Abstract
Colorectal cancer (CRC) is one of the most widespread tumor types, and it stands as the second leading cause of disease-related mortality globally. Due to its adverse effects, which lead to low patient adherence, new alternatives to conventional chemotherapy and radiotherapy treatments are being studied. Since, in most cases, platelets are positively involved in the persistence and progression of CRC, several elements of the platelet signaling pathway have been considered possible therapeutic targets. The present study assembles the main treatments for CRC and investigates the cellular mechanisms involved in the interaction between blood platelets and cancer cells. Additionally, this review cites other articles that propose possible therapeutic targets in the platelet activation pathways to be explored. Despite the reported benefits of antithrombotic therapy on CRC progression, some studies have warned about an increased bleeding risk and CRC incidence and highlight the importance of controlling this therapy through diagnostic tests. However, their high cost is still a significant obstacle to the population’s access from low Human Development Index (HDI) countries. Many research groups have studied platelet signaling pathways in depth to develop a safer, more effective, and affordable therapy for the population.
Keywords: Colorectal cancer, antineoplastic agents, tumor microenvironment, blood platelets, platelet activation, platelet aggregation inhibitors
Introduction
Colorectal cancer
Epidemiology, etiology, histological aspects
According to the World Health Organization (WHO), in 2018, there were 18.1 million cancer cases globally, with 9.6 million deaths, 90% due to metastasis. Cancer incidence and mortality rates are higher in males (9.5 million new cases; 5.4 million deaths) compared to females (8.6 million new cases; 4.2 million deaths). Colorectal cancer (CRC) is one of the most prevalent cancers in both sexes, ranking third in incidence (10.2%) after breast (11.6%) and lung cancers (11.6%), and second in mortality (9.2%) after lung cancer (18.4%) [1,2]. In Brazil, from 2005 to 2018, CRC incidence tripled (14.6 to 51.4 per 100,000 inhabitants), particularly among those aged 50-69. Incidence increased in those under 50 and over 70 due to health policies and lifestyle changes. The Human Development Index (HDI) inversely correlates with CRC occurrence, with the Brazilian Northeast having a lower HDI, showing the second-highest CRC incidence [3].
CRC originates from polyps in the colon or rectum, developing 10-35 years after their appearance [4-6]. Polyps smaller than 1 cm resemble normal cells, while larger ones have a higher cancer risk [4,5]. Inflammatory bowel diseases (IBD), like Crohn’s disease or ulcerative colitis, increase the risk of colitis-associated cancer (CAC), with an 18-20% higher risk in long-term ulcerative colitis patients [6]. While most CRC tumors are not inflammation-induced, inflammatory processes can trigger CRC of sporadic or hereditary origin, and anti-inflammatory drugs may prevent or delay the disease. Furthermore, in the consensus molecular subtype (CMS) 1, which is the immunogenic subtype and encompasses the majority of tumors with microsatellite instability (MSI), and the CMS 4 (mesenchymal subtype) cancers, a significant increase in the presence of lymphoid and myeloid-specific genes were observed [7]. Factors like tissue damage, mucus consistency changes, and certain bacteria (e.g., Clostridia) can cause inflammation and neoplasms through carcinogenic metabolites and pro-inflammatory cytokines (e.g., IL-17, IL-10) [6].
The predisposing hereditary factor is also an important cause of CRC, with Lynch Syndrome (LS) being the main cause of hereditary CRC. LS is an autosomal dominant disorder caused by germline missense, nonsense, or frameshift mutations with decreased expression of one or more genes responsible for the DNA mismatch repair (MMR) mechanism (MLH1, MSH2, MSH6, PMS2, and EPCAM), which leads to MSI [8-13]. The MMR (Mismatch Repair) proteins form heterodimeric complexes that detect and bind to DNA regions containing mispaired nucleotides, facilitating the repair of mismatches. The integrity of MMR proteins, as well as their functional loss, is modulated by microRNAs (miRNAs). Specifically, miR-155 has been shown to negatively regulate MSH2-MSH6 and MLH1-PMS2 complexes, contributing to the emergence of a mutant phenotype [9,11].
The loss of function of one of the MMR proteins causes an accumulation of errors, such as insertions and deletions in the microsatellite region of DNA, leading to genetic instability. MSI may have oncogenic potential when it occurs in the genome’s coding region with genes essential for cellular function or negative regulation of cancer (e.g., MRE11A, TGFBRII, BAX). Heterozygous mutations of MMR genes can also increase the susceptibility of the normal alleles to a secondary somatic mutation [8-13]. Germline mutations in the MLH1 and MSH2 genes account for approximately 70% of all hereditary mutations. Beyond traditional germline mutations, MLH1 and MSH2 are also susceptible to constitutional epimutations. Somatic alterations in the APC gene are more frequently observed in MSH2-associated tumors than those involving MLH1. Conversely, pathogenic variants in the CTNNB1 (catenin beta-1) gene are more prevalent in MLH1-related tumors. Differences in the development pathways of screen-identifiable and non-identifiable colorectal tumors were also observed. Mutations in the MLH1 and MSH2 genes present a greater risk for the development of CRC (30%-97% risk) and the early onset of the disease (27-42 years). Patients with LS are also at risk for the incidence of synchronous and metachronous tumors (e.g., endometrial cancer, gastric cancer) [8,9].
CRC causes macroscopic and microscopic tissue changes, including diminished mucus production and lesions like aberrant crypt foci (ACF). These ACFs, which can potentially progress into adenomas and carcinomas, are comprised of hyperplastic and dysplastic cells. Genetic differences distinguish hyperplastic ACFs from dysplastic ones, with some studies suggesting that hyperplastic ACFs may progress to dysplastic lesions. Additionally, the histological characteristics of tumors differ between the proximal (poorly differentiated cells) and distal (medium to high differentiation adenomas and adenocarcinomas) regions of the intestine [14].
Colorectal tumors contain tumor-initiating cells with stem cell-like characteristics, leading to therapy resistance through IL-22-induced STAT3 phosphorylation. Moreover, Paneth cell dysfunction in the small and proximal large intestine can trigger inflammation and cancer [7]. The non-muscleβ and γ-actin fibers are crucial for CRC cell migration and invasion. These fibers are found in submembrane rings, pseudopods, and proteins like ezrin and myosin [15].
Molecular characteristics
Seventy-five percent of colorectal cancer (CRC) cases occur due to the inactivation of suppressor genes p53, adenomatous polyposis coli (APC), and deleted in colon carcinoma (DCC) through loss of heterozygosity. Fifty percent results from point mutations in “ras” family proto-oncogenes, particularly K-ras, and a few from amplifying the myc proto-oncogene [14,16]. Additional mutations or increased expression of genes such as β-catenin, K-ras, transforming growth factor beta (TGF-β), and metalloproteinase 9 (MMP-9) are also observed, along with increased fatty acid synthesis and oxidation due to enzymes like iNOS, COX-2, and fatty acid synthase [14,17].
In the early stages of CRC, mutations in APC and ras genes are observed in polyps, while mutations in DCC and p53 appear later in malignant cells [18,19]. Proteins such as p53 induce apoptosis in cells with the potential to become cancerous. According to Ma et al., 2013 [20], p53 reduces the expression of proteins that promote cell survival (e.g., survivin) and cell death through an RNA fragment named miR-16. In CRC, miR-16 levels are reduced, and its expression is inversely correlated with the degree of cell differentiation. p53 increases protein levels of miR-16, which binds to the 3’untranslated region (3’UTR) of survivin, preventing its synthesis. Ultimately, increased expression of miR-16 eventually leads to p53 downregulation.
The Wnt signaling cascade is another pathway involved in many functions that affect the living organism, ranging from physiological processes to pathological conditions such as inflammation and cancer. This pathway supports regeneration and cell survival, facilitates the epithelial-to-mesenchymal transition (EMT) promoting metastasis, and modulates interactions between neoplastic cells and elements in the microenvironment, such as leukocytes and fibroblasts. A wide variety of cytokines activates the Wnt pathway, which is divided into two branches: (1) the Wnt/β-catenin pathway or classical pathway, which is involved in cell survival, differentiation, proliferation, and migration, and (2) the non-canonical β-catenin-independent pathway, which is encompassed by the pathway involved in planar cell polarity (Wnt-PCP) and the Wnt-Ca2+ pathway, which in turn modulate cell polarity and migration. When Wnt does not stimulate cells, β-catenin is phosphorylated by glycogen synthase kinase 3β (GSK-3β) and linked to a ubiquitin-dependent proteolytic pathway by the APC and Axin proteins [21-23].
In the classical pathway, Wnt binds to a complex of LRP5 or LRP6 receptors and proteins of the Fzd family. This binding leads to the recruitment of cytosolic proteins and disruption or prevention of the formation of the β-catenin/GSK-3β/Axin complex/APC. Consequently, there is an accumulation of β-catenin in the cytoplasm, facilitating its translocation to the nucleus, which regulates the expression of Wnt target genes. The Wnt/β-catenin pathway governs the division of the cells that form the intestinal crypts, and most colorectal tumors occur through modifications that increase the activation of this pathway. Furthermore, the Wnt/β-catenin pathway can synergize with the TGF-β and Notch pathways, favoring tumorigenesis and disseminating neoplastic cells throughout the body. The β-catenin-independent pathways contributing to CRC progression include (a) the receptor tyrosine kinase-like orphan receptor (ROR) signaling pathway; (b) the activation of the Wnt/β-catenin pathway by the transcription factor SP1 through the circular RNA circ_0026628; (c) the p38MAPK activation pathway; and (d) the MyD88-induced Wnt/β-catenin activation pathway. Additionally, there are inhibitory pathways that favor the tumor cell. Circular RNAs such as circ_0026628 and circ_0082182 capture micro RNAs, miR-346, miR-411, and miR-1205, which play negative regulatory roles in tumorigenesis. Another counterbalanced pathway involves the decreased expression of long non-coding RNAs such as ADAMTS9-AS1, which are negative modulators of the Wnt/β-catenin pathway [21,24-27].
β-catenin, a critical transcription factor for cell proliferation and adhesion, is negatively regulated by the APC gene and GSK-3β. β-catenin also inhibits the mechanisms that lead to cell apoptosis, such as the formation of autophagosomes and the synthesis of the p62/SQSTM1 protein through the TCF4 factor. In some conditions, such as hypoxia, it forms a complex with the LC3 protein, leading to the degradation and reduction of serum levels. Due to APC and β-catenin mutations and the increased activation of the signaling pathway induced by the Wnt protein, β-catenin accumulates in the nucleus where it associates with T cell factor (TCF) and promotes the activation of cyclin D1, c-myc, and PPAR-δ [14,21].
The K-ras gene mutation activates the PI3K/Akt and MEK/MAPK/ERK pathways, which are associated with increased expression of cyclin D1 and the c-erbB-2 receptor, as well as decreased expression of GTPase-activating protein expression. TGF-β, produced by adenomas and adenocarcinomas, facilitates bloodstream entry and negatively regulates leukocyte function [14]. Additionally, other factors, such as platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF), are also increased, promoting angiogenesis, cell proliferation, and glycolysis [28].
Differences in cellular kinetics, sensitivity to carcinogens and chemotherapeutics, and gene expression (K-ras, COX-2, PPAR-δ) are noted between the proximal and distal colon [14]. One distinction between the majority of CRCs and CAC is that, in the CRCs, mutations leading to the activation of the Wnt/β-catenin pathway, such as the inhibition of APC, GSK-3β, and mutations in β-catenin, often occur at an early stage of the disease’s progression. In contrast, in CAC, dysplasia is suspected to be caused by ROS produced during inflammation, and activation of the Wnt/β-catenin pathway occurs later, following mutations in the p53 and K-ras genes [6,21].
In both IBD and CRC, an increase in the activation of nuclear factor kappa B (NF-kB), a factor mainly regulated by the Tumor Necrosis Factor (TNF), is observed. This cytokine signaling pathway promotes the stabilization of the Snail factor, which is essential for tumor epithelial-mesenchymal transition (EMT) [29]. Moreover, in an inflammatory model induced by lipopolysaccharide (LPS), tumor cells benefit from activating the TNF-α/NF-kB pathway to survive and metastasize [30]. This evidence emphasizes that the inflammatory process is an essential factor in the positive modulation of the disease.
Interaction between CRC cells and the tumoral microenvironment
The tumor microenvironment (TME) comprises a dynamic interplay among various cell types, including immune cells (e.g., macrophages, CD4 cells, CD8 cells, Treg cells, and NK cells), extracellular matrix components, endothelial cells, and platelets. Inflammation attracts these cells, leading to an increase in the secretion of cytokines and chemokines. Cancer cells can connect to and regulate constituents of the TME in order to help them grow and evade the immune system [31,32]. In CRC, increased expression of NADPH oxidase 1 (NOX1) and generation of H2O2 by intestinal bacteria promotes neoplastic cell proliferation through activation of the Wnt/β-catenin pathway. Generation of CD45RO cells, a memory cell subpopulation of CD4 T lymphocytes, is directly proportional to tumor cell survival. The events that lead to increased generation of this population of cells are mainly microsatellite instabilities and LINE-1 gene methylation [32].
Regulatory T cells, another immune system element, can also positively modulate inflammation and tumor development by synthesizing and releasing the RAR-related orphan receptor type γ (RORγt) via the Wnt/β-catenin pathway. In addition, stromal cells can activate this signaling pathway and induce tumor cell proliferation by secreting the R-spondin-3 (RSPO3) protein. Conversely, CRC cells can produce phosphatidic acid through PLD2 activation, which causes the senescence of fibroblasts in the microenvironment, favoring cancer cell proliferation. Furthermore, the TME can inhibit the maturation of dendritic cells, causing them to assume a regulatory phenotype and promote tumor survival [21,32]. However, not all TME elements support the survival and proliferation of neoplastic cells; some play a crucial role in the defense against these cells. For example, in CRC, macrophage infiltration is related to a good prognosis. This is attributed to the disease regression being directly linked to an increase in the M1 macrophage subpopulation, exerting an anti-tumorigenic effect. Stage III CRC patients treated with the chemotherapy drug fluorouracil (5-FU) led to increased M1 macrophage infiltration [32].
Role of the platelets in the colorectal cancer
Platelet’s function
Platelets are the most minor functioning units, originating from megakaryocytes in the bone marrow, and are crucial for maintaining hemostasis. The production of platelets depends on the activity of thrombopoietin and the size of the megakaryocyte nucleus. Inflammatory mediators such as interleukin (IL)-6, IL-1, and TNF-α also activate platelet precursors. Under physiological conditions, when platelet levels in the peripheral circulation increase, the liver reduces the secretion of thrombopoietin, leading megakaryocytes to produce platelets through the fragmentation of their cytoplasm. However, in inflammatory conditions, cytokines like IL-6 stimulate increased platelet production by promoting the generation of thrombopoietin and its direct contact with megakaryocytes [33].
Platelets have various receptors that allow them to respond to different agonists associated with endothelial injury and interact with one another. They have GPIbα and GPVI, which are involved in thrombus formation. The GPIIbIIIa receptor, or αIIbβ3 integrin, participates in platelet adhesion and aggregation. Additionally, platelets contain other receptors, such as the toll-like receptor (TLR), essential in eliminating microorganisms [34]. In the submembrane region, actin filaments are responsible for platelet shape change, release of substances, and translocation of receptors in the cytoplasm to the cell surface [35]. Platelets are in constant contact with mediators in the plasma through the canalicular system. This channel is a means for releasing granular contents and assists in agonist-induced cell shape change. Platelets have three compartments in their cytoplasm: α, dense, and lysosomal granules. These granules contain important factors for coagulation and hemostasis (e.g., ADP, P-selectin, factor V, VWF), which, upon activation, are released into the cytoplasm or are transferred to the cell membrane [34,35].
Although they do not possess a nucleus, platelets have 3000-6000 transcripts among strands of pre-messenger RNA (pre-mRNA) and messenger RNA (mRNA) that are used in protein synthesis through the mTOR signaling pathway. Furthermore, they have non-coding fragments of RNA (miRNA), important in gene regulation and pathological processes. These fragments also lead to other effects, such as decreased ICAM-1 expression in the endothelium during myocardial infarction [36-38].
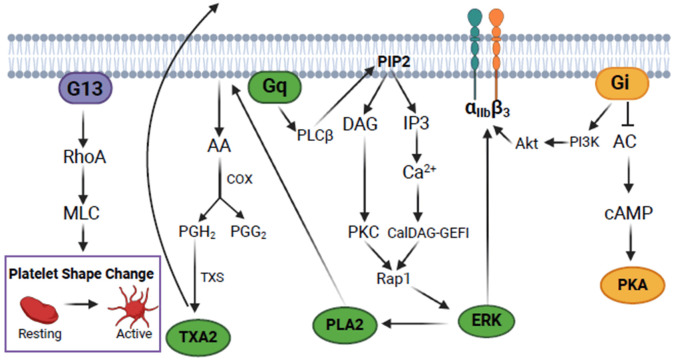
Platelets circulate in the plasma alone but activate and form aggregates in response to endothelial injury. GPIbα and GPVI bind to von Willebrand factor (VWF) and collagen in the exposed subendothelial layer, stabilizing the platelet adhesion. This process triggers a range of events that culminate in the propagation of the platelet activation signal through the secretion of agonists (e.g., ADP, TXA2), which promote cell activation through autocrine and paracrine pathways [34]. Many processes that promote activation and constitute the inside-out pathway are induced by G protein-coupled receptors G13, Gq, and Gi, such as ADP (P2Y1, P2Y12) and thrombin (PAR1, PAR4) receptors. In turn, the G13 protein promotes the activation of the GTPase RhoA, which inhibits the phosphatase of the myosin light chain kinase and increases its phosphorylation, leading to platelet shape change [39-42]. Gq protein participates in the activation of phospholipase C beta (PLC β), which converts phosphatidylinositol 4,5-bisphosphate (PIP2) from the plasma membrane into inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 binds to the dense tubular system, promoting Ca2+ release from the intracellular reserves, which leads to Ca2+ influx via the “store-operated calcium entry” (SOCE) pathway. DAG, in turn, activates protein kinase C (PKC), which leads to granular secretion [43,44].
The activation of the Gi protein results in adenylyl cyclase (AC) inhibition with a consequent decrease in cAMP synthesis, positively contributing to platelet activation [45]. Activation of the Gi protein also leads to the activation of the PI3K enzyme, which, together with its substrates PIP2 and PIP3, is responsible for the translocation of the Akt protein to the membrane, where it is phosphorylated on serine 473 (Ser473) by mTORC2 [46,47]. Subsequently, intracellular calcium, PKC, and Akt are essential for activating GPIIbIIIa. Integrin phosphorylation triggers the outside pathway, which results in the cytoskeleton reorganization and stabilization of the platelet aggregate [45,48-53]. Figure 1 illustrates these pathways.
Figure 1.
Platelet inside-out signaling triggered by activation of G protein-coupled receptors. AC, adenylyl cyclase; cAMP, cyclic adenosine monophosphate; PKA, cAMP-dependent protein kinase; PI3K, phosphoinositide 3-kinase; PLCβ, phospholipase C beta; PIP2, phosphatidylinositol 4,5-bisphosphate; DAG, diacylglycerol; PKC, protein kinase C; IP3, inositol triphosphate; CalDAG-GEFI, calcium and diacylglycerol-regulated guanine exchange factor 1; Rap1, ras-associated protein 1; ERK, extracellular signal-regulated kinase; PLA2, phospholipase A2; AA, arachidonic acid; COX, cyclooxygenase; PGH2, prostaglandin H2; PGG2, prostaglandin G2; TXS, thromboxane synthase; TXA2, thromboxane A2; RhoA, ras homolog family member A; MLC, myosin light chain. The figure was created with BioRender.com.
Beyond their role in regulating coagulation, platelets are also involved in other processes, such as cell proliferation and inflammation. This involvement has been well-documented in conditions like sepsis and atherosclerosis [54-56].
Platelets and cancer
Armand Trousseau established a link between platelets and cancer in 1865 [57]. Recent studies have confirmed that daily use of aspirin at a dose indicated for preventing cardiovascular diseases (75-100 mg) reduced the incidence and recurrence of various types of tumors. Moreover, one of the isoforms of COX, COX-2, is the primary source of prostanoid generation in inflammatory conditions and cancer [8,10,38,58]. However, the observed effect of the interaction between platelets and tumor cells remains controversial.
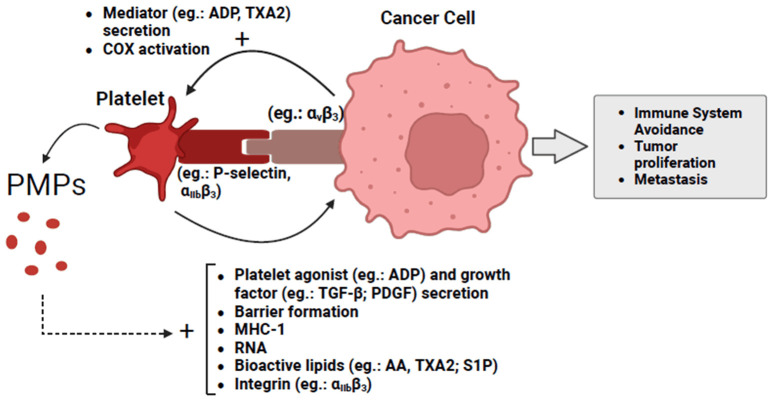
On the one hand, platelets contribute to cancer progression by negatively modulating leukocytes, directly and indirectly, and by secreting metalloproteinases and pro-angiogenic factors [57]. On the other hand, tumor cells can activate circulating platelets, which then support the tumor in spreading (Figure 2). This interaction occurs through direct interaction involving integrins αIIbβ3, αvβ3, and α6β1, as well selectins (e.g., P-selectin) present in the platelet’s membrane and tumor cells, or indirectly, through the release of angiogenic and procoagulant substances (e.g., ADP, thromboxane, VEGF) by neoplastic cells. Tumor cells can lead to thrombosis by several mechanisms, such as the tissue Factor (TF) and microparticles released from tumor cells that activate the coagulation cascade, and the platelets form the thrombus via the extrinsic route. Active platelets and phospholipids cleaved by tumor cells can also stimulate coagulation via the intrinsic pathway [34,59].
Figure 2.
Cancer-activated platelets help neoplastic cells avoid the immune system and promotes tumor growth and dissemination. PMPs, platelet microparticles; ADP, adenosine diphosphate; AA, arachidonic acid; TXA2, thromboxane A2; COX, cyclooxygenase; TGF-β, transforming growth factor beta; PDGF, platelet-derived growth factor; MHC-1, major histocompatibility complex class 1; S1P, sphingosine-1-phosphate. The figure was created with BioRender.com.
CRC can induce a hypercoagulable state, increasing the risk of venous thromboembolism (VTE), which may manifest as deep vein thrombosis or pulmonary embolism. This pathological condition is linked to the expression of TF by CRC cells. TF expression correlates with a worse prognosis, as evidenced by the significantly lower 3-year survival rate in TF-positive CRC patients compared to TF-negative patients (39% vs. 88%, respectively). Alternatively, TF is an important prognostic, predictive marker of CRC, as well as recurrent VTE within the context of cancer. Thromboembolism is a challenging condition to treat and has high morbidity and mortality. Patients with CRC have a 5% risk of developing VTE in the first six months after diagnosis. Neoplasia also increases the VTE recurrence risk compared to healthy individuals [60]. Patients who have a positive diagnosis of cancer at the time of VTE occurrence possess a lower one-year survival rate than those who do not have cancer [61]. The main factors influencing VTE incidence are the interaction between neoplastic cells, the hemostatic system, and the patient’s characteristics [62].
Tumor cells can also activate platelets through the activation of COX-1 and the synthesis of TXA2, which can contribute to tumorigenesis [38]. Sciulli et al., 2005 [63] observed that patients with CRC history had high urinary levels of the TXA2 metabolite, 11-dehydro-TXB2, significantly reduced with aspirin. There was also an increase in COX-2 expression in tumor cells, which led to increased activation of circulating platelets and tumorigenesis. COX-1 and COX-2 have the same substrates and produce the same types of prostanoids, but the latter requires a smaller substrate for this synthesis. For this reason, COX-2 has emerged as a promising target for treating CRC. A COX-2 inhibitor in patients can have a chemopreventive effect [38]. Additionally, the pro-angiogenic factor, podoplanin, is highly expressed in CRC cells, and its interaction with the platelet C-type lectin-like receptor 2 (CLEC-2) promotes platelet activation, contributing to tumor progression and metastasis formation [57].
ADP, TXA2, and thrombin promote platelet activation and contribute to tumor growth and angiogenesis. Furthermore, TF expressed by cancer cells enhances thrombin generation by bidding with the VIIa factor, forming a complex that promotes the activation of IX and X factors [57]. Circulating platelets activate and release VEGF upon binding to TF expressed on endothelial cells. Along with platelet microparticles (PMP), this factor promotes angiogenesis and induces tumor cells to express pro-angiogenic factors [34].
Platelets challenge the recognition and elimination of tumor cells by leukocytes. This is achieved by forming a protective barrier around the cancer cell, transferring Major Histocompatibility Complex Class 1 (MHC-I) to the neoplastic cell, and secretion of TGF-β. TGF-β, in turn, contributes to a reduction in the expression of the NKG2D receptor in natural killer (NK) cells. Additionally, platelets inhibit neutrophil function, which typically induces apoptosis of tumor cells through synthesizing H2O2. Platelet surface receptors also facilitate the adhesion of tumor cells to adhere to distant sites in the body, favoring metastasis. Platelet-secreted TGF-β also supports angiogenesis and the formation of premetastatic niches. The inhibition of TGF-β is related to decreased tumor growth by recruiting CD8 T lymphocytes, macrophages, neutrophils, and NK cells [34,38,57,58,64,65]. Conversely, platelets activate the TGF-β and NF-kB pathways in tumor cells, leading to EMT and metastasis [64].
Neoplastic cells can trigger the release of PMPs, which display a range of integrins on their surface, including αIIbβ3, and contain several bioactive lipids such as arachidonic acid (AA) and sphingosine one phosphate (S1P). The interaction between PMPs and specific cells can activate both platelets and leukocytes. Indeed, PMPs play a role in transferring genetic material to cancer cells, which can lead to increased invasiveness and allow them to evade tumor suppressive mechanisms [57,58,64]. Cancer cells can also transfer their RNA to platelets, creating what is known as tumor-educated platelets (TEP). This incorporated genetic material reduces the expression of genes related to immunity and protein synthesis while increasing the expression of genes linked to cancer [34]. Rodriguez-Martinez et al., 2022 [65] observed that the co-culture of SW480 cells with platelets resulted in a higher rate of substances from platelets to the neoplastic cells. This process appears dependent on physical contact between two cells. Furthermore, the co-culture of colon cells with platelets led to a decrease in the expression of epithelial cell adhesion molecule (EpCAM) after 24 h, and an increase in the expression of genes associated in EMT (e.g., VIMENTIN, SNAIL-1/2) and those which support the cellular structure (e.g., REX1, OCT4, and NANOG) after one hour of incubation [58].
During the colitis phase, Servais et al., 2018 [59] observed an increase in the expression of P-selectin and αIIbβ3 integrin on the platelet membrane, which normalized when cancer developed. Moreover, using clopidogrel reduced dysplasia, formation of adenomas and adenocarcinomas, disease activity index (DAI), diarrhea, and bleeding in the stool. This platelet inhibitor also reduced the accumulation of myeloid-derived suppressor cells (MDSC) in the blood and the expression of the Tgfb and Cebpb genes, which are related to the immunosuppressive activities of these cells. Haemmerle et al., 2017 [66] showed that incubation of ovarian and colon cancer cell lines with platelets for a period of 24-48 h led to a decrease in programmed cell death related to the lack of adhesion (anoikis) of neoplastic cells through S127 and S397 dephosphorylation and activation of the yes-associated protein 1 (YAP1) transcription factor, which is translocated to the nucleus. This process was mediated by the RhoA GTPase that interacted with the PP1 phosphatase at myosin phosphatase target subunit 1 (MYPT1), reducing inhibitory phosphorylation at T696 and T853 and leading to dephosphorylation of YAP1. This indicates that platelets are essential tools in the survival and spread of tumor cells throughout the body and participate in the inflammatory process that originates CRC.
Cancer-related platelet parameters
Several platelet indicators are associated with its activation and function, as well as assisting in the early diagnosis and prognosis of various types of cancer. Usually, four key platelet indices are measured: (1) number of circulating platelets (PLT); (2) platelet crit (PCT), which indicates the percentage of blood volume that is occupied by platelets; (3) platelet distribution width (PDW) and (4) mean platelet volume (MPV) [67]. An elevated blood platelet count serves as an indicator of metastasis presence and an unfavorable prognosis. Additionally, thromboembolism was noted in 15% of patients with a confirmed cancer diagnosis. This occurrence was particularly prevalent in the initial months post-diagnosis, with an escalated risk associated with factors like hyperviscosity syndrome, chemotherapy, infections, and surgical interventions [34,59,64].
Qian et al., 2019 [67] showed that although surgical tumor removal followed by chemotherapy treatment reduces PLT, PCT, and PDW levels, only changes in MPV levels seem to affect the overall survival of patients with CRC. MPV is another indicator that can be used in the prognosis of IBD, such as Crohn’s disease and most neoplastic disorders [33,68-71]. This parameter determines the size of the platelet as well as its activity. Platelets with a high MPV (>15 fl) are very active young cells consumed more quickly than others. A rise in MPV level is related to an increase in platelet aggregation and the release of TXA2 and β-thromboglobulin. This circumstance is also associated with the increase in the interaction of cytokines with megakaryocytes and the induction of pro-platelet release [33]. Numerous studies have demonstrated an increase in this parameter in patients with colorectal cancer (CRC), and the reduction of MPV following tumor removal is associated with a favorable prognosis and patient recovery [67,70,71]. However, reduced MPV has also been observed in some neoplastic diseases. Additionally, Karagöz et al., 2010 [72] observed that, although there was an increase in the number of platelets in cancer patients, no difference was observed in MPV values. Qian et al., 2019 [67] observed that maintaining unchanged MPV levels in CRC patients undergoing surgical procedures and chemotherapy treatment is desirable to obtain a reasonable prognosis of the pathological condition, and a decrease in MPV levels leads to the opposite effect. Complementing this data, a study showed that MPV reduction is associated with the presence of cytokines and growth factors (e.g., TNF-α, IL-1β, VEGF) secreted by several cells which cause an endothelial vascular thrombus and the fragmentation of large sized platelets [73].
Treatment of colorectal cancer
Diagnosis of Lynch syndrome-related colorectal cancer
To determine whether the adenoma or adenocarcinoma identified in routine tests is linked to a hereditary factor such as LS, until recently, the diagnosis consisted of surveying the patient’s medical and family history according to the Amsterdam and Bethesda criteria (e.g., family members diagnosed with CRC before the age of 50, individuals with synchronous or metachronous cancers, tumors with MMR genes deficiency by MSI or loss of protein expression), with the adjuvant use of clinical prediction models (e.g., PREMM5) which assist in the determination of the patients who will undergo genetic screening (e.g., immunohistochemistry, MSI test, multigene test). The results obtained from biopsies collected during colonoscopy exams determine whether surgical removal will be total or segmented. Since preoperative chemotherapy and radiotherapy may lead to a false negative result for LS, it is recommended that immunohistochemistry (IHC) be performed before therapy. However, due to the inability to carry out a more detailed survey of the patient’s medical and family history in clinical practice, their criteria are shown to be less accurate than those who worked on clinical trials [8,11,74-77]. Since 2017, the Amsterdam and Bethesda criteria for identifying and managing LS patients have been replaced by those of the National Institute for Clinical Excellence (NICE). According to this, all newly discovered CRCs should be analyzed for the identification of dMMR by IHC or the identification of MSI, and the results serve as a guide for evaluating the degree of LS; this leads to an improvement in the agility of diagnosis and the treatment efficacy [10].
Several signs are related to LS, such as skin lesions like sebaceous adenomas, sebaceous adenocarcinomas, and keratoacanthomas. These signs are most commonly associated with MLH1 and MSH2 mutations, and more than half of individuals with these skin lesions subsequently manifest internal malignancy [11]. LS patients have fewer precancerous polyps in the intestinal mucosa compared to individuals with other CRC-predisposing syndromes; furthermore, tumors that present the MLH1 pathogenic variant are generally not identifiable, while individuals carrying the pathogenic variant for MSH2 often develop adenomas that can be observed [8,9].
The diagnosis of LS is confirmed through comprehensive microscopic and molecular analyses, including germline testing, immunohistochemistry (IHC) to detect deficient expression of MMR proteins, and the identification of MSI via polymerase chain reaction (PCR). This process involves comparing the number of nucleotides repeats between normal and tumor tissue from the same individual using a panel of five microsatellite markers provided by the National Cancer Institute (NCI). According to the presence or absence and the number of unstable mononucleotides, tumors are classified as MSI with high instability (MSI-H) (≥ two mononucleotides), MSI with low instability (MSI-L) (1 mononucleotide) or stable (MSS). Tumors that present lesions with MSS can progress to MSI when polyps grow to >8-10 mm in diameter. Patients undergo genetic testing and counseling in case of an abnormal result [9-11,78].
The molecular analysis of MSI-H holds significant prognostic value. Patients with MSI-H tumors tend to exhibit an enhanced response to immunotherapy, such as anti-PD1 treatments, due to the elevated frequency of frameshift mutations that produce neoantigens and a heightened immune response. Consequently, these patients often experience a more favorable disease course. Also, using frameshift peptides (FSP) induces a specific cellular and humoral immune response in LS individuals, and detecting FSP-specific antibodies and T cells may be useful for the early diagnosis of cancer. Tumors can present frameshift mutations in genes such as HLA Class 1 and β2 microglobulin that grant immune system evasion. However, this genetic profile is generally associated with a more benign type of cancer [8,10,11].
In the absence of tumor tissue, IHC can be performed on adenomas. Abnormal loss of color, polyp size (>10 mm), and the presence of high-grade dysplasia impact the Lynch Syndrome diagnosis [13,79-81].
A pathogenic variant for MMR genes does not imply a standard diagnosis for all patients. Penetrance and expressivity impact the determination of the cancer development risk rate. Despite this, due to the high risk of early CRC onset, most guidelines for LS patients’ surveillance recommend that the minimum age at which investigation for the presence of CRC should be carried out in individuals with MLH1 and MSH2 mutations by an imaging test (e.g., colonoscopy) is 25 years. The frequency of monitoring should be done every 1 or 2 years [10]. In addition to periodic colonoscopy, the detection of high levels of CD3, CD8, and POXP3 positive T lymphocytes, as well as mutations in the EPCAM gene in adenomas can help in the early diagnosis of LS-associated CRC [8,10,13].
In the case of CRC, unrelated to any familial history or inherited DNA mutation, identification of MLH1 hypermethylation or mutation of the BRAF V600E gene can aid in early cancer diagnosis [82].
Chemotherapy
One class of adjuvant therapies frequently employed in the treatment of advanced CRC stages (III-IV), or in patients with elevated risk factors for metastasis, comprises chemotherapy agents such as capecitabine, 5-fluorouracil (5-FU), irinotecan, and oxaliplatin [83-86]. Genetic exams support the choice of the best therapeutic alternative. 5-FU is a pyrimidine analog, and it acts by inhibiting thymidylate synthase (TS), leading to the exchange of thymidine for fluorinated nucleotides in the DNA of the tumor cell, causing its death. TYMS has excellent prognostic value when determining the gene expression that encodes TS. Patients with low TS expression have a better survival rate than those with high enzyme expression [32].
Most chemotherapy drugs act on tumor cells undergoing cell division. As in CRC, there is a small fraction of dividing cells compared to leukemia and some types of lung cancer, and these cells multiply at a slower rate; this disease responds poorly to chemotherapy alone. Therefore, surgical removal of part of the tumor is performed to induce cancer cells from stage G0 to the cell division stage [87]. The use of chemotherapy can occur in the absence or presence of radiotherapy. Such being the case, the procedure is carried out before surgery to avoid the disease’s recurrence and possible damage to the intestinal mucosa that will be subjected to radiotherapy. The use of targeted therapy after the use of chemotherapy drugs has also been shown to be an effective method [32,85,86].
However, the disadvantage of using available chemotherapy drugs is a series of side effects such as diarrhea, nausea, vomiting, weakness, paresthesia, loss of taste, and alopecia, which lead to loss of compliance by the patient as well as the need to administer new medications, which in turn increases the total cost of treatment [85,86].
Targeted therapy
Because cancer cells can vary significantly in appearance and behavior, therapy targeting specific genes or proteins may offer a more tailored approach. This therapy is generally used in combination with chemotherapy by advanced-stage CRC patients or by patients who are not responding to chemotherapy alone. As an advantage over chemotherapy, using specific drugs has fewer side effects, which can be easily overcome. Among the most commonly used medications are angiogenesis inhibitors (e.g., bevacizumab, regorafenib, ramucirumab), epidermal growth factor receptor (EGFR) inhibitors (e.g., cetuximab, panitumumab) and BRAF inhibitors (e.g., encorafenib). In rare instances of CRC with NTRK gene fusions, specific drugs such as larotrectinib and entrectinib can be effective treatments [32,85].
Drug classes used for the inhibition of the Wnt/β-catenin pathway
Another important therapeutical target is the Wnt/β pathway. As in several types of cancer, dysregulation of the Wnt/β-catenin pathway is involved in regenerating the tumor stem cell population, cell proliferation, and differentiation [21].
Therapies used for the inhibition of the Wnt/β-catenin pathway nowadays consist of the use of natural components (e.g., vitamin D, curcumin, genistein), existing drugs (e.g., NSAIDs) and small molecule inhibitors (e.g., YW2065, OMP-18R5, anthocyanins) [21]. Many drugs that are being developed for the treatment of CRC act to reduce the expression of survivin (e.g., IWR-1), inhibit the Wnt/β-catenin pathway through mediators’ blockage such as CDC-like kinase (CLK) (e.g., SM08502) and porcupine (PORCN) (e.g., ETC-159) or produce genetic variants of the Wnt/β-catenin pathway that are involved in tumor cell gene suppression (e.g., SM08502) [21,88].
CRC inhibitory small molecules are divided into three groups: (1) those that bind to Dvl proteins and affect the stability of the complex that inhibits the degradation of β-catenin and the formation of the Axin/GSK3/APC complex, (2) those that interfere with the binding of β-catenin with TCF and (3) those that act as antagonists of transcription co-activators [21].
Equally relevant, natural products have marked the history of cancer treatment, and some compounds used today, such as irinotecan and camptothecin, originate from them [89]. Sishen Wan, a medicine listed in the Chinese Pharmacopoeia, through inhibition of the Wnt/β-catenin pathway, promotes the reduction of macro and microscopic lesions, leading to a decrease in colitis induced by 2,4,6-trinitrobenzene sulfonic acid (TNBS). Dendrobium officinale polysaccharide (DOP) is another Chinese medicinal product with the polysaccharide as the main active ingredient. DOP impairs the expression of genes involved in cancer cell survival and proliferation (e.g., Wnt2β, GSK3β, CyclinD1, and β-catenin) as well as inhibits pre-cancerous lesions of gastric cancer induced by 1-methyl-2-nitro-1-nitrosoguanidine (MNNG) through Wnt/β-catenin pathway modulation and modification of endogenous metabolites [21,90].
Immunotherapy
Immunotherapy is another treatment option for situations where the tumor is in an inoperable condition or metastasis. The drugs assist the immune system in combating neoplastic cells that generally present microsatellite instability (MSI-H) or mismatch repair deficiency (dMMR) through inhibition of the PD-1 receptor. Some examples of immunotherapeutic drugs are pembrolizumab, nivolumab, and dostarlimab [86]. Tumors that present a high rate of mutations are more susceptible to immunotherapy since these types of cells express a large amount of neoantigens, which increase the immunogenicity of the cells. The hypothesis is that certain chemotherapy drugs or targeted therapies increase the immunogenicity of the cancer cell, which the host immune system will destroy. Neoplastic cells that are destroyed immunogenically release surface markers (e.g., calreticulin) and factors (e.g., HMGB1) that induce an adaptive immune response that can benefit from the use of checkpoint inhibitor drugs (CPI). The VEGF factor negatively modulates the maturation of dendritic cells. Therefore, VEGF inhibitor drugs such as Bevacizumab can enhance the immune response and increase patient survival. Although the development of monoclonal antibodies has improved the patient’s response to treatment, the resistance of neoplastic cells to drugs still proves to be an obstacle to be overcome since, for metastasizing CRC, the 5-year survival rate is still a little more than 12% [32].
Factors such as surgical interventions can reduce the effectiveness of locally acting drugs. To overcome this issue, several groups are developing clinical trials based on bispecific antibody models that simultaneously bind to tumor proteins (e.g., CD20, Neu, EGFR) and immune system cells [32,91-93].
Despite being considered one of the leading causes of resistance to chemotherapy therapy and disease relapse, slow-cycling cells (SCC) have received positive attention in cancer immunotherapy. Sun et al., 2012 [94] demonstrated that vaccinating colon cancer-bearing mice with SCCs led to a decrease in tumor volume and an increase in survival.
Chemotherapy drug resistance factors
Cells at the G0 stage and cancer stem cells
Since most cancer-related deaths are linked to the appearance of metastases, the study of new drugs that have an inhibitory effect on the progression and recurrence of the tumor has become quite common [6,84]. A significant challenge for the healthcare team is the resistance of specific neoplastic cells to treatment. One key factor in this resistance contributing to cancer recurrence is the presence of SCCs. Although these cells are in the G0 stage, when faced with certain stimuli, such as the stroma induction secreted protein, CoCo, they can become active and enter cell division [95,96].
In addition to the ability to divide, the property of a neoplastic cell to originate another cell type is another aspect linked to the appearance of disease relapses. CRC tumors are built by a heterogeneous population of cells with cancer stem cells (CSC). The characteristics that make up these cells are the presence of adhesion proteins (e.g., EpCAM, DC44), the hyperactivation of the β-catenin, the TGF-β and the Notch and hedgehog genes signaling pathways, and the expression of efflux pumps similar to that belonging to the family of ATP-binding cassette transporters. Because CSCs have a low replication rate, they are not affected by treatments that eliminate their progeny and, therefore, tumors can recur. The resistance of stem cells to chemotherapy treatment and their regeneration capacity are important prognostic markers [21,32,97].
Genetic modifications
Changes in the karyotype, such as the presence of an extra pair of small chromosomes (double minute chromosomes) and the existence of a uniformly stained region interspersed between the places where the regular chromosomes are located, are also related to the tumor resistance to multiple antineoplastic therapies. Tumors that present a diminished MMR gene expression, like the LS-related CRCs, respond poorly to cytotoxic chemotherapy agents since these drug classes require the proper function of the MMR mechanism to induce tumor death. Specific genes, such as mdr-1, hinder the entry of fat-soluble drugs by encoding an ATPase [11,98,99]. More recent studies in CRC have shown that tumors with mutations in the RAS or BRAF genes do not respond adequately to EGFR inhibitors but are susceptible to treatment with BRAF inhibitors (e.g., encorafenib). On the other hand, a mutation in the G13D portion of K-ras is related to increased tumor sensitivity to anti-EGFR therapy [32,85].
Epigenetic modifications such as histone acetylation are involved in tumor resistance to irinotecan. Furthermore, inhibiting FGF2, FGF9, MECOM, PLA2G4C, and PRKACB seems to improve the response to chemotherapy [32]. Oxaliplatin resistance factors are linked to the genes responsible for the nucleotide excision repair pathway, the WBSCR22 protein, and the secretion of TGF-β1 by tumor microenvironment cells [32,100]. Moreover, metastatic CRC cells show resistance against anti-VEGF therapy. This aspect is linked to an increase in the expression of the VEGFR1 receptor and a decrease in the levels of hepatocyte growth factor (HGF) [32].
Cells that comprise the tumoral microenvironment
Cells present in the microenvironment can contribute to increased tumor resistance to chemotherapy. Matrix stiffness is an essential parameter for studying the cellular response of cancer cells in the TME [101]. It was shown that in CRC-induced liver metastasis, there is the deposition of extracellular matrix and an increased fatty acid oxidation pathway activation, which is involved with increased tumor resistance to anti-angiogenic agents (e.g., bevacizumab). Inhibition of fatty acid oxidation with etomoxir improved the efficacy of anti-angiogenic drug treatment. Hepatic stellate cells are involved in matrix stiffness and fatty acid oxidation through the activation of the focal adhesion kinase (FAK)/yes-associated protein (YAP) pathway, which leads to lipolysis and fatty acid secretion [102].
Long-term treatment with oxaliplatin can cause this drug to be captured by cancer-associated fibroblasts, leading to increased TGF-β pathway activation and production of multiple factors, such as IL-11 and the POSTNi4 gene, which enhance the tumor aggressiveness [103]. Also, when in contact with neoplastic cells, Wnt secreted by fibroblasts can cause their differentiation and increase their resistance to therapy. Also, the interaction of elements of the Wnt pathway of the neoplastic cell with the immune system cells present in the microenvironment limits their anti-tumor response and favors the increase of the cancer cell tolerance against the immune response. However, this association is also essential for renewing the leukocyte population and maintaining damaged ones [21].
Bacteria present in the intestinal microbiota can also interfere with immunotherapy against CRC. Jiang et al., 2023 [104] observed that Fusobacterium nucleatum can decrease the efficacy of anti-PD-1 immune checkpoint blockade therapy through succinic acid secretion, which inhibits the cyclic GMP-AMP synthase (cGAS)-interferon β pathway and decreases the CD8 cells flow to the TME. The use of metronidazole increased tumor sensitivity to anti-PD-1. Conversely, bacteria that make up the gut microbiome are essential for certain anti-tumor drugs’s effects support. Studies have shown that commensal microorganisms of the Bacteriodales and Burkholderiales species are essential for maintaining the anti-tumor effect of ipilimumab and that antibiotics can impair its therapeutic effect [32].
Enzymes involved in the degradation of chemotherapeutic drugs
Endogenous enzymes are involved in the degradation of chemotherapy drugs. Therefore, they interfere with their mechanism of action. Thymidine phosphorylase, uridine phosphorylase, orotate phosphoribosyl transferase, and dihydropyrimidine dehydrogenase can carry out the metabolism and degradation of 5-FU. Similarly, carboxylesterases, uridine diphosphate glucuronosyltransferase, CYP3A, β-glucuronidase, and the ATP-binding cassette transporter protein (ABC) are involved in the sequestration and metabolism of irinotecan [32].
Drugs that affect platelet activation
Potential therapeutic approach for CRC based on inhibition of platelet activation
As seen previously, tumor cells have the ability to activate circulating platelets. This activation can benefit neoplastic cells, hindering their recognition by immune system cells, helping in their proliferation, and facilitating their dissemination throughout the body. Treatment of different types of tumors with a series of drugs already being marketed and acting on platelet activation has shown promising results. Since an increase in TXA2 synthesis was observed in CRC by identifying the 11-dihydro-TXB2 metabolite, one of the approaches was to investigate the effect of daily use of aspirin, a COX inhibitor, on the disease progression [38,58,63,105]. Aspirin can reduce tumor growth by inhibiting the expression of oncoproteins on the surface of the neoplastic cell induced by platelets (e.g., c-MYC). However, the antitumor effect of aspirin may also come from interaction with other vascular cells or tumor cells themselves [106].
Determining the positive effects of aspirin on cancer prevention is a recent achievement, and some studies have presented controversial results. In 2017 and 2018, several prospective studies were carried out to determine the effect of the drug on the incidence of different types of cancer, some of the most prominent being carried out by the steering committees of the studies “Aspirin in Reducing Events in the Elderly” (ASPREE) and “Aspirin to Reduce Risk of Initial Vascular Events” (ARRIVE). Although the latter showed an increase in the incidence of cancer linked to the use of the drug, ASPREE indicated a minimal increase in the incidence of several types of cancer, including CRC, compared to the number of deaths linked to the disease and also not observed an increase in mortality in patients who already had the condition [106].
On the one hand, it was shown that the inhibition of the platelet P2Y12 receptor and the decrease in the integrin αIIbβ3 activation, induced by clopidogrel, was related to improving the cancer-associated colitis in C57BL/6J mice [59]. On the other hand, a study carried out by the American FDA showed a link between prasugrel, another P2Y12 receptor inhibitor, and a significant increase in the incidence of colon cancer compared to the use of clopidogrel. However, considering the number of cases, a non-significant difference was noted between the two groups (thirteen vs. four patients) [106].
Although drugs that block platelet αIIbβ3 integrin or its respective ligand on the tumor cell membrane are associated with a decreased incidence of metastasis [107], their chronic use is not recommended as this may lead to an increased risk of bleeding. Since the αIIbβ3 integrin is activated in the presence of the tumor cell, a safer and more effective approach to treatment is to perform molecular imaging tests to identify the tumor’s exact location. Another treatment option is the use of drugs that block proteins that are not essential for the execution of a physiological function of the platelet but that are involved in its interaction with tumor cells. One example is the inhibition of the signaling pathway induced by the platelet CLEC-2 receptor, which is involved in the interaction with tumor podoplanin by Btk protein inhibitors (e.g., ibrutinib) [106].
Takahashi et al., 2017 [108] performed a retrospective cohort study where they evaluated the effect of the use of antithrombotic therapy (ATT), consisting of antiplatelet drugs (aspirin, and clopidogrel) and anticoagulants (warfarin, edoxaban, rivaroxaban, apixaban, dabigatran) on the progression of CRC. The use of ATT promoted a reduction in disease recurrence and the need for chemotherapy. ATT also improved the overall survival of patients with stage 0-III CRC, which makes it positively related to the prevention of the disease and the occurrence of micrometastasis. The “clopidogrel for high atherothrombotic risk and ischemic stabilization, management and avoidance” (CHARISMA) clinical trial showed that the combined use of two antiplatelet drugs (aspirin and clopidogrel) led to more excellent prevention of cancer incidence than the use of aspirin alone [109,110]. However, a study with the benefit of dual antiplatelet therapy, where prasugrel was one of the drugs tested, showed an increased risk of the appearance of new cancers. This probably occurred by eliminating the platelet barrier, essential for blocking the advancement of neoplastic cells in the vasculature [111].
According to Symeonidis et al., 2012 [105], in addition to aspirin and clopidogrel, the use of other antiplatelet drugs, such as ticlopidine and dipyridamole, appears to improve the early detection of CRC, as it reduces the incidence of the disease in a more advanced stage (stage IV, according to the American Joint Committee on Cancer classification). Another possible platelet target that could be explored in cancer treatment is the PKCs. Irie et al., 2012 [112] observed that the natural compound bryostatin 1 and its less lipophilic analog, along-1, suppress tumor growth by activating PKCδ.
The anticoagulant heparin has also been associated with reducing metastasis by blocking the binding of platelet P-selectin to sialyl Lewis-carrying mucins present on the surface of the neoplastic cell [106]. In another sense, it can also inhibit tumor-induced thrombin release. However, it can cause angiogenesis by inhibiting VEGF, tissue factor, and platelet-activating factor (PAF) [108].
Certain tumors can trigger thrombopoiesis by causing the liver to release IL-6 and stimulating thrombopoietin secretion. It has been reported that the use of siltuximab, an antibody against IL-6, led to a decrease in the growth of ovarian tumors. Excluding situations where the patient uses myelotoxic treatment or has an immune response or infection-associated thrombocytopenia, the use of anti-thrombopoietin drugs and a decrease in the number of circulating platelets to levels still within physiological standards seems to be a safer treatment than those who use medications that have a systemic effect [106].
The use of antiplatelet drugs (e.g., aspirin, ticlopidine) is indicated for the treatment of vascular complications caused by CRC, such as VTE. However, this drug class can increase the risk of bleeding in the gastrointestinal tract, lungs, and brain, especially in cancer individuals. Therefore, developing antiplatelet drugs with fewer side effects is crucial [60,113]. Resveratrol, a natural polyphenolic compound derived from grapes, blueberries, and peanuts, has been shown to reduce thrombus formation and stricture incidence in colon cancer patients. This occurred through the increase in cGMP synthesis and VASP phosphorylation and the inhibition of Akt and p38MAPK phosphorylation, which led to the reduction of PLC activation, DAG, and IP3 production with a consequent decrease in [Ca2+] and inhibition of platelet activation, adhesion and aggregation. As this inhibitory effect occurs without affecting platelet viability, this minimizes the risk of thrombocytopenia and bleeding, which makes resveratrol a safer option for VTE treatment [113].
The increase in platelet activation and the risk of thrombotic events are intricately associated not only with the progression of cancer but also with the administration of specific chemotherapy drugs, particularly those derived from platinum. New chemotherapy drugs derived from other metallic elements have been developed to address this issue. Shyu et al., 2018 [114] developed a drug derived from Iridium, Ir-3. This drug promoted a significant but reversible decrease in collagen-induced platelet aggregation, ATP secretion, intraplatelet calcium mobilization, and P-selectin expression. Also, Ir-3 inhibited PLCγ2, impacting the activation of PKC, the phosphorylation of the Akt protein, and the activation of c-jun N-terminal protein kinase 1 (JNK1).
TRAIL, another cytokine belonging to the TNF family, presents characteristics similar to TNF-α, such as pro-apoptotic properties through the activation of DR4 and DR5 death domain-coupled receptors or pro-tumorogenic, through the activation of kinases. Wu et al., 2019 [115] observed that the treating platelets co-incubated with HT29 and HCT116 with TRAIL decreased the invasiveness of these cells. This reduction is likely due to TRAIL binding to the DR4 receptor and decreasing platelet secretion of TGF-β1. Notably, TRAIL did not affect the clotting ability of other circulating platelets, suggesting it could be a promising and safe therapeutic option for treating CRC.
Future perspectives for CRC treatment based on platelet modulation
Despite their role in facilitating the proliferation of neoplastic cells, platelets have been investigated to propose various experimental models for chemotherapy drug transport. Using platelets loaded with chemotherapy drugs (e.g., doxorubicin) combined with immunotherapy aims to enhance treatment specificity. The overarching goal is to mitigate potential side effects and to thwart tumor evasion from the immune system [116,117].
The platelet signaling pathways of cancer are complex and have not yet been determined. The use of antiplatelet drugs as a preventive method for various types of cancer is a recent practice. Many studies have shown controversial results, with some offering a benefit from the use of these drugs and others warning about the increased risk of tumor incidence and recurrence. As there is a wide variety of CRC types with different etiologies and behaviors, this suggests that a complex web of signaling pathways encompassing other cells that are important for the protection and correct functioning of our body is involved. A significant challenge in researching new treatment alternatives for CRC is determining a pathway that is exclusively engaged in the pathogenesis of the disease. The adjuvant use of diagnostic tests can improve treatment by helping to choose the most appropriate drug and, thus, avoid possible adverse effects.
To this end, improving the population’s economic conditions and public health policies can increase patient’s access to these tests and, in this way, promote early detection of the disease and increase their quality of life [3]. It is also worthwhile to explore the interaction of neoplastic cells with platelets to develop new transport systems for chemotherapy drugs. Going deeper, the evidence proving the involvement of PKC in platelet activation in cancer makes it a relevant target to be explored in developing new therapies for CRC.
Acknowledgements
We thank Tristan Torriani for revising the English version of our manuscript. This study was supported by the National Council for Scientific and Technological Development (CNPq) [Grant scholarship number #302557/2021-0 for R.F.L.]. B.A.G.R. (co-author) received a postdoctoral scholarship from support for Education, Research and Extension Support (FAEPEX), University of Campinas. L.M.G. (coauthor) received a doctoral scholarship from The São Paulo Research Foundation (FAPESP).
Disclosure of conflict of interest
None.
Abbreviations
- AA
arachidonic acid
- ABC
ATP-binding cassette
- AC
adenylyl cyclase
- ACF
aberrant crypt foci
- ADP
adenosine diphosphate
- APC
adenomatous polyposis coli
- ARRIVE
Aspirin to Reduce Risk of Initial Vascular Events
- ASPREE
Aspirin in Reducing Events in the Elderly
- ATT
antithrombotic therapy
- CAC
colitis-associated cancer
- CalDAG-GEFI
calcium and diacylglycerol-regulated guanine exchange factor 1
- cAMP
cyclic adenosine monophosphate
- CHARISMA
clopidogrel for high atherothrombotic risk and ischemic stabilization, management and avoidance
- CLEC-2
C-type lectin-like receptor 2
- CLK
CDC-like kinase
- CMS
consensus molecular subtype
- COX
cyclooxygenase
- CPI
checkpoint inhibitor
- CRC
Colorectal cancer
- CSC
cancer stem cells
- DAG
diacylglycerol
- DAI
disease activity index
- DCC
deleted in colon carcinoma
- dMMR
mismatch repair deficiency
- DOP
Dendrobium officinale polysaccharide
- EGFR
epidermal growth factor receptor
- EMT
epithelial-to-mesenchymal transition
- ERK
extracellular signal-regulated kinase
- 5-FU
5-fluorouracil
- GAP
GTPase-activating protein
- GSK-3β
glycogen synthase kinase 3β
- HDI
Human Development Index
- HGF
hepatocyte growth factor
- IBD
Inflammatory bowel diseases
- IL
interleukin
- IP3
inositol triphosphate
- LPS
lipopolysaccharide
- Mdr-1
multidrug resistance 1
- MDSC
myeloid-derived suppressor cells
- MHC-I
Major Histocompatibility Complex Class 1
- MLC
myosin light chain
- MMP-9
matrix metalloproteinase 9
- MNNG
1-methyl-2-nitro-1-nitrosoguanidine
- MPV
mean platelet volume
- mRNA
messenger RNA
- MYPT1
myosin phosphatase target subunit 1
- MSI
microsatellite instability
- NF-kB
nuclear factor kappa B
- NOX1
NADPH oxidase 1
- PAF
platelet-activating factor
- PAR
protease-activated receptor
- PCP
planar cell polarity
- PCT
platelet crit
- PDGF
platelet-derived growth factor
- PDW
platelet distribution width
- PGG2
prostaglandin G2
- PGH2
prostaglandin H2
- PI3K
phosphoinositide 3-kinase
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PKA
cAMP-dependent protein kinase
- PKC
protein kinase C
- PLA2
phospholipase A2
- PLC β
phospholipase C beta
- PMP
platelet microparticles
- PORCN
porcupine
- Rap1
ras-associated protein 1
- RhoA
ras homolog family member A
- ROR
receptor tyrosine kinase-like orphan receptor
- RORγt
RAR-related orphan receptor type γ
- ROS
reactive oxygen species
- RSPO3
R-spondin-3
- S1P
sphingosine 1 phosphate
- SCC
slow-cycling cells
- Ser473
serine 473
- SOCE
store-operated calcium entry
- STAT
signal transducer and activator of transcription
- TCF
T cell factor
- TEP
tumor-educated platelets
- TF
tissue factor
- TGF-β
transforming growth factor beta
- TLR
toll-like receptor
- TME
tumor microenvironment
- TNBS
2,4,6-trinitrobenzene sulfonic acid
- TNF
Tumor Necrosis Factor
- TS
thymidylate synthase
- TXA2
thromboxane A2
- TXS
thromboxane synthase
- 3’UTR
3’untranslated region
- VEGF
vascular endothelial growth factor
- VWF
von Willebrand factor
- WHO
World Health Organization
- YAP1
yes-associated protein 1
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149:778–789. doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Sampaio APN, de Souza LP, de Lima Moreira JP, Luiz RR, Fogaça HS, de Souza HS. Geographic distribution and time trends of colorectal cancer in Brazil from 2005 to 2018. Dig Dis Sci. 2022;67:4708–4718. doi: 10.1007/s10620-021-07357-9. [DOI] [PubMed] [Google Scholar]
- 4.Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36:2251–70. doi: 10.1002/cncr.2820360944. [DOI] [PubMed] [Google Scholar]
- 5.Fleming M, Ravula S, Tatishchev SF, Wang HL. Colorectal carcinoma: pathologic aspects. J Gastrointest Oncol. 2012;3:153–173. doi: 10.3978/j.issn.2078-6891.2012.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grivennikov SI. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol. 2013;35:229–44. doi: 10.1007/s00281-012-0352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long AG, Lundsmith ET, Hamilton KE. Inflammation and colorectal cancer. Curr Colorectal Cancer Rep. 2017;13:341–351. doi: 10.1007/s11888-017-0373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dal Buono A, Puccini A, Franchellucci G, Airoldi M, Bartolini M, Bianchi P, Santoro A, Repici A, Hassan C. Lynch syndrome: from multidisciplinary management to precision prevention. Cancers (Basel) 2024;16:849. doi: 10.3390/cancers16050849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nolano A, Medugno A, Trombetti S, Liccardo R, De Rosa M, Izzo P, Duraturo F. Hereditary colorectal cancer: state of the art in lynch syndrome. Cancers (Basel) 2023;15:75. doi: 10.3390/cancers15010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams MH, Hadjinicolaou AV, Norton BC, Kader R, Lovat LB. Lynch syndrome: from detection to treatment. Front Oncol. 2023;13:1166238. doi: 10.3389/fonc.2023.1166238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtius K, Gupta S, Boland CR. Review article: lynch syndrome-a mechanistic and clinical management update. Aliment Pharmacol Ther. 2022;55:960–977. doi: 10.1111/apt.16826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maratt JK, Elena S. Identification of lynch syndrome. Gastrointest Endosc Clin N Am. 2022;32:45–58. doi: 10.1016/j.giec.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Huth C, Kloor M, Voigt AY, Bozukova G, Evers C, Gaspar H, Tariverdian M, Schirmacher P, von Knebel Doeberitz M, Bläker H. The molecular basis of EPCAM expression loss in lynch syndrome-associated tumors. Mod Pathol. 2012;25:911–6. doi: 10.1038/modpathol.2012.30. [DOI] [PubMed] [Google Scholar]
- 14.Perse M, Cerar A. Morphological and molecular alterations in 1,2 dimethylhydrazine and azoxymethane induced colon carcinogenesis in rats. J Biomed Biotechnol. 2011;2011:473964. doi: 10.1155/2011/473964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simiczyjew A, Mazur AJ, Dratkiewicz E, Nowak D. Involvement of β- and γ-actin isoforms in actin cytoskeleton organization and migration abilities of bleb-forming human colon cancer cells. PLoS One. 2017;12:e0173709. doi: 10.1371/journal.pone.0173709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, Srinivasan P, Gao J, Chakravarty D, Devlin SM, Hellmann MD, Barron DA, Schram AM, Hameed M, Dogan S, Ross DS, Hechtman JF, DeLair DF, Yao J, Mandelker DL, Cheng DT, Chandramohan R, Mohanty AS, Ptashkin RN, Jayakumaran G, Prasad M, Syed MH, Rema AB, Liu ZY, Nafa K, Borsu L, Sadowska J, Casanova J, Bacares R, Kiecka IJ, Razumova A, Son JB, Stewart L, Baldi T, Mullaney KA, Al-Ahmadie H, Vakiani E, Abeshouse AA, Penson AV, Jonsson P, Camacho N, Chang MT, Won HH, Gross BE, Kundra R, Heins ZJ, Chen HW, Phillips S, Zhang H, Wang J, Ochoa A, Wills J, Eubank M, Thomas SB, Gardos SM, Reales DN, Galle J, Durany R, Cambria R, Abida W, Cercek A, Feldman DR, Gounder MM, Hakimi AA, Harding JJ, Iyer G, Janjigian YY, Jordan EJ, Kelly CM, Lowery MA, Morris LGT, Omuro AM, Raj N, Razavi P, Shoushtari AN, Shukla N, Soumerai TE, Varghese AM, Yaeger R, Coleman J, Bochner B, Riely GJ, Saltz LB, Scher HI, Sabbatini PJ, Robson ME, Klimstra DS, Taylor BS, Baselga J, Schultz N, Hyman DM, Arcila ME, Solit DB, Ladanyi M, Berger MF. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703–713. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radziwon-Balicka A, Santos-Martinez MJ, Corbalan JJ, O’Sullivan S, Treumann A, Gilmer JF, Radomski MW, Medina C. Mechanisms of platelet-stimulated colon cancer invasion: role of clusterin and thrombospondin 1 in regulation of the P38MAPK-MMP-9 pathway. Carcinogenesis. 2014;35:324–32. doi: 10.1093/carcin/bgt332. [DOI] [PubMed] [Google Scholar]
- 18.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–32. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 19.Saito M, Yamaguchi A, Goi T, Tsuchiyama T, Nakagawara G, Urano T, Shiku H, Furukawa K. Expression of DCC protein in colorectal tumors and its relationship to tumor progression and metastasis. Oncology. 1999;56:134–41. doi: 10.1159/000011954. [DOI] [PubMed] [Google Scholar]
- 20.Ma Q, Wang X, Li Z, Li B, Ma F, Peng L, Zhang Y, Xu A, Jiang B. microRNA-16 represses colorectal cancer cell growth in vitro by regulating the p53/survivin signaling pathway. Oncol Rep. 2013;29:1652–8. doi: 10.3892/or.2013.2262. [DOI] [PubMed] [Google Scholar]
- 21.Zhao H, Ming T, Tang S, Ren S, Yang H, Liu M, Tao Q, Xu H. Wnt signaling in colorectal cancer: pathogenic role and therapeutic target. Mol Cancer. 2022;21:144. doi: 10.1186/s12943-022-01616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Tewari D, Bawari S, Sharma S, DeLiberto LK, Bishayee A. Targeting the crosstalk between canonical Wnt/β-catenin and inflammatory signaling cascades: a novel strategy for cancer prevention and therapy. Pharmacol Ther. 2021;227:107876. doi: 10.1016/j.pharmthera.2021.107876. [DOI] [PubMed] [Google Scholar]
- 24.Shi J, Li F, Luo M, Wei J, Liu X. Distinct roles of Wnt/β-catenin signaling in the pathogenesis of chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Mediators Inflamm. 2017;2017:3520581. doi: 10.1155/2017/3520581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon C, Cheng P, King IN, Andersen P, Shenje L, Nigam V, Srivastava D. Notch post-translationally regulates β-catenin protein in stem and progenitor cells. Nat Cell Biol. 2011;13:1244–51. doi: 10.1038/ncb2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li N, Li J, Mi Q, Xie Y, Li P, Wang L, Binang H, Wang Q, Wang Y, Chen Y, Wang Y, Mao H, Du L, Wang C. Long non-coding RNA ADAMTS9-AS1 suppresses colorectal cancer by inhibiting the Wnt/β-catenin signalling pathway and is a potential diagnostic biomarker. J Cell Mol Med. 2020;24:11318–11329. doi: 10.1111/jcmm.15713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu R, Deng P, Zhang Y, Wang Y, Peng C. Circ_0082182 promotes oncogenesis and metastasis of colorectal cancer in vitro and in vivo by sponging miR-411 and miR-1205 to activate the Wnt/β-catenin pathway. World J Surg Oncol. 2021;19:51. doi: 10.1186/s12957-021-02164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moench R, Grimmig T, Kannen V, Tripathi S, Faber M, Moll EM, Chandraker A, Lissner R, Germer CT, Waaga-Gasser AM, Gasser M. Exclusive inhibition of PI3K/Akt/mTOR signaling is not sufficient to prevent PDGF-mediated effects on glycolysis and proliferation in colorectal cancer. Oncotarget. 2016;7:68749–68767. doi: 10.18632/oncotarget.11899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–28. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Nenkov M, Ma Y, Gaßler N, Chen Y. Metabolic reprogramming of colorectal cancer cells and the microenvironment: implication for therapy. Int J Mol Sci. 2021;22:6262. doi: 10.3390/ijms22126262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van der Jeught K, Xu HC, Li YJ, Lu XB, Ji G. Drug resistance and new therapies in colorectal cancer. World J Gastroenterol. 2018;24:3834–3848. doi: 10.3748/wjg.v24.i34.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korniluk A, Koper-Lenkiewicz OM, Kamińska J, Kemona H, Dymicka-Piekarska V. Mean Platelet Volume (MPV): new perspectives for an old marker in the course and prognosis of inflammatory conditions. Mediators Inflamm. 2019;2019:9213074. doi: 10.1155/2019/9213074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meikle CK, Kelly CA, Garg P, Wuescher LM, Ali RA, Worth RG. Cancer and thrombosis: the platelet perspective. Front Cell Dev Biol. 2017;4:147. doi: 10.3389/fcell.2016.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White JG. Platelet structure. In: Michelson AD, editor. Platelets. 2nd ed. Burlington: 2007. pp. 45–74. [Google Scholar]
- 36.Nagalla S, Shaw C, Kong X, Kondkar AA, Edelstein LC, Ma L, Chen J, McKnight GS, López JA, Yang L, Jin Y, Bray MS, Leal SM, Dong JF, Bray PF. Platelet microRNA-mRNA coexpression profiles correlate with platelet reactivity. Blood. 2011;117:5189–97. doi: 10.1182/blood-2010-09-299719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rowley JW, Schwertz H, Weyrich AS. Platelet mRNA: the meaning behind the message. Curr Opin Hematol. 2012;19:385–91. doi: 10.1097/MOH.0b013e328357010e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dovizio M, Alberti S, Guillem-Llobat P, Patrignani P. Role of platelets in inflammation and cancer: novel therapeutic strategies. Basic Clin Pharmacol Toxicol. 2014;114:118–27. doi: 10.1111/bcpt.12156. [DOI] [PubMed] [Google Scholar]
- 39.Jin J, Daniel JL, Kunapuli SP. Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J Biol Chem. 1998;273:2030–4. doi: 10.1074/jbc.273.4.2030. [DOI] [PubMed] [Google Scholar]
- 40.Jantzen HM, Gousset L, Bhaskar V, Vincent D, Tai A, Reynolds EE, Conley PB. Evidence for two distinct G-protein-coupled ADP receptors mediating platelet activation. Thromb Haemost. 1999;81:111–7. [PubMed] [Google Scholar]
- 41.Moers A, Nieswandt B, Massberg S, Wettschureck N, Grüner S, Konrad I, Schulte V, Aktas B, Gratacap MP, Simon MI, Gawaz M, Offermanns S. G13 is an essential mediator of platelet activation in hemostasis and thrombosis. Nat Med. 2003;9:1418–22. doi: 10.1038/nm943. [DOI] [PubMed] [Google Scholar]
- 42.De Candia E. Mechanisms of platelet activation by thrombin: a short history. Thromb Res. 2012;129:250–6. doi: 10.1016/j.thromres.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Chung SH, Polgár J, Reed GL. Protein kinase C phosphorylation of syntaxin 4 in thrombin-activated human platelets. J Biol Chem. 2000;275:25286–91. doi: 10.1074/jbc.M004204200. [DOI] [PubMed] [Google Scholar]
- 44.Pólgar J, Lane WS, Chung SH, Houng AK, Reed GL. Phosphorylation of SNAP-23 in activated human platelets. J Biol Chem. 2003;278:44369–76. doi: 10.1074/jbc.M307864200. [DOI] [PubMed] [Google Scholar]
- 45.Moheimani F, Fackson DE. P2Y12 receptor: platelet thrombus formation and medical interventions. Int J Hematol. 2012;96:572–87. doi: 10.1007/s12185-012-1188-5. [DOI] [PubMed] [Google Scholar]
- 46.Bynagari-Settipalli YS, Lakhani P, Jin J, Bhavaraju K, Rico MC, Kim S, Woulfe D, Kunapuli SP. Protein Kinase C isoform epsilon (ε) negatively regulates ADP-induced calcium mobilization and thromboxane generation in platelets. Arterioscler Thromb Vasc Biol. 2012;32:1211–9. doi: 10.1161/ATVBAHA.111.242388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Badolia R, Manne BK, Dangelmaier C, Chernoff J, Kunapuli SP. Gq-mediated Akt translocation to the membrane: a novel PIP3-independent mechanism in platelets. Blood. 2015;125:175–84. doi: 10.1182/blood-2014-05-576306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shattil SJ. Signaling through platelet integrin alpha IIb beta 3: inside-out, outside-in, and sideways. Thromb Haemost. 1999;82:318–25. [PubMed] [Google Scholar]
- 49.Li Z, Xi X, Du X. A mitogen-activated protein kinase-dependent signaling pathway in the activation of platelet integrin alpha IIbbeta3. J Biol Chem. 2001;276:42226–32. doi: 10.1074/jbc.M106129200. [DOI] [PubMed] [Google Scholar]
- 50.Stefanini L, Bergmeier W. CalDAG-GEFI and platelet activation. Platelets. 2010;21:239–43. doi: 10.3109/09537101003639931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Z, Delaney MK, O’Brien KA, Du X. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol. 2010;30:2341–49. doi: 10.1161/ATVBAHA.110.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen B, Delaney MK, Du X. Inside-out, outside-in, and inside-outside-in: G protein signaling in integrin-mediated cell adhesion, spreading, and retraction. Curr Opin Cell Biol. 2012;24:600–6. doi: 10.1016/j.ceb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sebastiano M, Momi S, Falcinelli E, Bury L, Hoylaerts MF, Gresele P. A novel mechanism regulating human platelet activation by MMP-2-mediated PAR1 biased signaling. Blood. 2017;129:883–895. doi: 10.1182/blood-2016-06-724245. [DOI] [PubMed] [Google Scholar]
- 54.Badimon L, Padró T, Vilahur G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur Heart J Acute Cardiovasc Care. 2012;1:60–74. doi: 10.1177/2048872612441582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas MR, Storey RF. The role of platelets in inflammation. Thromb Haemost. 2015;114:449–458. doi: 10.1160/TH14-12-1067. [DOI] [PubMed] [Google Scholar]
- 56.Zhang L, Xie YH, Lin BR. Effects of washed platelets vs platelet-rich plasma on the proliferation and mineralization of rat dental pulp cells. Genet Mol Res. 2015;14:9486–96. doi: 10.4238/2015.August.14.12. [DOI] [PubMed] [Google Scholar]
- 57.Plantureux L, Crescence L, Dignat-George F, Panicot-Dubois L, Dubois C. Effects of platelets on cancer progression. Thromb Res. 2018;164(Suppl 1):S40–S47. doi: 10.1016/j.thromres.2018.01.035. [DOI] [PubMed] [Google Scholar]
- 58.Contursi A, Grande R, Dovizio M, Bruno A, Fullone R, Patrignani P. Platelets in cancer development and diagnosis. Biochem Soc Trans. 2018;46:1517–1527. doi: 10.1042/BST20180159. [DOI] [PubMed] [Google Scholar]
- 59.Servais L, Wéra O, Dibato Epoh J, Delierneux C, Bouznad N, Rahmouni S, Mazzucchelli G, Baiwir D, Delvenne P, Lancellotti P, Oury C. Platelets contribute to the initiation of colitis-associated cancer by promoting immunosuppression. J Thromb Haemost. 2018;16:762–777. doi: 10.1111/jth.13959. [DOI] [PubMed] [Google Scholar]
- 60.Rees PA, Clouston HW, Duff S, Kirwan CC. Colorectal cancer and thrombosis. Int J Colorectal Dis. 2018;33:105–108. doi: 10.1007/s00384-017-2909-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846–1850. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 62.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122:1712–1723. doi: 10.1182/blood-2013-04-460121. [DOI] [PubMed] [Google Scholar]
- 63.Sciulli MG, Filabozzi P, Tacconelli S, Padovano R, Ricciotti E, Capone ML, Grana M, Carnevale V, Patrignani P. Platelet activation in patients with colorectal cancer. Prostaglandins Leukot Essent Fatty Acids. 2005;72:79–83. doi: 10.1016/j.plefa.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 64.Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126:582–8. doi: 10.1182/blood-2014-08-531582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodriguez-Martinez A, Simon-Saez I, Perales S, Garrido-Navas C, Russo A, de Miguel-Perez D, Puche-Sanz I, Alaminos C, Ceron J, Lorente JA, Molina MP, Gonzalez C, Cristofanilli M, Ortigosa-Palomo A, Real PJ, Rolfo C, Serrano MJ. Exchange of cellular components between platelets and tumor cells: impact on tumor cells behavior. Theranostics. 2022;12:2150–2161. doi: 10.7150/thno.64252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haemmerle M, Taylor ML, Gutschner T, Pradeep S, Cho MS, Sheng J, Lyons YM, Nagaraja AS, Dood RL, Wen Y, Mangala LS, Hansen JM, Rupaimoole R, Gharpure KM, Rodriguez-Aguayo C, Yim SY, Lee JS, Ivan C, Hu W, Lopez-Berestein G, Wong ST, Karlan BY, Levine DA, Liu J, Afshar-Kharghan V, Sood AK. Platelets reduce anoikis and promote metastasis by activating YAP1 signaling. Nat Commun. 2017;8:310. doi: 10.1038/s41467-017-00411-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qian W, Ge XX, Wu J, Gong FR, Wu MY, Xu MD, Lian L, Wang WJ, Li W, Tao M. Prognostic evaluation of resectable colorectal cancer using platelet-associated indicators. Oncol Lett. 2019;18:571–580. doi: 10.3892/ol.2019.10388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu S, Ren J, Han G, Wang G, Gu G, Xia Q, Li J. Mean platelet volume: a controversial marker of disease activity in Crohn’s disease. Eur J Med Res. 2012;17:27. doi: 10.1186/2047-783X-17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zubcevic N, Mesihovic R, Zubcevic S. Usefulness of laboratory data in estimation of Crohn’s disease activity. Med Arh. 2010;64:33–6. [PubMed] [Google Scholar]
- 70.Li N, Yu Z, Zhang X, Liu T, Sun YX, Wang RT, Yu KJ. Elevated mean platelet volume predicts poor prognosis in colorectal cancer. Sci Rep. 2017;7:10261. doi: 10.1038/s41598-017-11053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tuncel T, Ozgun A, Emirzeoglu L, Celik S, Bilgi O, Karagoz B. Mean platelet volume as a prognostic marker in metastatic colorectal cancer patients treated with bevacizumab-combined chemotherapy. Asian Pac J Cancer Prev. 2014;15:6421–3. doi: 10.7314/apjcp.2014.15.15.6421. [DOI] [PubMed] [Google Scholar]
- 72.Karagöz B, Sücüllü İ, Sayan Ö, Bilgi O, Tuncel T, Filiz Aİ, Yücel E, Ozgun A, Erikçi AA, Alacacioğlu A, Kandemir EG. Platelet indices in patients with colorectal cancer. Cent Eur J Med. 2010;5:365–368. [Google Scholar]
- 73.Mutlu H, Berk V, Karaca H, Erden A, Aslan T, Akca Z. Treatment regimen with bevacizumab decreases mean platelet volume in patients with metastatic colon cancer. Clin Appl Thromb Hemost. 2012;18:546–8. doi: 10.1177/1076029611430958. [DOI] [PubMed] [Google Scholar]
- 74.Einarsson H, Runarsdottir JR, Tryggvason T, Snaebjornsson P, Smaradottir A, Stefansdottir V, Thoroddsen A, Arngrimsson R, Jonasson JG, Haraldsdottir S. Universal tumor screening in a population with MSH6- and PMS2-associated Lynch syndrome. Genet Med. 2022;24:999–1007. doi: 10.1016/j.gim.2022.01.012. [DOI] [PubMed] [Google Scholar]
- 75.Leclerc J, Vermaut C, Buisine MP. Diagnosis of Lynch syndrome and strategies to distinguish lynch-related tumors from sporadic MSI/dMMR tumors. Cancers (Basel) 2021;13:467. doi: 10.3390/cancers13030467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moller P, Seppälä T, Bernstein I, Holinski-Feder E, Sala P, Evans DG, Lindblom A, Macrae F, Blanco I, Sijmons R, Jeffries J, Vasen H, Burn J, Nakken S, Hovig E, Rodland EA, Tharmaratnam K, de Vos Tot Nederveen Cappel WH, Hill J, Wijnen J, Green K, Lalloo F, Sunde L, Mints M, Bertario L, Pineda M, Navarro M, Morak M, Renkonen-Sinisalo L, Frayling IM, Plazzer JP, Pylvanainen K, Sampson JR, Capella G, Mecklin JP, Möslein G Mallorca Group (http://mallorca-group.eu) Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut. 2017;66:464–472. doi: 10.1136/gutjnl-2015-309675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baglietto L, Lindor NM, Dowty JG, White DM, Wagner A, Gomez Garcia EB, Vriends AH Dutch Lynch Syndrome Study Group. Cartwright NR, Barnetson RA, Farrington SM, Tenesa A, Hampel H, Buchanan D, Arnold S, Young J, Walsh MD, Jass J, Macrae F, Antill Y, Winship IM, Giles GG, Goldblatt J, Parry S, Suthers G, Leggett B, Butz M, Aronson M, Poynter JN, Baron JA, Le Marchand L, Haile R, Gallinger S, Hopper JL, Potter J, de la Chapelle A, Vasen HF, Dunlop MG, Thibodeau SN, Jenkins MA. Risks of Lynch syndrome cancers for MSH6 mutation carriers. J Natl Cancer Inst. 2010;102:193–201. doi: 10.1093/jnci/djp473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang W, Li L, Ke CF, Wang W, Xiao BY, Kong LH, Tang JH, Li Y, Wu XD, Hu Y, Guo WH, Wang SZ, Wan DS, Xu RH, Pan ZZ, Ding PR. Universal germline testing among patients with colorectal cancer: clinical actionability and optimised panel. J Med Genet. 2022;59:370–376. doi: 10.1136/jmedgenet-2020-107230. [DOI] [PubMed] [Google Scholar]
- 79.Shia J, Holck S, DePetris G, Greenson JK, Klimstra DS. Lynch syndrome-associated neoplasms: a discussion on histopathology and immunohistochemistry. Fam Cancer. 2013;12:241–260. doi: 10.1007/s10689-013-9612-4. [DOI] [PubMed] [Google Scholar]
- 80.Bonadona V, Bonaiti B, Olschwang S, Grandjouan S, Huiart L, Longy M, Guimbaud R, Buecher B, Bignon YJ, Caron O, Colas C, Noguès C, Lejeune-Dumoulin S, Olivier-Faivre L, Polycarpe-Osaer F, Nguyen TD, Desseigne F, Saurin JC, Berthet P, Leroux D, Duffour J, Manouvrier S, Frébourg T, Sobol H, Lasset C, Bonaïti-Pellié C French Cancer Genetics Network. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304–10. doi: 10.1001/jama.2011.743. [DOI] [PubMed] [Google Scholar]
- 81.Ligtenberg MJ, Kuiper RP, Geurts van Kessel A, Hoogerbrugge N. EPCAM deletion carriers constitute a unique subgroup of Lynch syndrome patients. Fam Cancer. 2013;12:169–74. doi: 10.1007/s10689-012-9591-x. [DOI] [PubMed] [Google Scholar]
- 82.Thiel A, Heinonen M, Kantonen J, Gylling A, Lahtinen L, Korhonen M, Kytölä S, Mecklin JP, Orpana A, Peltomäki P, Ristimäki A. BRAF mutation in sporadic colorectal cancer and Lynch syndrome. Virchows Arch. 2013;463:613–21. doi: 10.1007/s00428-013-1470-9. [DOI] [PubMed] [Google Scholar]
- 83.Otani K, Ishihara S, Hata K, Murono K, Sasaki K, Yasuda K, Nishikawa T, Tanaka T, Kiyomatsu T, Kawai K, Nozawa H, Yamaguchi H, Watanabe T. Colorectal cancer with venous tumor thrombosis. Asian J Surg. 2018;41:197–202. doi: 10.1016/j.asjsur.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 84.Shin AE, Giancotti FG, Rustgi AK. Metastatic colorectal cancer: mechanisms and emerging therapeutics. Trends Pharmacol Sci. 2023;44:222–236. doi: 10.1016/j.tips.2023.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Cutsem E, Cervantes A, Nordlinger B, Arnold D ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii1–9. doi: 10.1093/annonc/mdu260. [DOI] [PubMed] [Google Scholar]
- 86.Johdi NA, Sukor NF. Colorectal cancer immunotherapy: options and strategies. Front Immunol. 2020;11:1624. doi: 10.3389/fimmu.2020.01624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loeb LA, Loeb KR, Anderson JP. Multiple mutations and cancer. Proc Natl Acad Sci U S A. 2003;100:776–81. doi: 10.1073/pnas.0334858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tam BY, Chiu K, Chung H, Bossard C, Nguyen JD, Creger E, Eastman BW, Mak CC, Ibanez M, Ghias A, Cahiwat J, Do L, Cho S, Nguyen J, Deshmukh V, Stewart J, Chen CW, Barroga C, Dellamary L, Kc SK, Phalen TJ, Hood J, Cha S, Yazici Y. The CLK inhibitor SM08502 induces anti-tumor activity and reduces Wnt pathway gene expression in gastrointestinal cancer models. Cancer Lett. 2020;473:186–197. doi: 10.1016/j.canlet.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 89.Huang M, Lu JJ, Ding J. Natural products in cancer therapy: past, present and future. Nat Prod Bioprospect. 2021;11:5–13. doi: 10.1007/s13659-020-00293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao Y, Li B, Wang G, Ge S, Lan X, Xu G, Liu H. Dendrobium officinale polysaccharides inhibit 1-Methyl-2-Nitro-1-nitrosoguanidine induced precancerous lesions of gastric cancer in rats through regulating Wnt/β-catenin pathway and altering serum endogenous metabolites. Molecules. 2019;24:2660. doi: 10.3390/molecules24142660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xuan C, Steward KK, Timmerman JM, Morrison SL. Targeted delivery of interferon-alpha via fusion to anti-CD20 results in potent antitumor activity against B-cell lymphoma. Blood. 2010;115:2864–71. doi: 10.1182/blood-2009-10-250555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang X, Zhang X, Fu ML, Weichselbaum RR, Gajewski TF, Guo Y, Fu YX. Targeting the tumor microenvironment with interferon-β bridges innate and adaptive immune responses. Cancer Cell. 2014;25:37–48. doi: 10.1016/j.ccr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tabernero J, Melero I, Ros W, Argiles G, Marabelle A, Rodriguez-Ruiz ME, Albanell J, Calvo E, Moreno V, Cleary JM, Eder JP, Karanikas V, Bouseida S, Sandoval F, Sabanes D, Sreckovic S, Hurwitz H, Paz-Ares LG, Suarez JMS, Segal NH. Phase Ia and Ib studies of the novel carcinoembryonic antigen (CEA) T-cell bispecific (CEA CD3 TCB) antibody as a single agent and in combination with atezolizumab: preliminary efficacy and safety in patients with metastatic colorectal cancer (mCRC) J. Clin. Oncol. 2017;35:3002. [Google Scholar]
- 94.Sun Q, Zhong Y, Wu F, Zhou C, Wang D, Ma W, Zhang Y, Zhang S. Immunotherapy using slow-cycling tumor cells prolonged overall survival of tumor-bearing mice. BMC Med. 2012;10:172. doi: 10.1186/1741-7015-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Linde N, Fluegen G, Aguirre-Ghiso JA. The relationship between dormant cancer cells and their microenvironment. Adv Cancer Res. 2016;132:45–71. doi: 10.1016/bs.acr.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Davis JE Jr, Kirk J, Ji Y, Tang DG. Tumor dormancy and slow-cycling cancer cells. Adv Exp Med Biol. 2019;1164:199–206. doi: 10.1007/978-3-030-22254-3_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Niethammer AG, Wodrich H, Loeffler M, Lode HN, Emmerich K, Abdollahi A, Krempien R, Debus J, Huber PE, Reisfeld RA. Multidrug resistance-1 (MDR-1): a new target for T cell-based immunotherapy. FASEB J. 2005;19:158–9. doi: 10.1096/fj.04-2355fje. [DOI] [PubMed] [Google Scholar]
- 99.Duesberg P, Li R, Sachs R, Fabarius A, Upender MB, Hehlmann R. Cancer drug resistance: the central role of the karyotype. Drug Resist Updat. 2007;10:51–8. doi: 10.1016/j.drup.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 100.Gnoni A, Russo A, Silvestris N, Maiello E, Vacca A, Marech I, Numico G, Paradiso A, Lorusso V, Azzariti A. Pharmacokinetic and metabolism determinants of fluoropyrimidines and oxaliplatin activity in treatment of colorectal patients. Curr Drug Metab. 2011;12:918–31. doi: 10.2174/138920011798062300. [DOI] [PubMed] [Google Scholar]
- 101.Chauhan VP, Boucher Y, Ferrone CR, Roberge S, Martin JD, Stylianopoulos T, Bardeesy N, DePinho RA, Padera TP, Munn LL, Jain RK. Compression of pancreatic tumor blood vessels by hyaluronan is caused by solid stress and not interstitial fluid pressure. Cancer Cell. 2014;26:14–5. doi: 10.1016/j.ccr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zheng Y, Zhou R, Cai J, Yang N, Wen Z, Zhang Z, Sun H, Huang G, Guan Y, Huang N, Shi M, Liao Y, Bin J, Liao W. Matrix stiffness triggers lipid metabolic cross-talk between tumor and stromal cells to mediate bevacizumab resistance in colorectal cancer liver metastases. Cancer Res. 2023;83:3577–3592. doi: 10.1158/0008-5472.CAN-23-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Linares J, Sallent-Aragay A, Badia-Ramentol J, Recort-Bascuas A, Méndez A, Manero-Rupérez N, Re DL, Rivas EI, Guiu M, Zwick M, Iglesias M, Martinez-Ciarpaglini C, Tarazona N, Varese M, Hernando-Momblona X, Cañellas-Socias A, Orrillo M, Garrido M, Saoudi N, Elez E, Navarro P, Tabernero J, Gomis RR, Batlle E, Pisonero J, Cervantes A, Montagut C, Calon A. Long-term platinum-based drug accumulation in cancer-associated fibroblasts promotes colorectal cancer progression and resistance to therapy. Nat Commun. 2023;14:746. doi: 10.1038/s41467-023-36334-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jiang SS, Xie YL, Xiao XY, Kang ZR, Lin XL, Zhang L, Li CS, Qian Y, Xu PP, Leng XX, Wang LW, Tu SP, Zhong M, Zhao G, Chen JX, Wang Z, Liu Q, Hong J, Chen HY, Chen YX, Fang JY. Fusobacterium nucleatum-derived succinic acid induces tumor resistance to immunotherapy in colorectal cancer. Cell Host Microbe. 2023;31:781–797. e9. doi: 10.1016/j.chom.2023.04.010. [DOI] [PubMed] [Google Scholar]
- 105.Symeonidis D, Koukoulis G, Christodoulidis G, Mamaloudis I, Chatzinikolaou I, Tepetes K. Impact of antiplatelet treatment on colorectal cancer staging characteristics. World J Gastrointest Endosc. 2012;4:409–413. doi: 10.4253/wjge.v4.i9.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tao DL, Tassi Yunga S, Williams CD, McCarty OJT. Aspirin and antiplatelet treatments in cancer. Blood. 2021;137:3201–3211. doi: 10.1182/blood.2019003977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Palacios-Acedo AL, Mezouar S, Mége D, Crescence L, Dubois C, Panicot-Dubois L. P2RY12-inhibitors reduce cancer-associated thrombosis and tumor growth in pancreatic cancers. Front Oncol. 2021;11:704945. doi: 10.3389/fonc.2021.704945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Takahashi K, Ito H, Hashimoto M, Mita K, Asakawa H, Hayashi T, Fujino K. Does antithrombotic therapy improve survival with colorectal cancer? World J Surg Oncol. 2017;15:161. doi: 10.1186/s12957-017-1235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, Flather MD, Haffner SM, Hamm CW, Hankey GJ, Johnston SC, Mak KH, Mas JL, Montalescot G, Pearson TA, Steg PG, Steinhubl SR, Weber MA, Brennan DM, Fabry-Ribaudo L, Booth J, Topol EJ CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–17. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 110.Bhatt DL, Topol EJ Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance Executive Committee. Clopidogrel added to aspirin versus aspirin alone in secondary prevention and high-risk primary prevention: rationale and design of the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. Am Heart J. 2004;148:263–268. doi: 10.1016/j.ahj.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 111.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 112.Irie K, Yanagita RC, Nakagawa Y. Challenges to the development of bryostatin-type anticancer drugs based on the activation mechanism of protein kinase Cδ. Med Res Rev. 2012;32:518–35. doi: 10.1002/med.20220. [DOI] [PubMed] [Google Scholar]
- 113.Yu Z, Zhu W, Lu F, Liu H, Sun H, Dong J, Zhang Y, Wang H. Inhibitory effects of resveratrol on platelet activation and thrombosis in colon cancer through regulation of the MAPK and cGMP/VASP pathways. Thromb Res. 2024;241:109111. doi: 10.1016/j.thromres.2024.109111. [DOI] [PubMed] [Google Scholar]
- 114.Shyu KG, Velusamy M, Hsia CW, Yang CH, Hsia CH, Chou DS, Jayakumar T, Sheu JR, Li JY. Novel iridium (III) derived organometallic compound for the inhibition of human platelet activation. Int J Mol Med. 2018;41:2589–600. doi: 10.3892/ijmm.2018.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu LS, Wang XW, He W, Ma XT, Wang HY, Han M, Li BH. TRAIL inhibits platelet-induced colorectal cancer cell invasion. J Int Med Res. 2019;47:962–972. doi: 10.1177/0300060518820785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu P, Zuo H, Zhou R, Wang F, Liu X, Ouyang J, Chen B. Doxorubicin-loaded platelets conjugated with anti-CD22 mAbs: a novel targeted delivery system for lymphoma treatment with cardiopulmonary avoidance. Oncotarget. 2017;8:58322–58337. doi: 10.18632/oncotarget.16871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang C, Sun W, Ye Y, Hu Q, Bomba HN, Gu Z. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nat Biomed Eng. 2017;1:0011. [Google Scholar]