically resected extrahepatic cholangiocarcinoma (EHCC) tissue samples were collected from the Department of General Surgery of Huadong Hospital Affiliated to Fudan University from March 2019 to May 2020. None of the patients had received adjuvant therapy, such as chemotherapy or radiotherapy, prior to surgery. Another 7 patients with benign bile duct disease composed the control group. All specimens and subsequent experiments were approved by the hospital ethics committee. The patients, aged 45 to 83 years old, included 11 males and 5 females. Postoperative pathological reports confirmed the diagnosis of EHCC.
After collecting EHCC tissue samples, we adopted a refined experimental design strategy to ensure the scientific and reliable outcomes of our research. Despite having a larger number of samples at our disposal, we specifically selected six pairs of specimens for an in-depth analysis for Figure 3. The reasons for choosing these specimens are as follows:
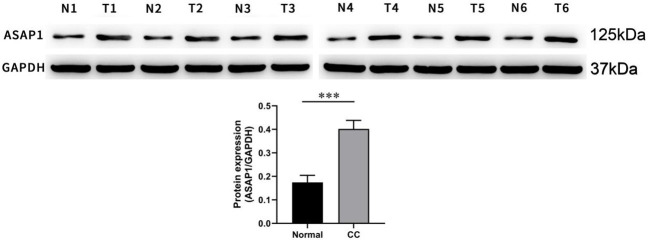
Figure 3.
ASAP1 protein expression in cholangiocarcinoma and its adjacent tissues. Notes: CC: cholangiocarcinoma; N: normal tissue; T: tumor tissue; ***P<0.001.
1. High ASAP1 Expression Levels: These six pairs demonstrated the highest levels of ASAP1 protein expression, making them ideal for studying ASAP1’s role in EHCC. 2. Pathological Confirmation: All selected samples were carefully diagnosed by pathologists, ensuring the accuracy and representativeness of the samples. 3. Complete Clinical Information: The patients from whom these samples were obtained had complete clinical information records, which is crucial for statistical analysis and clinical correlation studies. 4. Good Tissue Quality: The selected samples maintained high tissue quality throughout the collection, processing, and storage procedures, avoiding degradation or contamination. 5. Consistent Treatment Background: None of the selected patients had received chemotherapy or radiotherapy, ensuring that the treatment effects did not influence the experimental outcomes. 6. Ethical Review and Patient Consent: The use of all samples was approved by the ethics committee of Huadong Hospital Affiliated to Fudan University and consented to by the patients, ensuring the ethical compliance of the study.
Five strains of cholangiocarcinoma cells - QBC939, HuH28, RBE, HCCC 9810, LICCF and normal bile duct epithelial cells - were purchased from the Cell Bank of the Chinese Academy of Sciences. Primary HIBEC human bile duct epithelial cells were purchased from American Type Culture Collection (ATCC). All specimens were examined by the hospital’s pathology department and pathologically diagnosed with EHCC.
The study was conducted following the Declaration of Helsinki and received approval from the Ethics Committee of Shanghai Huadong Hospital.
Q-PCR was used to measure ASAP1 expression in both control and CC tissue samples. The specimens were collected immediately after surgical excision and placed in cryopreservation tubes within 30 minutes. The specimens were labeled and stored in a -80°C freezer for RNA and protein detection. During the preservation of sample tissues, repeated freezing and thawing should be avoided to prevent damage to tissue structure and degradation of RNA and protein.
Collection and analysis of follow-up data
According to the case inclusion criteria, case data, including age, sex, tumor differentiation status, lymph node metastasis status, and tumor stage, were collated (Table 1). The prognosis was evaluated by reviewing the patients’ detailed records with a normal follow-up protocol.
Table 1.
The clinicopathological parameters in patients with high and low ASAP1 expression
| Clinical parameters | N | ASAP1 Expression | ||
|---|---|---|---|---|
|
| ||||
| Low (%) | High (%) | P-value | ||
| Total | 16 | 9 (37.5) | 7 (62.5) | P>0.05 |
| Gender | ||||
| Male | 11 | 7 (63.6) | 4 (36.4) | P>0.05 |
| Female | 5 | 2 (40.0) | 3 (60.0) | P>0.05 |
| Age, years | ||||
| <70 | 8 | 6 (75.5) | 2 (25.5) | P>0.05 |
| ≥70 | 8 | 3 (25.5) | 5 (75.5) | P>0.05 |
| Tumor differentiation | ||||
| Well | 6 | 4 (66.7) | 2 (33.3) | P>0.05 |
| Moderate | 8 | 4 (50.0) | 4 (50.5) | P>0.05 |
| Poor | 2 | 1 (50.0) | 1 (50.0) | P>0.05 |
| Lymph metastasis | ||||
| Negative | 9 | 5 (55.6) | 4 (44.4) | P>0.05 |
| Positive | 7 | 4 (57.1) | 3 (42.9) | P>0.05 |
| Early or late stage | ||||
| I/II | 9 | 5 (55.6) | 4 (44.4) | P>0.05 |
| III/V | 7 | 4 (57.1) | 3 (42.9) | P>0.05 |
Bioinformatics analysis
Bioinformatics analysis of the relative expression of ASAP1 in CC patients in the Gene Expression Omnibus (GEO) CCA database was performed, and gene enrichment analysis of the relationship between high ASAP1 expression and Wnt/β-catenin signaling pathway activation in CC was conducted.
Cell lines and culture
The cell lines QBC939, HuH28, and LICCF were purchased from Jin Shao Yuan (Shanghai) Biotechnology Co., Ltd. (Shanghai, China). The FRH0201 cell line was obtained from Ying Wan Biotechnology Co., Ltd. (Shanghai, China). Additionally, human intrahepatic biliary epithelial cell (HIBEpiC), CCLP-1, and other cell types were maintained for the study. QBC939 cells and HIBEpiC cells were grown in DMEM (Gibco, Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Rockville, MD, USA) and 1% penicillin/streptomycin. RPMI-1640 (Gibco, Rockville, MD, USA) supplemented with 10% FBS (Gibco, Rockville, MD, USA) and 1% penicillin/streptomycin was used to cultivate RBE, FRH0201, and HCCC9810 cells. All cell lines were kept at 37°C with 5% CO2 in a humidified incubator.
Construction of shRNA-transfected and ASAP1-knockdown cell lines
CCLP-1 cells, which express relatively high levels of ASAP1, were selected for transfection. A preliminary experiment was conducted to determine the multiplicity of infection (MOI) value (MOI = 60) and optimal conditions for lentiviral infection. One day before transfection, 3-5×103 cells were inoculated in 6-well plates. Transfection was performed at 37°C for 16-24 hours when the cells reached approximately 30% confluency. Before transfection, the old medium was removed, and 1 ml of fresh medium was added. At the same time, the corresponding virus infection enhancer HiTransG P was added for approximately 4 h. According to the MOI value and titer, the corresponding amount of virus was added according to the following formula. Virus volume = MOI × number of cells/virus titer. When MOI = 60, approximately 3 µl of virus stock solution was added to each well, and the medium was adjusted to 2 ml. The mixture was incubated at 37°C and 95% humidity for 12-16 hours, after which the medium was replaced with fresh culture medium. If cell morphology changes were observed under the microscope, the duration of fluid exchange could be adjusted accordingly. The medium was also refreshed during culture as needed to maintain cell viability. After 72 hours, the infection efficiency was observed under a fluorescence microscope. Then, the resistant cells were screened with treminomycin (the concentration was determined to be 2 g/µl in the prescreening experiment) for 1 week. The selected positive cells were reserved for subsequent experiments.
RNA extraction and qRT-PCR
Using an RNA-PCR kit from TaKaRa (Tokyo, Japan), 2 µg of total RNA was reverse transcribed to produce first-strand cDNA. Total RNA was extracted from tissue samples and cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Following the manufacturer’s instructions, a SYBR Green PCR Master Mix kit (Applied Biosystems, Foster City, CA, USA) was used to perform real-time RT-PCR on the generated cDNA. The primers for ASAP1 were as follows: forward, 5’-GATTGCAGAACCCAAGTCAAGT-3’ and reverse, 5’-CTATTGGCGCCAAAATAGTCAG-3’. The primers for PTEN were as follows: forward, 5’-GACCAGAGACAAAAAGGGAGTA-3’ and reverse, 5’-ACAAACTGAGGATTGCAAGTTC-3’. Relative quantification was performed by the 2-ΔΔCt method.
Western blot analysis
Cell lysates were prepared using RIPA lysis buffer (Beyotime, Shanghai, China), and the total protein content was measured using a BCA protein detection kit (Pierce Biotechnology, Rockford, IL, USA). SDS-PAGE (salt dodecyl sulfate-polyacrylamide gel electrophoresis) was used to separate the target proteins, and the isolated proteins were then transferred to polyvinylidene fluoride (PVDF) membranes. Primary antibodies were incubated with the PVDF membranes at 4°C overnight after blocking with 5% bovine serum albumin for 1 hour. The membranes were then washed with TBST and incubated with secondary antibodies for one hour. The signals were detected using an enhanced chemiluminescence (ECL) detection reagent (Beyotime, Shanghai, China).
Cell proliferation and colony formation
Transfected cells were plated in 96-well plates at a density of 3×103 cells per well and incubated at 37°C for 24, 48, 72, 96, or 120 hours to assess cell proliferation. After that, 10 µg of the cell counting kit-8 (CCK-8) reagent from Dojindo (Kumamoto, Japan) was added to each well, which was then incubated at 37°C for 1 hour. The absorbance at 450 nm was measured with a microplate reader. For the colony formation assay, 500 cells per well were seeded into 6-well plates and cultured in DMEM supplemented with 10% FBS for 14 days. The colonies were fixed with methanol and stained with 0.5% crystal violet, after which visible colonies were counted.
EdU pulse-chase incorporation
Using the Cell-Light EdU DNA cell proliferation kit (RiboBio, Guangzhou, China), a 5-ethynyl-2’-deoxyuridine (EdU) experiment was carried out to evaluate cell proliferation. Transfected cells were cultured in 30-mm Petri plates until they reached a typical growth stage. Then, cell fixation, permeabilization, and EdU detection were carried out using a Click-iTTM EdU flow cytometry kit (Invitrogen, Carlsbad, CA, USA), as directed by the manufacturer. In 10 randomly chosen fields for each plate, EdU-labeled cells were manually counted, and the percentages were computed.
Subcutaneous tumorigenesis experiment in nude mice
Animal experiments were conducted in the Experimental Animal Center of East China Normal University with the approval of the Experimental Animal Ethics Committee of East China Normal University. Immunodeficient BALB/C nude mice (Shanghai Lake) aged 4 weeks and weighing 14±2 g, were divided into a no-load cell control group and an ASAP1 stable interference group. Each group consisted of 6 mice (3 males and 3 females). All the animals were housed in a laminar flow cabinet under SPF-grade conditions with free access to food and water. After 1 week of adaptive feeding, stable cells with logarithmic growth were collected, and 1×106 cells (either no-load cells or ASAP1 stable interference cells) in 100 μL of PBS were injected into the tail vein of each mouse to establish a model of subcutaneous tumors in nude mice. The survival of the nude mice was checked every three days. After six weeks, the subcutaneous tumors were removed, and the tissues were imaged. Both HE and IHC staining were performed on the tumor tissues for further analysis.
Euthanasia method
In this study, euthanasia was conducted in accordance with the American Veterinary Medical Association (AVMA) guidelines and approved by our Institutional Animal Care and Use Committee (IACUC) to ensure the most humane and ethical treatment of mice. Mice were euthanized using carbon dioxide (CO2) asphyxiation, a method recognized for its rapid induction of unconsciousness and minimal distress. The euthanasia chamber was maintained at ambient temperature, and mice were individually housed to prevent stress. CO2 was introduced gradually to reach a final concentration of 70-80%, ensuring a humane process. Continuous observation confirmed unconsciousness and death, which were verified by the absence of respiratory movements and heartbeat. Euthanasia details, including date, time, and conditions, were documented. Post-mortem, carcasses were handled with respect and in accordance with established protocols.
Statistical analysis
The Statistical Product and Service Solutions (SPSS) 17.0 statistical software package was used for statistical analyses (IBM, Armonk, NY, USA). Results were expressed as the mean ± standard deviation (SD). Unless otherwise noted, every experiment was carried out three times. A t test was used to assess the associations between ASAP1 and clinicopathological traits. A P value less than 0.05 was considered with statistical significance.
Results
Expression and clinical value of ASAP1 in cholangiocarcinoma
Differences in ASAP1 mRNA expression between cholangiocarcinoma tissues and normal tissues
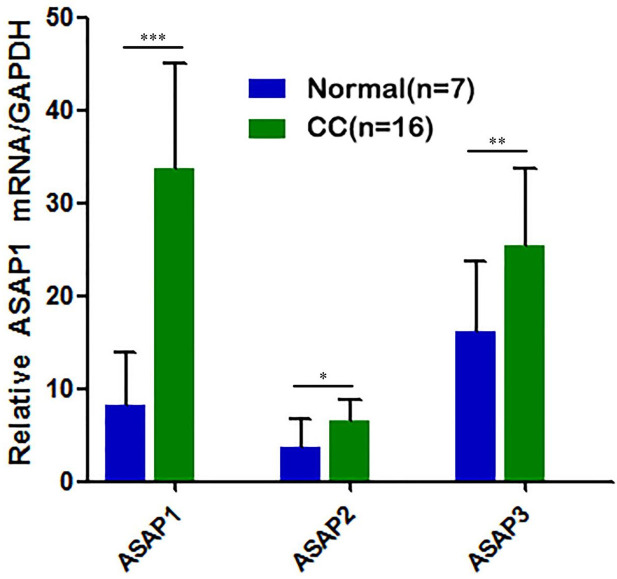
In the 16 CC and benign bile duct tissues, the expression of three members of the ADP ribosylation factor GTP-activated protein family was detected using Q-PCR, and ASAP1 was found to have the most significant difference in expression between CC and normal tissues (Figure 1).
Figure 1.
Expression of ASAP in cholangiocarcinoma (CC) tissues. Notes: *P<0.05, **P<0.01, ***P<0.001.
Results of the bioinformatics analysis
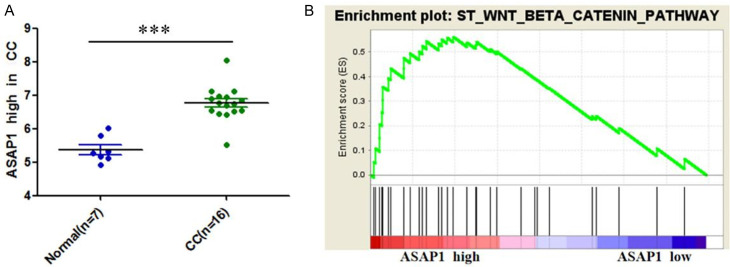
Bioinformatics analysis of the GEO CCA database (GSE32879) revelated a relatively high level of ASAP1 expression in CC. Gene enrichment analysis further demonstrated that elevated ASAP1 expression was positively associated with Wnt/β-catenin signaling activation in CC (Figure 2). This suggests that ASAP1 may contribute to the invasion and metastasis of CC through the activation of the Wnt/β-catenin signaling pathway.
Figure 2.
Bioinformatics analysis based on GEO CCA database (No. GSE32879). A. ASAP1 is highly expressed in the CC tissues; B. ASAP1 is positively correlated with Wnt/β-catenin signaling pathway activation. Notes: CC: cholangiocarcinoma; ***P<0.001.
Differences in ASAP1 protein expression levels in cholangiocarcinoma and adjacent tissues
Six pairs of specimens with the highest protein expression level of ASAP1 were selected for further analysis. Western blotting was used to detect the protein expression levels of the ASAP1in these specimens. The results showed that ASAP1protein expression was significantly higher in the CC tissues compared to the corresponding normal adjacent tissues (Figure 3).
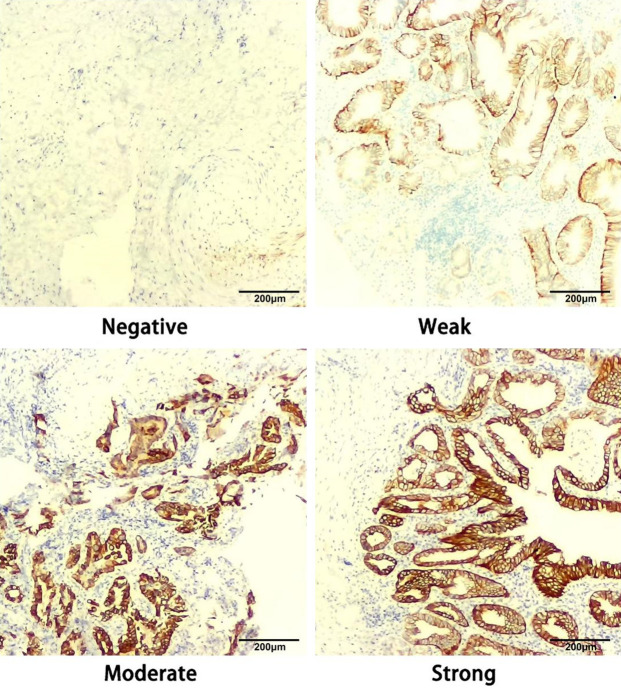
ASAP1 protein expression in CC tissues detected using immunohistochemistry
The protein expression of ASAP1 in 16 EHCC tissue samples was explored using immunohistochemical staining. The results showed that ASAP1 protein was mainly expressed in the cytoplasm of cancer cells. The intensity of staining was categorized as follows: 0 (negative), 1 (weak), 2 (moderate), and 3 (strong) (Figure 4). Based on ASAP1 expression intensity, the patients were divided into either a high or a low ASAP1 expression group. No significant differences were observed in clinical characteristics or survival rates between the high and low groups (Figure 5).
Figure 4.
Representative immunohistochemical images of ASAP1 expression in CC tissues. Note: CC: cholangiocarcinoma.
Figure 5.
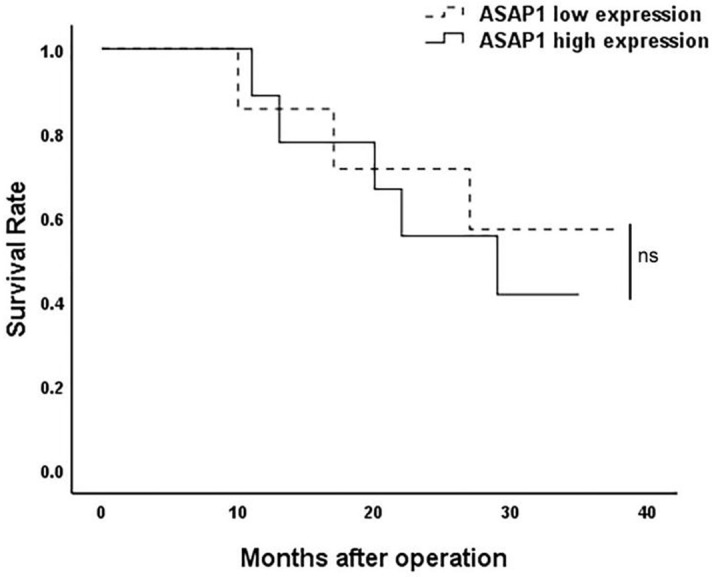
The survival rate of patients with low and high ASAP1 expression (ns, P>0.05).
Effect of regulating ASAP1 expression on the biological behaviors of CC cells
ASAP1 mRNA expression level in various bile duct cancer cell lines and normal bile duct epithelial cell lines
The mRNA expression of ASAP1 in 5 bile duct cancer cell lines (QBC939, HuH28, RBE, HCCC 9810, and CCLP-1) and normal bile duct epithelial cell line HIBEpiC was detected by RT-PCR. ASAP1 mRNA expression was dramatically higher in CCLP-1 cells than that in HIBEpiC, RBE and HuH28 cells. Among the bile duct cancer cell lines, QBC939, HuH28, RBE, and HCCC 9810 showed lower ASAP1 expression levels compared to CCLP-1 (Figure 6).
Figure 6.
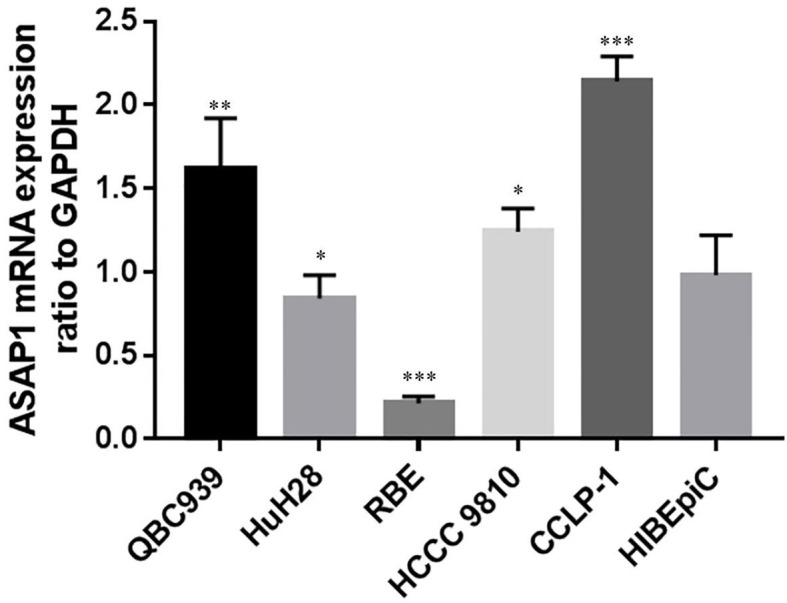
Expression of ASAP1 in different bile duct cancer cell lines and human intrahepatic biliary epithelial HIBEpiC cells. Notes: *P<0.05, **P<0.01, ***P<0.001.
Effect of ASAP1 on the biological behavior of bile duct cancer cells in vitro
Efficacy of ASAP1 knockdown or overexpression in hilar cholangiocarcinoma cells
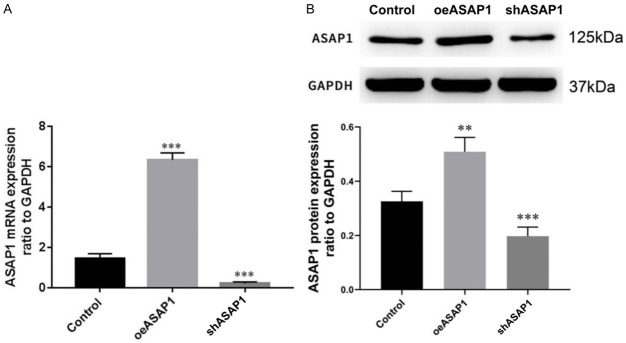
The cholangiocarcinoma cell line CCLP-1, which express high levels of ASAP1, were selected for further functional studies. CCLP-1 cells were divided into three groups: blank control group (Control), oeASAP1 group (cells with stable ASAP1 overexpression), and shASAP1 group (cells with stable ASAP1 knockdown) by transfection with corresponding lentiviruses. The transfection efficiency was detected by real-time quantitative PCR and Western blotting (Figure 7).
Figure 7.
Verification of lentiviruses transfection efficiency. A. The mRNA level detected by real-time fluorescence quantitative PCR; B. The protein level detected by Western Blotting. OeASAP-1: overexpression of ASAP-1; shASAP-1: interference of ASAP-1; **P<0.01, ***P<0.001.
Effect of ASAP1 on the proliferation of bile duct carcinoma cells
Cell growth curve of each experimental group was plotted according to the CCK-8 assay results. As shown in Figure 8, overexpression of ASAP1 induced cell proliferation, while knockdown of ASAP1 inhibited cell activity, indicating that ASAP1 can stimulate the proliferation of bile duct cancer cells in vitro.
Figure 8.
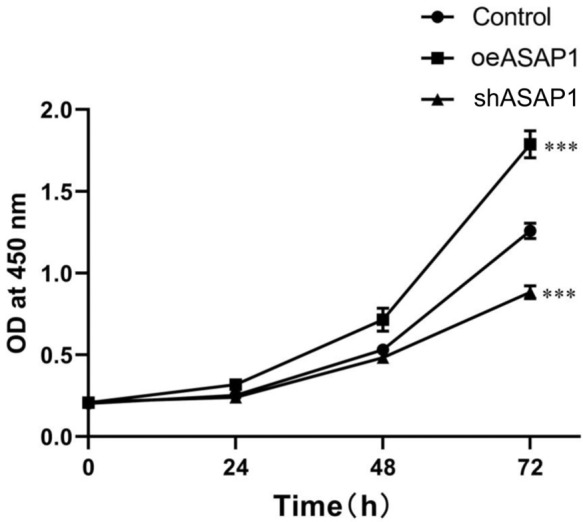
Cell proliferation detected by CCK-8 assay. ***P<0.001.
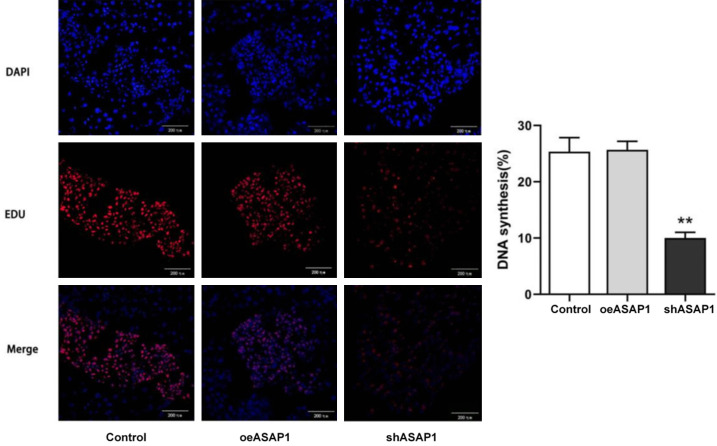
EdU pulse-chase incorporation assay
To further explore the effects of ASAP1 on cell proliferation, an EdU assay was performed. Compared with those in the Control group, ASAP1 knockdown inhibited the proliferation of CCLP-1 cells, while ASAP1 overexpression seems to have no significant effect on the proliferation of CCLP-1 cells (Figure 9). This finding also suggested that ASAP1 can accelerate the proliferation of bile duct cancer cells in vitro.
Figure 9.
Cell proliferation detected by EdU staining. **P<0.01, vs. Control group.
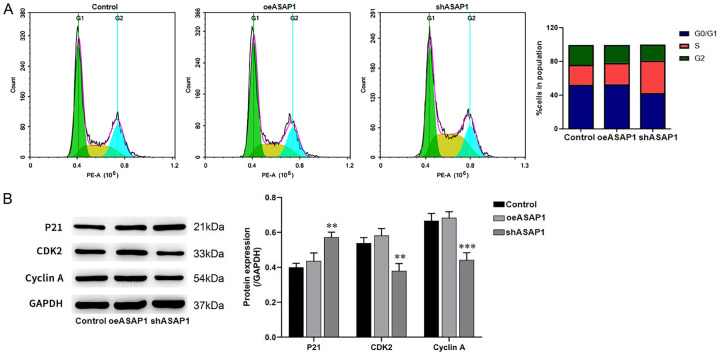
Regulation of the cycle of bile duct cancer cells by ASAP1
The above experiments confirmed the effect of ASAP1 on the proliferation of bile duct cancer cells. Given that cell cycle is closely related to cell proliferation, we examined impact of ASAP1 on the cell cycle using flow cytometry. After ASAP1 knockdown, the cell cycle was arrested in the S phase (Figure 10A). Western blot analysis revealed a decrease in the expression of CDK2 and Cyclin A, both of which are key regulators of the S phase, and an increase in the CDK inhibitor p21 (Figure 10B). These findings suggest that ASAP1 regulates bile duct cancer cell proliferation by influencing the cell cycle.
Figure 10.
Impact of ASAP1 on cell cycle progression and protein expression of cell cycle-related markers. A. Cell cycle distribution after ASAP1 knockdown in CCLP-1 cells; B. Expression of cell cycle related proteins detected by WB. **P<0.01, ***P<0.001, vs. Control group.
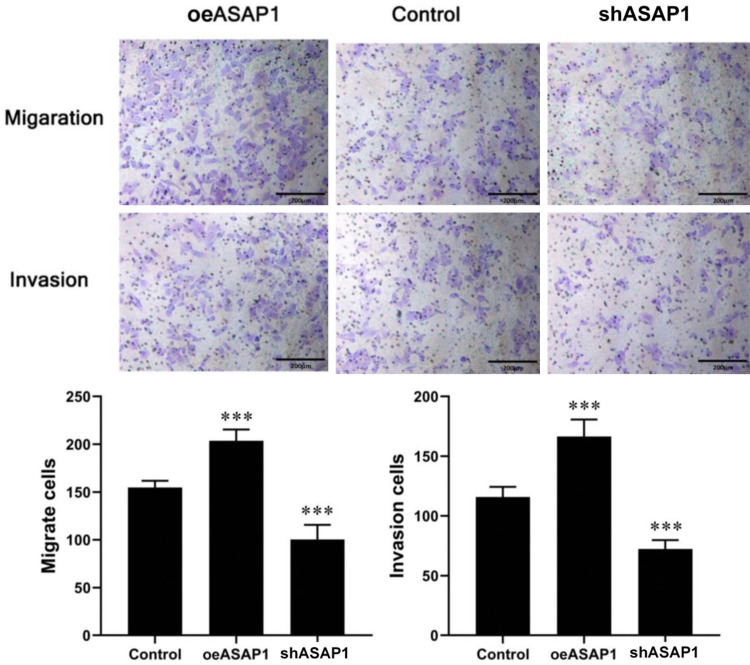
Knockdown of ASAP1 inhibited the migration of bile duct cancer cells
The migration ability of bile duct cancer cells was assessed using Transwell assays. The results revealed that compared the Control group, shASAP1 group exhibited reduced number of cells penetrating the Transwell membrane; However, oeASAP1 showed significantly increased number of cells penetrating the chamber (all P<0.001) (Figure 11).
Figure 11.
Effect of ASAP1 on cell migration and invasion ability detected by Transwell assay. ***P<0.001, vs. Control.
Effect of ASAP1 on the invasion of bile duct cancer cells
Transwell invasion assay revealed that, compared with those in the Control group, the invasion ability of CCLP1 cells was significantly decreased after ASAP1 knockdown but enhanced after ASAP1 overexpression (Figure 11). This finding implies that ASAP1 may promote the aggressiveness of bile duct cancer cells by enhancing cell invasion.
Effect of ASAP1 on the growth of subcutaneous transplanted hilar cholangiocarcinoma tumors in nude mice
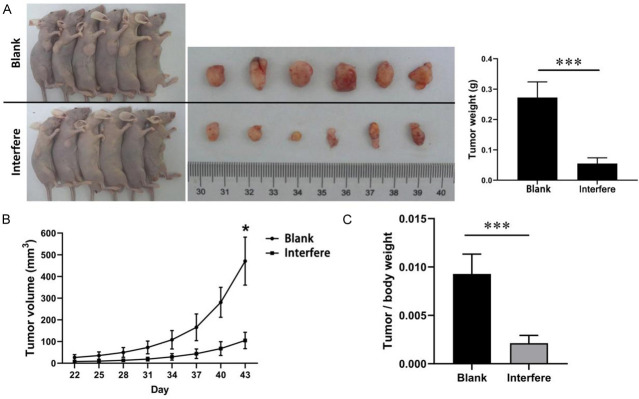
Previous in vitro experiments confirmed that ASAP1 promotes the proliferation of cholangiocarcinoma cells. However, the in vivo environment is more complex, and the role of ASAP1 in tumor formation in vivo remains unclear. To further investigate ASAP1’s role in promoting tumor proliferation in vivo, a subcutaneous tumor formation experiment was performed in nude mice using CCLP1 cells. Mice were subcutaneously injected with CCLP-1 cells with stable ASAP1 knockdown (interference group) or control CCLP-1 cells (blank group) (Figure 12A). The experimental outcomes demonstrated a substantial difference in tumor growth between the two groups. Tumors in the interference group grew at a much slower rate, with significantly smaller volume and weight compared to those in the blank group (Figure 12B, 12C) (P<0.01). These findings suggest that ASAP1 plays a crucial role in promoting tumor growth in vivo.
Figure 12.
Tumor growth and weight in mice bearing CCLP1 cell-transplanted xenograft. A. The growth and weight of transplanted tumors in nude mice; B. Growth curve of transplanted tumors; C. Tumor/body weight ratio. *P<0.05, ***P<0.001.
Potential molecular mechanisms by which ASAP1 regulates cell proliferation and apoptosis
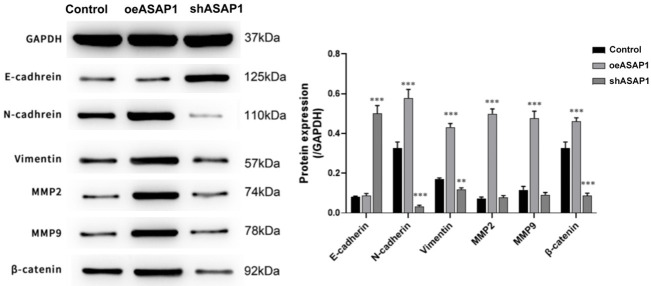
To explore how ASAP1 regulates cell proliferation and apoptosis, Western blotting was used to examine the expression levels of β-catenin, E-cadherin, and N-cadherin after ASAP1 overexpression or knockdown. The results indicated that oeASAP1 enhanced N-cadherin expression, but not strongly affected E-cadherin expression; shASAP1 decreased N-cadherin expression and significantly increased E-cadherin expression, and there are due to the role of ASAP1 in the activation of the Wnt/β-catenin signaling pathway (Figure 13). These findings suggest that ASAP1 promotes cell proliferation and invasion, partially by modulating the Wnt/β-catenin pathway and affecting cadherin-mediated cell adhesion.
Figure 13.
Effect of ASAP1 on protein expression of β-catenin, E-cadherin and N-cadherin. **P<0.01, ***P<0.001, vs. Control group.
Discussion
Early diagnosis and treatment of cholangiocarcinoma (CC) remain challenging due to its insidious onset and progression. For advanced CC, the treatment options are limited and often ineffective, leading to dismal prognosis for patients diagnosed at advanced stage. ASAP1 has been implicated in the development and progression of several solid tumors [13,14,20]. Aberrant ASAP1 expression is viewed as an independent unfavorable prognostic factor. The role of ASAP1 in cholangiocarcinoma, particularly in its expression patterns and associations with clinical features, remains unclear. Notably, no clear correlation between ASAP1 expression and factors such as age, sex, metastasis, or tumor differentiation has been established in patients.
In this study, we explored the biological activity and expression levels of ASAP1 in EHCC, noting its overexpression in tumor tissue compared to adjacent normal bile duct tissue. Although no significant differences were observed in clinical characteristics, pathological staging, or survival curves between high and low ASAP1 expression groups, which is likely due to our limited sample size, we observed a trend indicating longer survival periods for patients with lower ASAP1 expression. Echoing findings from acute myeloid leukemia and various solid tumors, including prostate cancer [17], colorectal cancer [21], and hepatocellular carcinoma [12], our results position ASAP1 as a pivotal factor in tumorigenesis. These insights, corroborated by our in vivo and in vitro experiments, underscore the potential of ASAP1 as a therapeutic target. Therefore, we advocate for future research in larger cohorts to fully elucidate the role of ASAP1 in CC and its potential as a treatment target.
In prostate cancer, downregulation of ASAP1 expression results in cell cycle arrest, apoptosis, and suppression of cell proliferation [17]. In colorectal cancer, ASAP1 expression is significantly upregulated, correlating with poor metastasis-free survival and prognosis [21]. In gastric cancer, ASAP1 promotes tumor progression and chemotherapy resistance. ASAP1 knockdown dramatically suppressed the growth and migration of gastric cancer cells, demonstrating its oncogenic role [16]. In this study, we used lentiviral particles to create a stable ASAP1-knockdown cell line to investigate the biological role of ASAP1 in CC cells. Our findings showed that blocking ASAP1 expression prevented the growth of CC cells both in vitro and in vivo. Conversely, overexpression of ASAP1 enhanced tumor cell proliferation in vitro. Additionally, ASAP1 downregulation caused cell cycle arrest in the S phase and promoted cell death, reinforcing its role in cancer cell survival.
Previous studies have shown that ASAP1 can promote invasion and metastasis via the EGFR-GEP100-Arf6 signaling pathway in breast cancer [31]. Activation of Arf6 by GEP100 disrupts E-cadherin-based cell-cell adhesion, and E-cadherin is a key protein in Wnt/β-catenin pathway. Increasing evidence demonstrates the important role of Wnt/β-catenin signaling in the development of liver cancer, including hepatocellular carcinoma (HCC) and cholangiocarcinoma (CAA), although the underlying mechanisms are complex [30]. Our bioinformatics analysis revealed that high ASAP1 expression was positively associated with Wnt/β-catenin signaling activation in CC (Figure 2). To further investigate this relationship, we examined the expression of β-catenin under different expression conditions of ASAP1 (Figure 13). The results showed that overexpression of ASAP1 significantly upregulated the expression of β-catenin, while inhibition of ASAP1 expression led to a significant downregulation of β-catenin expression, to a certain extent validating the bioinformatics findings. Subsequent in vitro experiments showed that ASAP1 can activate the Wnt/β-catenin pathway, promote the expression of N-cadherin and restrain the expression of E-cadherin. The in vitro experiments verified the results described above.
The role of Arf GTPase-activating protein 1 (ASAP1) in carcinogenesis has been extensively studied, particularly in colorectal and prostate cancers, where it is implicated in cell proliferation and invasion [11-17]. However, the specific role of ASAP1 in CC progression, as elucidated in this study, presents a novel perspective. Unlike its established function in other cancer types, our findings indicate that in CC, ASAP1 modulates the Wnt/β-catenin signaling pathway, which is critical for cell cycle regulation, apoptosis, and tumorigenesis [25-28]. This pathway’s dysregulation has been linked to poor prognosis in CC, suggesting that ASAP1’s influence on Wnt/β-catenin signaling may be a key driver of CC malignancy.
Our study reveals distinct mechanisms by which ASAP1 contributes to CC pathogenesis compared to other cancers. While in colorectal cancer, ASAP1 is reported to enhance cell viability and aggressiveness [21], our findings show that in CC, it directly affects cell cycle progression and apoptosis by modulating Wnt/β-catenin activity. This distinction underscores the complexity of ASAP1’s role across different cancer types and highlights the need for tissue-specific therapeutic strategies.
Conclusion
This study confirms that ASAP1 plays a proto-oncogene role in extrahepatic cholangiocarcinoma, both in vitro and in vivo. ASAP1 may regulate cell proliferation, affect the cell cycle progression, and modulate apoptosis by regulating the Wnt/β-catenin signaling pathway.
Acknowledgements
We deeply appreciate the support of all participants.
Informed consent has been obtained from human subjects for related experiments.
Disclosure of conflict of interest
None.
References
- 1.Sarcognato S, Sacchi D, Fassan M, Fabris L, Cadamuro M, Zanus G, Cataldo I, Capelli P, Baciorri F, Cacciatore M, Guido M. Cholangiocarcinoma. Pathologica. 2021;113:158–169. doi: 10.32074/1591-951X-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ilyas SI, Affo S, Goyal L, Lamarca A, Sapisochin G, Yang JD, Gores GJ. Cholangiocarcinoma - novel biological insights and therapeutic strategies. Nat Rev Clin Oncol. 2023;20:470–486. doi: 10.1038/s41571-023-00770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moris D, Palta M, Kim C, Allen PJ, Morse MA, Lidsky ME. Advances in the treatment of intrahepatic cholangiocarcinoma: an overview of the current and future therapeutic landscape for clinicians. CA Cancer J Clin. 2023;73:198–222. doi: 10.3322/caac.21759. [DOI] [PubMed] [Google Scholar]
- 4.Ruff SM, Diaz DA, Pitter KL, Hartwell BC, Pawlik TM. Multidisciplinary management in the treatment of intrahepatic cholangiocarcinoma. CA Cancer J Clin. 2023;73:346–352. doi: 10.3322/caac.21779. [DOI] [PubMed] [Google Scholar]
- 5.Elvevi A, Laffusa A, Scaravaglio M, Rossi RE, Longarini R, Stagno AM, Cristoferi L, Ciaccio A, Cortinovis DL, Invernizzi P, Massironi S. Clinical treatment of cholangiocarcinoma: an updated comprehensive review. Ann Hepatol. 2022;27:100737. doi: 10.1016/j.aohep.2022.100737. [DOI] [PubMed] [Google Scholar]
- 6.Randazzo PA, Hirsch DS. Arf GAPs: multifunctional proteins that regulate membrane traffic and actin remodelling. Cell Signal. 2004;16:401–413. doi: 10.1016/j.cellsig.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Nie Z, Hirsch DS, Luo R, Jian X, Stauffer S, Cremesti A, Andrade J, Lebowitz J, Marino M, Ahvazi B, Hinshaw JE, Randazzo PA. A BAR domain in the N terminus of the Arf GAP ASAP1 affects membrane structure and trafficking of epidermal growth factor receptor. Curr Biol. 2006;16:130–139. doi: 10.1016/j.cub.2005.11.069. [DOI] [PubMed] [Google Scholar]
- 8.Brown MT, Andrade J, Radhakrishna H, Donaldson JG, Cooper JA, Randazzo PA. ASAP1, a phospholipid-dependent arf GTPase-activating protein that associates with and is phosphorylated by Src. Mol Cell Biol. 1998;18:7038–7051. doi: 10.1128/mcb.18.12.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Loijens JC, Martin KH, Karginov AV, Parsons JT. The association of ASAP1, an ADP ribosylation factor-GTPase activating protein, with focal adhesion kinase contributes to the process of focal adhesion assembly. Mol Biol Cell. 2002;13:2147–2156. doi: 10.1091/mbc.E02-01-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Randazzo PA, Andrade J, Miura K, Brown MT, Long YQ, Stauffer S, Roller P, Cooper JA. The Arf GTPase-activating protein ASAP1 regulates the actin cytoskeleton. Proc Natl Acad Sci U S A. 2000;97:4011–4016. doi: 10.1073/pnas.070552297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richards JR, Shin D, Pryor R, Sorensen LK, Sun Z, So WM, Park G, Wolff R, Truong A, McMahon M, Grossmann AH, Harbour JW, Zhu W, Odelberg SJ, Yoo JH. Activation of NFAT by HGF and IGF-1 via ARF6 and its effector ASAP1 promotes uveal melanoma metastasis. Oncogene. 2023;42:2629–2640. doi: 10.1038/s41388-023-02792-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bang S, Jee S, Son H, Cha H, Sim J, Kim Y, Park H, Myung J, Kim H, Paik S. Clinicopathological implications of ASAP1 expression in hepatocellular carcinoma. Pathol Oncol Res. 2022;28:1610635. doi: 10.3389/pore.2022.1610635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei Y, Lu C, Zhou P, Zhao L, Lyu X, Yin J, Shi Z, You Y. EIF4A3-induced circular RNA ASAP1 promotes tumorigenesis and temozolomide resistance of glioblastoma via NRAS/MEK1/ERK1-2 signaling. Neuro Oncol. 2021;23:611–624. doi: 10.1093/neuonc/noaa214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang N, Yang Y, Zhao G, Yuan Q, Liu Z, Wang X, Geng Z, Jia M, Zheng J, Lu X, Yue J, Fan Y. Knockout of ASAP1 induces autophagy in papillary thyroid carcinoma by inhibiting the mTOR signaling pathway. Pathol Res Pract. 2020;216:152950. doi: 10.1016/j.prp.2020.152950. [DOI] [PubMed] [Google Scholar]
- 15.Gowrikumar S, Primeaux M, Pravoverov K, Wu C, Szeglin BC, Sauve CG, Thapa I, Bastola D, Chen XS, Smith JJ, Singh AB, Dhawan P. A Claudin-based molecular signature identifies high-risk, chemoresistant colorectal cancer patients. Cells. 2021;10:2211. doi: 10.3390/cells10092211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie W, Han Z, Zuo Z, Xin D, Chen H, Huang J, Zhu S, Lou H, Yu Z, Chen C, Chen S, Hu Y, Huang J, Zhang F, Ni Z, Shen X, Xue X, Lin K. ASAP1 activates the IQGAP1/CDC42 pathway to promote tumor progression and chemotherapy resistance in gastric cancer. Cell Death Dis. 2023;14:124. doi: 10.1038/s41419-023-05648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin D, Watahiki A, Bayani J, Zhang F, Liu L, Ling V, Sadar MD, English J, Fazli L, So A, Gout PW, Gleave M, Squire JA, Wang YZ. ASAP1, a gene at 8q24, is associated with prostate cancer metastasis. Cancer Res. 2008;68:4352–4359. doi: 10.1158/0008-5472.CAN-07-5237. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, Luo Q, Feng R, Yang F, Xu Q, Chen X, Yang S. ADP ribosylation factor guanylate kinase 1 promotes the malignant phenotype of gastric cancer by regulating focal adhesion kinase activation. Life Sci. 2021;273:119264. doi: 10.1016/j.lfs.2021.119264. [DOI] [PubMed] [Google Scholar]
- 19.Schreiber C, Gruber A, Rosswag S, Saraswati S, Harkins S, Thiele W, Foroushani ZH, Munding N, Schmaus A, Rothley M, Dimmler A, Tanaka M, Garvalov BK, Sleeman JP. Loss of ASAP1 in the MMTV-PyMT model of luminal breast cancer activates AKT, accelerates tumorigenesis, and promotes metastasis. Cancer Lett. 2022;533:215600. doi: 10.1016/j.canlet.2022.215600. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Yerushalmi GM, Grigera PR, Parsons JT. Mislocalization or reduced expression of Arf GTPase-activating protein ASAP1 inhibits cell spreading and migration by influencing Arf1 GTPase cycling. J Biol Chem. 2005;280:8884–8892. doi: 10.1074/jbc.M412200200. [DOI] [PubMed] [Google Scholar]
- 21.Muller T, Stein U, Poletti A, Garzia L, Rothley M, Plaumann D, Thiele W, Bauer M, Galasso A, Schlag P, Pankratz M, Zollo M, Sleeman JP. ASAP1 promotes tumor cell motility and invasiveness, stimulates metastasis formation in vivo, and correlates with poor survival in colorectal cancer patients. Oncogene. 2010;29:2393–2403. doi: 10.1038/onc.2010.6. [DOI] [PubMed] [Google Scholar]
- 22.Furman C, Short SM, Subramanian RR, Zetter BR, Roberts TM. DEF-1/ASAP1 is a GTPase-activating protein (GAP) for ARF1 that enhances cell motility through a GAP-dependent mechanism. J Biol Chem. 2002;277:7962–7969. doi: 10.1074/jbc.M109149200. [DOI] [PubMed] [Google Scholar]
- 23.Cougoule C, Carréno S, Castandet J, Labrousse A, Astarie-Dequeker C, Poincloux R, Le Cabec V, Maridonneau-Parini I. Activation of the lysosome-associated p61Hck isoform triggers the biogenesis of podosomes. Traffic. 2005;6:682–694. doi: 10.1111/j.1600-0854.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 24.Webb BA, Eves R, Crawley SW, Zhou S, Côté GP, Mak AS. PAK1 induces podosome formation in A7r5 vascular smooth muscle cells in a PAK-interacting exchange factor-dependent manner. Am J Physiol Cell Physiol. 2005;289:C898–907. doi: 10.1152/ajpcell.00095.2005. [DOI] [PubMed] [Google Scholar]
- 25.Song P, Gao Z, Bao Y, Chen L, Huang Y, Liu Y, Dong Q, Wei X. Wnt/beta-catenin signaling pathway in carcinogenesis and cancer therapy. J Hematol Oncol. 2024;17:46. doi: 10.1186/s13045-024-01563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue W, Yang L, Chen C, Ashrafizadeh M, Tian Y, Sun R. Wnt/beta-catenin-driven EMT regulation in human cancers. Cell Mol Life Sci. 2024;81:79. doi: 10.1007/s00018-023-05099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, Zhou Z, Shu G, Yin G. Wnt/beta-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7:3. doi: 10.1038/s41392-021-00762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selvaggi F, Catalano T, Lattanzio R, Cotellese R, Aceto GM. Wingless/It/beta-catenin signaling in liver metastasis from colorectal cancer: a focus on biological mechanisms and therapeutic opportunities. World J Gastroenterol. 2023;29:2764–2783. doi: 10.3748/wjg.v29.i18.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lozano E, Sanchon-Sanchez P, Morente-Carrasco A, Chinchilla-Tabora LM, Mauriz JL, Fernandez-Palanca P, Marin JJG, Macias RIR. Impact of aberrant beta-catenin pathway on cholangiocarcinoma heterogeneity. Cells. 2023;12:1141. doi: 10.3390/cells12081141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He S, Tang S. WNT/beta-catenin signaling in the development of liver cancers. Biomed Pharmacother. 2020;132:110851. doi: 10.1016/j.biopha.2020.110851. [DOI] [PubMed] [Google Scholar]
- 31.Sabe H, Hashimoto S, Morishige M, Ogawa E, Hashimoto A, Nam JM, Miura K, Yano H, Onodera Y. The EGFR-GEP100-Arf6-AMAP1 signaling pathway specific to breast cancer invasion and metastasis. Traffic. 2009;10:982–993. doi: 10.1111/j.1600-0854.2009.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]