Abstract
Introduction: EpCAM (epithelial cell adhesion molecule) protein expression was detected in 45 to 90% of breast cancers in different studies, and high expression levels were associated with poor outcomes in several retrospective analyses. This study aims to investigate the relationship between EpCAM and clinicopathological parameters and survival in breast cancer. Methodology: This study was conducted as a Quasi-Experimental Cohort Study to explore 100 breast cancer patients. After the surgical excision of breast cancer, pathology blocks were deparaffinized and subjected to IHC (immunohistochemistry) for EpCAM examination. Using a Roche VENTANA Benchmark GX automated staining instrument and a well-established IHC staining protocol, the expression of EpCAM in breast cancer tissue was assessed. Independent sample T-test and Chi squared and Logistic Regression test with STATA version 17 software were used for data analysis. Results: The difference in the distribution of the negative state of biomarkers (ER = estrogen receptor, PR = Progesterone receptor) and EPCAM positive group was significant (P-value = 0.002) (P-value = 0.006). A statistically insignificant distinction was observed in the distribution of the HER2 (human epidermal growth factor receptor) and EPCAM groups (P-value = 0.198). With 30.95% of those in the EPCAM-positive cohort experienced metastasis or recurrence. ER+ and PR+ decreased the chance of EPCAM positive by 0.25 and 0.29, respectively. HER2+ and Basal like breast cancer increase the chances of EPCAM being positive by 1.9 and 2.08, respectively. Basal like breast cancer increases the odds of EpCAM positive 2.19 times. Similarly, N2 and stage 3 increase the odds of EpCAM positive by 1.95 and 0.5 times, respectively. Conclusion: We found that Basal like breast cancer, HER2+, and stage 3 increase the chance of EpCAM positivity. It seems that EPCAM positive cancer has more chance for recurrence and metastasis.
Keywords: Breast cancer, epithelial cell adhesion molecule, survival, recurrence, metastasis
Introduction
Breast cancer constitutes 25% of all diagnosed cancers, which is the most common cause of cancer-related death in the women worldwide [1]. The trends and principles of early diagnosis and treatment of breast cancer have improved in recent years, leading to better survival rates [2]. Due to many characteristics of the illness, researchers have explored several biomarkers to better comprehend their contributions to the development and advancement of breast cancer, as well as their ability to predict patient outcomes. These biomarkers include luminal A, luminal B, HER2 positive, and TNBC (Triple Negative Breast Cancer) [3]. Among these, epithelial cell adhesion molecules (EpCAMs) in breast cancer carcinogenesis, particularly in the therapeutic targets, radiation resistance, and prognostic value with lymphatic and metastasis processes, are of paramount importance [4].
The cell adhesion molecule EpCAM has garnered attention due to its potential involvement in metastasis in breast and other cancers [5]. Its physiological functions include cellular differentiation, and maturation during embryonic growth and tissue regeneration after inflammatory responses [6,7]. In cancer, EpCAM exhibits altered expression patterns [8].
However, despite the aggressive nature of EpCAM in breast cancer, previous studies on its prognostic and roles as well as its contribution to disease staging, extent of surgery, need for preoperative chemotherapy, and postoperative radiotherapy have been equivocal.
The expression of EpCAM in a large group of breast tumors with long-term follow-up was analyzed. The importance of the prognostic value of this protein in pathological clinical outcomes, such as relapse or metastasis in breast cancer patients who underwent surgery in Rasul Akram (pbuh) and Khatam al-Anbiya (pbuh) hospitals, was analyzed and described.
Materials and methods
Subject requirements
This study was conducted as a Quasi-Experimental Cohort Study to explore the occurrence of EpCAM expression, and its association between clinic pathological parameters, and outcomes (survival, recurrence, or metastasis) in 100 breast cancer patients who underwent surgery with ethics code IR.IUMS.REC.1401.1029 at Rasoul Akram and Khatam Anbiya Hospitals at 2022-2023 in Tehran, Iran. Entry criteria included patients with any early or locally advanced breast cancer with Invasive ductal carcinoma NST histological type. Patients with Lobular or specific type of breast cancer, inadequate patient data, or incapacity to withstand surgery or chemotherapy because of old age or other medical problems were among the exclusion criteria. After the surgical excision of breast cancer, pathology blocks were deparaffinized and subjected to IHC for EpCAM examination (Figures 1, 2). Patients were informed that their test results will be used to conduct a research project.
Figure 1.
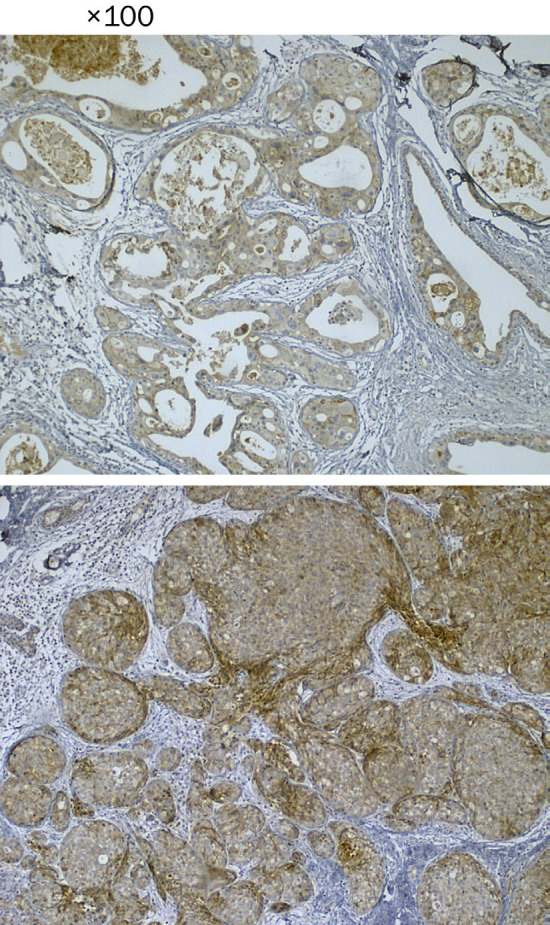
Sections show tumoral tissue, both carcinoma in situ and invasive components show moderate staining of EpCAM in membranous pattern using immunohistochemistry. Magnification ×100.
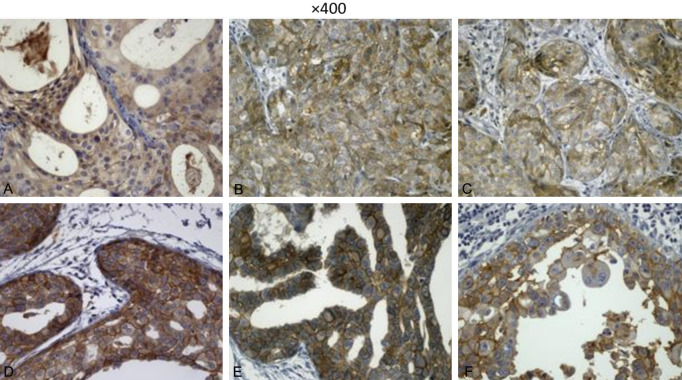
Figure 2.
Sections show tumoral tissue. A. This image shows weak staining of tumoral cells in cytoplasmic area. B and C. These images show moderate staining of tumoral cells in membranous pattern. D-F. Show strong complete membranous staining with in situ components using immunohistochemistry. Magnification ×400.
Immunohistochemical analysis
Using the well-established IHC (immunohistochemical) staining methodology and the EpCAM-specific antibody (AA 40-260) (Reactivity: Human) (Catalog No. ABIN3021638) [9] by Roche VENTANA Benchmark GX automated staining system, the expression of EpCAM was assessed in breast tissues. Four expression subgroups (no stain, weak, moderate, intense) were defined. EpCAM overexpression was defined for tissues that showed moderate or intense staining.
For the first three months after surgery, follow-ups were done monthly; after that, they were done every three to six months. The data were analyzed after preparing the demographic and clinicopathological checklist.
Statistical analysis
Mean and standard deviation were used to analyze quantitative data and ratios were used for qualitative data. In the part of analytical analysis and investigation of the relationship between variables, related statistical models such as independent t-test were used to evaluate the mean difference between two variables, Chi squared test to determine the relationship between qualitative variables and logistic regression was used to predict, and determine the relevance and control of confounders. STATA version 17 software was utilized for data analysis. All analyses were performed with a 95% confidence level.
Results
The average age of the 100 patients is 51.46 years (SD: _+11/14). The youngest is 34 years, and the oldest is 78 years. All patients were female. The highest mean age was in the positive EpCAM group and this difference was not significant (P-value <0.388). The association between EpCAM expression and clinical pathology parameters are shown in Table 1.
Table 1.
Association between EpCAM expression and clinicopathological parameters
| Clinicopathological parameters | EpCAM positive | EpCAM negative | P value | ||
|---|---|---|---|---|---|
| Mean age at diagnosis (years) | 52.59 | 10.66% | 50.63 | 11.49% | <0.388 |
| Tumor grade | <0.446 | ||||
| I | 0 | 0.00% | 2 | 3.64% | |
| II | 24 | 57.14% | 29 | 52.73% | |
| III | 18 | 42.86% | 24 | 43.64% | |
| Tumor Stage | <0.276 | ||||
| I | 10 | 23.81% | 7 | 12.73% | |
| II | 23 | 54,76% | 38 | 69.09% | |
| III | 9 | 21.43% | 10 | 18.18% | |
| Size | <0.402 | ||||
| <2 cm | 11 | 26.19% | 9 | 15.79% | |
| 2-5 cm | 29 | 69.05% | 46 | 80.70% | |
| ≥5 cm | 2 | 4.76% | 2 | 3.51% | |
| Estrogen receptor | <0.002 | ||||
| ER+ | 9 | 21.43% | 30 | 51.72% | |
| ER- | 33 | 78.57% | 28 | 48.28% | |
| Progesterone receptor | <0.006 | ||||
| PR+ | 9 | 21.43% | 28 | 28.48% | |
| PR- | 33 | 78.57% | 30 | 51.72% | |
| HER2 | <0.198 | ||||
| HER2+ | 10 | 23.81% | 8 | 13.79% | |
| HER2- | 32 | 76.19% | 50 | 86.21% | |
| Ki67 | <0.658 | ||||
| <14% | 6 | 14.29% | 10 | 17.24% | |
| 14-29% | 5 | 11.90% | 10 | 17.24% | |
| ≥30% | 31 | 73.81% | 38 | 65.52% | |
| LVI | <0.892 | ||||
| N1 | 33 | 78.57% | 47 | 81.03% | |
| N2 | 5 | 11.90% | 7 | 12.07% | |
| N3 | 4 | 9.52% | 4 | 6.90% | |
| TNBC | <0.073 | ||||
| TNBC+ | 25 | 59.52% | 24 | 41.38% | |
| TNBC- | 17 | 40.48% | 34 | 58.62% | |
| Met/Recurrence | <0.002 | ||||
| Yes | 13 | 30.95% | 4 | 7.14% | |
| No | 29 | 69.05% | 52 | 92.86% | |
Abbreviations: EpCAM = epithelial cell adhesion molecule; ER = estrogen receptor; HER2 = human epidermal growth factor receptor; LVI = lymph vascular invasion; TNBC = Triple Negative Breast Cancer.
The difference in the distribution of the negative state of biomarkers (respectively ER-PR) and the EPCAM-positive group was significant (P-value = 0.002) (P-value = 0.006). There was no significant difference between the distribution of HER2 and EPCAM groups (P-value = 0.198). Out of the total EPCAM-positive patients (42 patients), 25 (59.52%) had TNBC. The rest of the patients were EpCAM-negative (n = 58), 24 (41.38) % had TNBC, which indicates a significant relationship between EPCAM-positive and TNBC (P-value = 0.073).
Furthermore, 13% (30.95) of EpCAM-positive patients had relapse or metastasis, while in the EpCAM negative group, 4 patients (7.14%) had recurrence or metastasis, which indicates a significant relationship between being EpCAM-positive and recurrence or metastasis (P-value = 0.002). Among the 13 patients who tested positive for EpCAM and had recurrence and metastasis, 6 of them developed bone metastases. Additional metastases were found in the lung, liver, neck lymph nodes, brain, and thyroid, in that order. Additional data is provided in Table 1. Based on the univariate logistic regression model, ER+ and PR+ decreased the chance of EpCAM positive by 0.25 and 0.29, respectively. In other words, ER+ and PR+ are protective factors (P-value <0.003) (P-value <0.007).
Moreover, HER2+ and TNBC (Basal like breast cancer) increase the chances of EpCAM being positive by 1.9 and 2.08, respectively (P-value <0.203) (P-value <0.075). Table 2 displays the effect of additional pathological factors on EPCAM using a univariate logistic regression model. In the group of EpCAM-positive patients, the mean Ki67 index was 41.28 (SD: +/- 22.28), which indicates a high proliferative index. The average Ki67 index between positive and negative EpCAM is not significant (P-value = 0.670).
Table 2.
Association between EpCAM expression and clinicopathological parameters based on univariate logistic regression
| Variable | OR | Std.err | P-value | Conf-interval (95%) |
|---|---|---|---|---|
| ER+ | 0.25 | 0.11 | 0.003 | 0.10-0.062 |
| PR+ | 0.29 | 0.13 | 0.007 | 0.11-0.71 |
| HER2+ | 1.9 | 1.02 | 0.203 | 0.69-5.47 |
| TNBC | 2.08 | 0.85 | 0.075 | 0.92-4.67 |
| SIZE | 0.51 | 0.26 | 0.193 | 0.190-1.39 |
| STAGE 3 | 0.63 | 0.42 | 0.493 | 0.16-2.35 |
| N2 (LN = 3-9) | 1.6 | 0.88 | 0.363 | 0.56-4.69 |
In Table 3, we measured the effect of pathologic variables on the odds of EpCAM positivity based on a (adjusted) multivariate logistic regression model. The presence of TNBC raises the likelihood of EpCAM positivity by a factor of 2.19. Similarly, the presence of N2 increases the likelihood of EpCAM positivity by a factor of 1.95, whereas stage 3 increases it by a factor of 0.5.
Table 3.
Association between EpCAM expression and clinicopathological parameters based on adjusted logistic regression
| Variable | OR | Std.err | P-value | Conf-interval (95%) |
|---|---|---|---|---|
| TNBC | 2.19 | 0.96 | 0.073 | 0.93-5.18 |
| SIZE | 0.82 | 0.50 | 0.755 | 0.24-2.73 |
| STAGE 3 | 0.50 | 0.31 | 0.273 | 0.14-1.71 |
| N2 (LN = 3-9) | 1.95 | 0.83 | 0.116 | 0.84-4.52 |
Discussion
The results of our study showed that Basal like breast cancer increases the chance of being EpCAM positive more than 2 times. Thus, HER2+ increases the chance of positive EpCAM. The recurrence and metastasis in the EpCAM-positive group was more than 4 times that of EpCAM negative group. The highest prevalence of metastasis in the group of EpCAM-positive patients was bone metastasis.
The preponderance of patients (80%) in this study exhibited N1 (0-3) involvement. Involvement of one unit more, with N2 lymph nodes in comparison to N1, increases the probability of EpCAM positivity by 1.95. The probability of EpCAM positivity increases as stage rises. At stage 3, the probability of an EpCAM positive result increases by 0.6 times. Most of the patients had stage (2-5 cm), so tumor size is still a protective factor even in the conditions of equalizing confounding factors. The analysis of these cases can be in terms of the association with TNBC or HER2 positivity with increasing tumor size and lymph node involvement, where the patient was first sent to neoadjuvant chemotherapy, and then surgery was performed, and then the lymph node size decreased. In other words, people with larger size are usually ER/PR positive patients.
The standard chemotherapy treatments in TNBC patients was without immunotherapy, and in HER2-positive patients, a single therapy was used (without dual therapy). EpCAM protein expression was detected in 45 to 90% of breast cancers in different studies. In recent years, different adhesive and migratory phenotypes have been discussed in EpCAM and potentially explain at least part of the appearance. Inconsistency does appear between different studies [10]. Therefore, reliable biomarkers for diagnosis, prognosis, and treatment of breast cancer patients are essential.
The EpCAM family is commonly prevalent in epithelial tumors and interacts with many cell types [11]. Expression of this protein is likely related with chromosomal instability and aneuploidy, and hence, tumor metastasis [4]. The occurrence of EpCAM was discussed in studies in carcinomas of various origins, including breast, ovarian, prostate, lung, colon, pancreas, stomach, and head and neck tissues [12]. They said that overexpression is associated with aggressive and metastatic behavior in various tumor types, including gastrointestinal, ovarian, bladder, and breast tumors, by regulating anti-apoptotic factors and inducing abnormal functions of other cell adhesion molecules [13]. The biological significance and predictive potential of EpCAM, especially in breast cancer, are under investigation [14].
Other research found that EpCAM expression was higher in high-grade poorly differentiated tumors than in low-grade tumors, particularly in breast and prostate cancer [15]. Another study stated that the expression of this protein is related to tumor stage, which is consistent with our results [16]. Its overexpression was reported to induce proliferation and upregulation of Pro-oncogenic signals that lead to metastasis and decreased survival especially in node-positive breast cancer patients [17], which was in agreement with the results of our recent study. Nevertheless, instances of EPCAM association have been documented in negative lymph nodes containing gene mutations (KRAS, TP53, ERBB1) [18]. Studies showed that the presence of high-expressing EPCAM circulating tumor cells (CTC) in the blood of prostate and breast cancer patients is strongly associated with poor prognosis [19]. In our study, a similar result was obtained.
Because of its widespread expression, particularly in the patients with a high risk of metastasis, the detection of EpCAM could improve the identification of Breast CTCs, which is why combined detection using antibodies for EpCAM and HER2 may be beneficial [20]. Recent studies noted that overexpression of EpCAM in breast cancer causes resistance to radiotherapy, metastasis, and worse conditions of the disease. Therefore, the patients with EpCAM expression may benefit from mastectomy treatment, and this requires further studies [21].
Additionally, another study showed that [22] catumaxomab in conjunction with activated T-cells could potentially serve as an effective therapeutic approach for chemoresistant EpCAM-positive TNBC cells in patients. Previous studies suggest that EpCAM may have a regulatory role in epithelial-mesenchymal transition (EMT) in breast cancer cells [23]. The presence of CD31+/EpCAM+ results in resistance to neoadjuvant chemotherapy and a poorer prognosis. Recent studies have shown that more circulating endothelial cells (CEC) in the circulation enable metastasis and resistance to chemotherapy [24].
The predictive value of EpCAM expression in basal-like and luminal B and HER2+ subtype breast cancer, which is associated with poor prognosis, has been investigated [23]. These findings are in line with our recent study findings. Another study was conducted that investigated the relationship between EpCAM expression and pathologic response, clinical outcomes, and biomarker expression in a significant cohort of breast tumors, with special emphasis on HER2-positive tumors and TNBC [15]. There are numerous parallels between the findings of his research and our own. The overexpression of this protein may lead to proliferation, migration, and invasion of breast cancer cells. These studies indicate its association with tumors and suggest it is a potential target for immunotherapy [25,26].
Also, patients, especially TNBC and EPCAM positive, are the best candidates for immunotherapy with catomaxumab (Anti-EpCAM - Anti-CD3) in patients with breast cancer resistant to chemotherapy [22]. Overexpression of this protein is independently associated with poor survival in node-positive patients, particularly in the TNBC subgroup. This finding is consistent with the results of our recent research.
In conclusion, these cases introduce EpCAM as a promising therapeutic target in this patient population [4]. Additionally, combination therapy with adecatumumab, an EpCAM antibody, and docetaxel is safe, effective, and feasible even in advanced stages of previously treated breast cancer [22,27]. Furthermore, the important role of EpCAM in promoting the behavior of tumor cells and metastatic cells of breast cancer was suggested. Another study showed that epithelial cell phenotypes expressing EpCAM contribute to the development of bone metastasis, especially after bone entry, and blood flow [28]. This is consistent with our study.
Conclusion
We found that Basal like breast cancer, HER2+, and being in STAGE 3 increase the chance of EpCAM positivity. It seems that EpCAM positive cancer has more chance for recurrence and metastasis. The limitations of this study were a lack of specialized EpCAM measurement kits, due to financial limitations, with limited access to the pathology laboratory, and most importantly, this being a retrospective study in with a limited sample size. It is important to note that most of the conclusions may be more broadly applied if this study is conducted prospectively with a greater number of samples.
Acknowledgements
The authors would like to acknowledge the Breast Cancer and Health Research Center of Iran University of Medical Sciences.
Disclosure of conflict of interest
None.
References
- 1.Brooks JD, Mah A, Christensen RAG, Arneja J, Eisen A, Chiarelli AM. Validation of the International Breast Cancer Intervention Study (IBIS) model in the High Risk Ontario Breast Screening Program: a retrospective cohort study. Genet Med. 2023;25:100820. doi: 10.1016/j.gim.2023.100820. [DOI] [PubMed] [Google Scholar]
- 2.Milroy MJ. Breast cancer screening. S D Med. 2015:69–73. [PubMed] [Google Scholar]
- 3.Perez AA, Balabram D, Rocha RM, da Silva Souza Á, Gobbi H. Co-expression of p16, Ki67 and COX-2 is associated with basal phenotype in high-grade ductal carcinoma in situ of the breast. J Histochem Cytochem. 2015;63:408–416. doi: 10.1369/0022155415576540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng L, Deng X, Zhong J, Yuan L, Tao X, Zhang S, Zeng Y, He G, Tan P, Tao Y. Prognostic value of biomarkers EpCAM and αB-crystallin associated with lymphatic metastasis in breast cancer by iTRAQ analysis. BMC Cancer. 2019;19:831. doi: 10.1186/s12885-019-6016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bill R, Christofori G. The relevance of EMT in breast cancer metastasis: correlation or causality? FEBS Lett. 2015;589:1577–1587. doi: 10.1016/j.febslet.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Pauken CM, Kenney SR, Brayer KJ, Guo Y, Brown-Glaberman UA, Marchetti D. Heterogeneity of circulating tumor cell neoplastic subpopulations outlined by single-cell transcriptomics. Cancers (Basel) 2021;13:4885. doi: 10.3390/cancers13194885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan X, Ma F, Li C, Wu S, Hu S, Huang J, Sun X, Wang J, Luo Y, Cai R, Fan Y, Li Q, Chen S, Zhang P, Li Q, Xu B. The prognostic and therapeutic implications of circulating tumor cell phenotype detection based on epithelial-mesenchymal transition markers in the first-line chemotherapy of HER2-negative metastatic breast cancer. Cancer Commun (Lond) 2019;39:1. doi: 10.1186/s40880-018-0346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu M, Qian G, Xie F, Shi C, Yan L, Yu L, Zheng T, Wei L, Yang J. Expression of epithelial cell adhesion molecule associated with elevated ductular reactions in hepatocellar carcinoma. Clin Res Hepatol Gastroenterol. 2014;38:699–705. doi: 10.1016/j.clinre.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Weimholt RC, Sharifai N, Abro B, Vij K, Bernadt C. Reactivity with the EpCAM-specific antibodies MOC-31 and Ber-Ep4 in plasma cell neoplasms: a potential diagnostic pitfall in cytology samples. J Am Soc Cytopathol. 2019;8:265–269. doi: 10.1016/j.jasc.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Fagotto F, Aslemarz A. EpCAM cellular functions in adhesion and migration, and potential impact on invasion: a critical review. Biochim Biophys Acta Rev Cancer. 2020;1874:188436. doi: 10.1016/j.bbcan.2020.188436. [DOI] [PubMed] [Google Scholar]
- 11.Gao S, Sun Y, Liu X, Zhang D, Yang X. EpCAM and COX-2 expression are positively correlated in human breast cancer. Mol Med Rep. 2017;15:3755–3760. doi: 10.3892/mmr.2017.6447. [DOI] [PubMed] [Google Scholar]
- 12.Ruan Y, Chen L, Xie D, Luo T, Xu Y, Ye T, Chen X, Feng X, Wu X. Mechanisms of cell adhesion molecules in endocrine-related cancers: a concise outlook. Front Endocrinol (Lausanne) 2022;13:865436. doi: 10.3389/fendo.2022.865436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chimonidou M, Strati A, Malamos N, Kouneli S, Georgoulias V, Lianidou E. Direct comparison study of DNA methylation markers in EpCAM-positive circulating tumour cells, corresponding circulating tumour DNA, and paired primary tumours in breast cancer. Oncotarget. 2017;8:72054–72068. doi: 10.18632/oncotarget.18679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gires O, Pan M, Schinke H, Canis M, Baeuerle PA. Expression and function of epithelial cell adhesion molecule EpCAM: where are we after 40 years? Cancer Metastasis Rev. 2020;39:969–987. doi: 10.1007/s10555-020-09898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vishnubalaji R, Alajez NM. Single-cell transcriptome analysis revealed heterogeneity and identified novel therapeutic targets for breast cancer subtypes. Cells. 2023;12:1182. doi: 10.3390/cells12081182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao J, Liu X, Yang F, Liu T, Yan Q, Yang X. By inhibiting Ras/Raf/ERK and MMP-9, knockdown of EpCAM inhibits breast cancer cell growth and metastasis. Oncotarget. 2015;6:27187–98. doi: 10.18632/oncotarget.4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thakur C, Qiu Y, Zhang Q, Carruthers NJ, Yu M, Bi Z, Fu Y, Wadgaonkar P, Almutairy B, Seno A, Stemmer PM, Chen F. Deletion of mdig enhances H3K36me3 and metastatic potential of the triple negative breast cancer cells. iScience. 2022;25:105057. doi: 10.1016/j.isci.2022.105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Treitschke S, Weidele K, Varadarajan AR, Feliciello G, Warfsmann J, Vorbeck S, Polzer B, Botteron C, Hoffmann M, Dechand V, Mederer T, Weber F, Werner-Klein M, Robold T, Hofmann HS, Werno C, Klein CA. Exvivo expansion of lung cancer-derived disseminated cancer cells from lymph nodes identifies cells associated with metastatic progression. Int J Cancer. 2023;153:1854–1867. doi: 10.1002/ijc.34658. [DOI] [PubMed] [Google Scholar]
- 19.de Wit S, Manicone M, Rossi E, Lampignano R, Yang L, Zill B, Rengel-Puertas A, Ouhlen M, Crespo M, Berghuis AMS, Andree KC, Vidotto R, Trapp EK, Tzschaschel M, Colomba E, Fowler G, Flohr P, Rescigno P, Fontes MS, Zamarchi R, Fehm T, Neubauer H, Rack B, Alunni-Fabbroni M, Farace F, De Bono J, IJzerman MJ, Terstappen LWMM. EpCAMhigh and EpCAMlow circulating tumor cells in metastatic prostate and breast cancer patients. Oncotarget. 2018;9:35705–35716. doi: 10.18632/oncotarget.26298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nini A, Hoffmann MJ, Lampignano R, Große Siemer R, van Dalum G, Szarvas T, Cotarelo CL, Schulz WA, Niederacher D, Neubauer H, Stoecklein NH, Niegisch G. Evaluation of HER2 expression in urothelial carcinoma cells as a biomarker for circulating tumor cells. Cytometry B Clin Cytom. 2020;98:355–367. doi: 10.1002/cyto.b.21877. [DOI] [PubMed] [Google Scholar]
- 21.Mal A, Bukhari AB, Singh RK, Kapoor A, Barai A, Deshpande I, Wadasadawala T, Ray P, Sen S, De A. EpCAM-mediated cellular plasticity promotes radiation resistance and metastasis in breast cancer. Front Cell Dev Biol. 2021;8:597673. doi: 10.3389/fcell.2020.597673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubo M, Umebayashi M, Kurata K, Mori H, Kai M, Onishi H, Katano M, Nakamura M, Morisaki T. Catumaxomab with activated T-cells efficiently lyses chemoresistant EpCAM-positive triple-negative breast cancer cell lines. Anticancer Res. 2018;38:4273–4279. doi: 10.21873/anticanres.12724. [DOI] [PubMed] [Google Scholar]
- 23.Soysal SD, Muenst S, Barbie T, Fleming T, Gao F, Spizzo G, Oertli D, Viehl CT, Obermann EC, Gillanders WE. EpCAM expression varies significantly and is differentially associated with prognosis in the luminal B HER2+, basal-like, and HER2 intrinsic subtypes of breast cancer. J Cancer. 2013;108:1480–1487. doi: 10.1038/bjc.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma G, Jiang Y, Liang M, Li J, Wang J, Mao X, Veeramootoo JS, Xia T, Liu X, Wang S. Dynamic monitoring of CD45-/CD31+/DAPI+ circulating endothelial cells aneuploid for chromosome 8 during neoadjuvant chemotherapy in locally advanced breast cancer. Ther Adv Med Oncol. 2020;12:1758835920918470. doi: 10.1177/1758835920918470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jäger M, Schoberth A, Ruf P, Hess J, Hennig M, Schmalfeldt B, Wimberger P, Ströhlein M, Theissen B, Heiss M, Lindhofer H. Immunomonitoring results of a phase II/III study of malignant ascites patients treated with the trifunctional antibody catumaxomab (anti-EpCAM× anti-CD3) Cancer Res. 2012;72:24–32. doi: 10.1158/0008-5472.CAN-11-2235. [DOI] [PubMed] [Google Scholar]
- 26.Kurbacher CM, Horn O, Kurbacher JA, Herz S, Kurbacher AT, Hildenbrand R, Bollmann R. Outpatient intraperitoneal catumaxomab therapy for malignant ascites related to advanced gynecologic neoplasms. Oncologist. 2015;20:1333–1341. doi: 10.1634/theoncologist.2015-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt M, Scheulen ME, Dittrich C, Obrist P, Marschner N, Dirix L, Schmidt M, Rüttinger D, Schuler M, Reinhardt C, Awada A. An open-label, randomized phase II study of adecatumumab, a fully human anti-EpCAM antibody, as monotherapy in patients with metastatic breast cancer. Ann Oncol. 2010;21:275–282. doi: 10.1093/annonc/mdp314. [DOI] [PubMed] [Google Scholar]
- 28.Hiraga T, Ito S, Nakamura H. EpCAM expression in breast cancer cells is associated with enhanced bone metastasis formation. Int J Cancer. 2016;138:1698–1708. doi: 10.1002/ijc.29921. [DOI] [PubMed] [Google Scholar]