Abstract
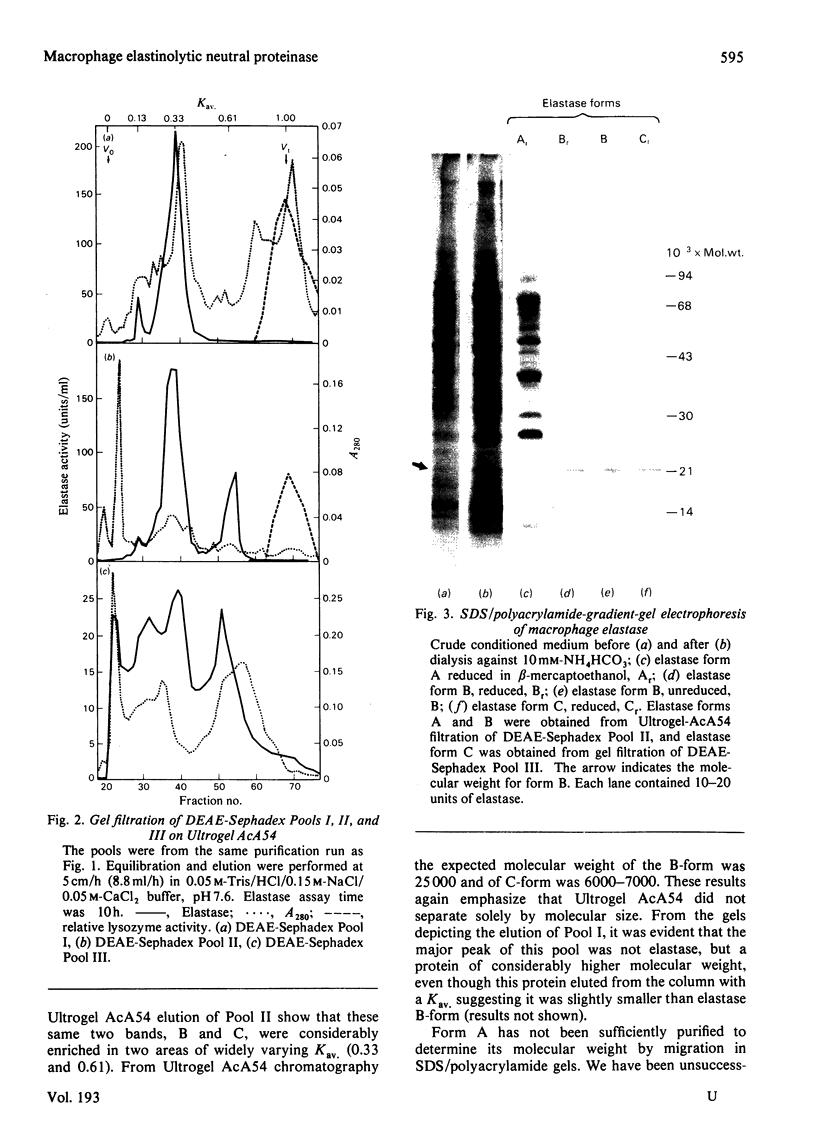
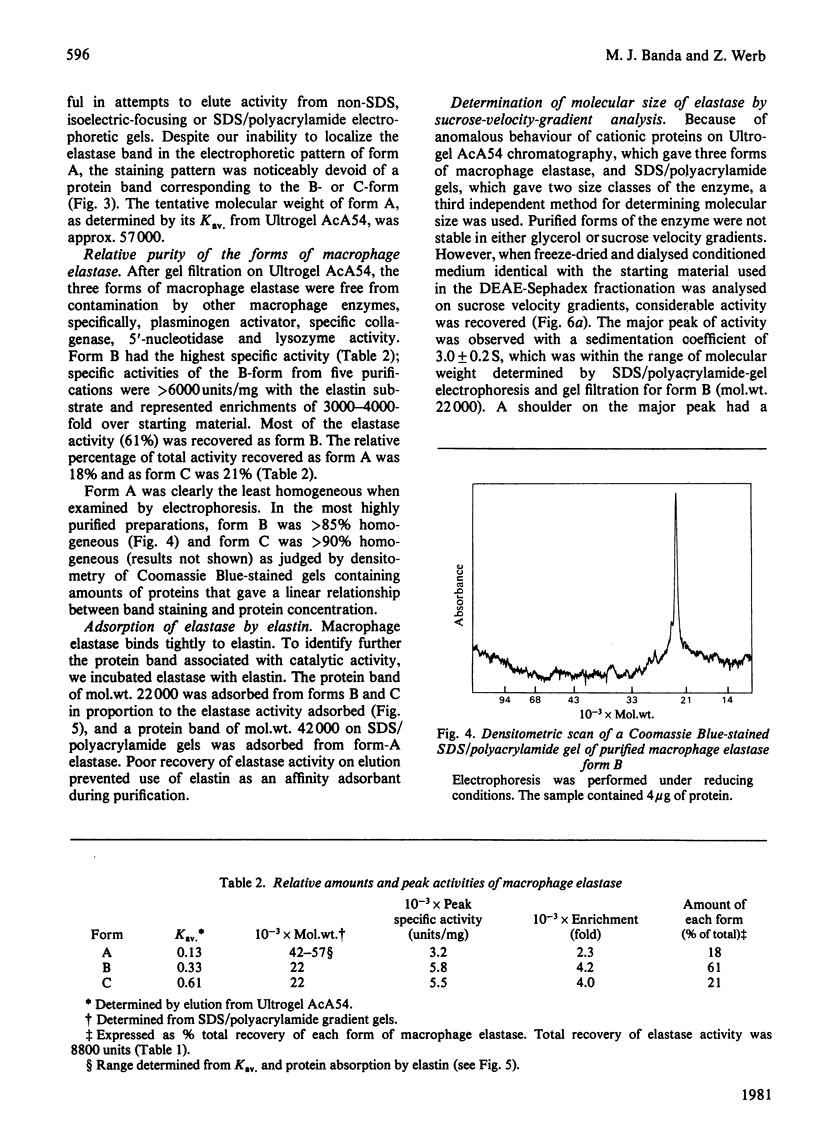
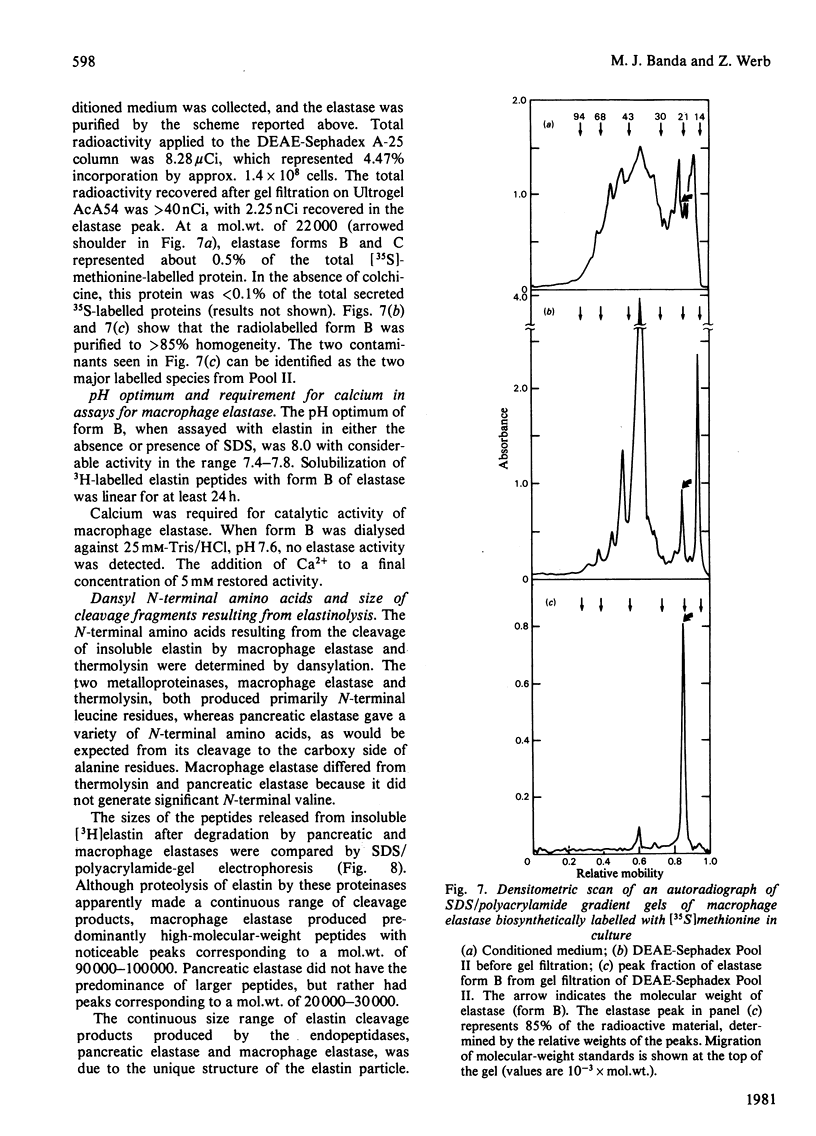
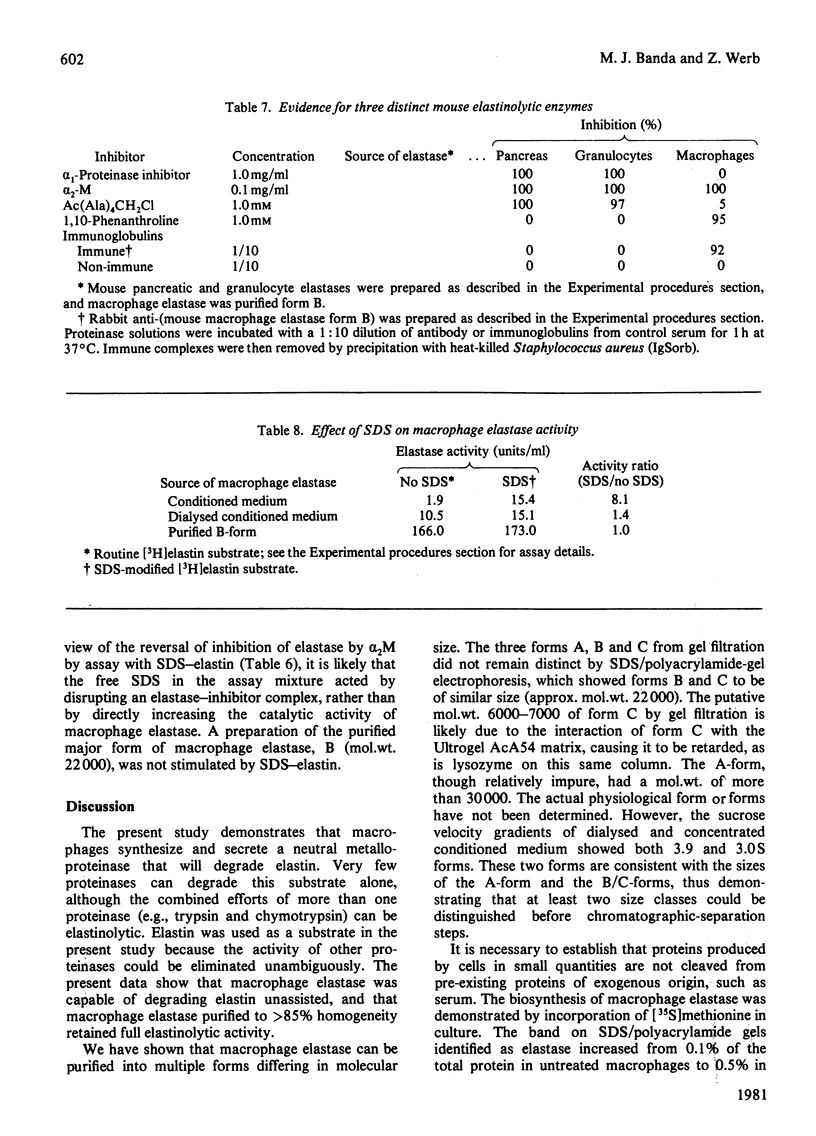
Macrophage elastase was purified from tissue-culture medium conditioned by inflammatory mouse peritoneal macrophages. Characterized as a secreted neutral metalloproteinase, this enzyme was shown to be catalytically and immunochemically distinct from the mouse pancreatic and mouse granulocyte elastases, both of which are serine proteinases. Inhibition profiles, production of nascent N-terminal leucine residues and sodium dodecyl sulphate/polyacrylamide-gel electrophoresis of degraded elastin indicated that macrophage elastase is an endopeptidase, with properties of a metalloproteinase, rather than a serine proteinase. Macrophage elastase was inhibited by alpha 2-macroglobulin, but not by alpha 1-proteinase inhibitor. Macrophage elastase was resolved into three chromatographically distinct forms. The predominant form had mol.wt. 22 000 and was purified 4100-fold. Purification of biosynthetically radiolabelled elastase indicated that this form represented less than 0.5% of the secreted protein of macrophages. Approx. 800% of the starting activity was recovered after purification. Evidence was obtained for an excess of an endogenous inhibitor masking more than 80% of the secreted activity.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardelt W. Inactivation of some pancreatic and leucocyte elastases by peptide chloromethyl ketones and alkyl isocyanates. FEBS Lett. 1976 Aug 15;67(2):156–160. doi: 10.1016/0014-5793(76)80355-9. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. The many forms and functions of cellular proteinases. Fed Proc. 1980 Jan;39(1):9–14. [PubMed] [Google Scholar]
- Bieth J., Spiess B., Wermuth C. G. The synthesis and analytical use of a highly sensitive and convenient substrate of elastase. Biochem Med. 1974 Dec;11(4):350–357. doi: 10.1016/0006-2944(74)90134-3. [DOI] [PubMed] [Google Scholar]
- Colman P. M., Jansonius J. N., Matthews B. W. The structure of thermolysin: an electron density map at 2-3 A resolution. J Mol Biol. 1972 Oct 14;70(3):701–724. doi: 10.1016/0022-2836(72)90569-4. [DOI] [PubMed] [Google Scholar]
- Gordon S., Werb Z. Secretion of macrophage neutral proteinase is enhanced by colchicine. Proc Natl Acad Sci U S A. 1976 Mar;73(3):872–876. doi: 10.1073/pnas.73.3.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. C., Cocking E. C. High-efficiency liquid-scintillation counting of 14C-labelled material in aqueous solution and determination of specific activity of labelled proteins. Biochem J. 1965 Sep;96(3):626–633. doi: 10.1042/bj0960626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser P., Vaes G. Degradation of cartilage proteoglycans by a neutral proteinase secreted by rabbit bone-marrow macrophages in culture. Biochem J. 1978 May 15;172(2):275–284. doi: 10.1042/bj1720275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A. L., Kelman J. A., Crystal R. G. Activation of alveolar macrophage collagenase by a neutral protease secreted by the same cell. Nature. 1976 Dec 23;264(5588):772–774. doi: 10.1038/264772a0. [DOI] [PubMed] [Google Scholar]
- Janoff A. Neutrophil proteases in inflammation. Annu Rev Med. 1972;23:177–190. doi: 10.1146/annurev.me.23.020172.001141. [DOI] [PubMed] [Google Scholar]
- Kagan H. M. Changes in the state of ionization of carboxyl groups in elastin in response to the binding of sodium dodecyl sulfate. Connect Tissue Res. 1978;6(3):167–169. doi: 10.3109/03008207809152627. [DOI] [PubMed] [Google Scholar]
- Kagan H. M., Crombie G. D., Jordan R. E., Lewis W., Franzblau C. Proteolysis of elastin-ligand complexes. Stimulation of elastase digestion of insoluble elastin by sodium dodecyl sulfate. Biochemistry. 1972 Aug 29;11(18):3412–3418. doi: 10.1021/bi00768a014. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- MANDL I., COHEN B. B. Bacterial elastase. I. Isolation, purification and properties. Arch Biochem Biophys. 1960 Nov;91:47–53. doi: 10.1016/0003-9861(60)90453-7. [DOI] [PubMed] [Google Scholar]
- MORIHARA K. PRODUCTION OF ELASTASE AND PROTEINASE BY PSEUDOMONAS AERUGINOSA. J Bacteriol. 1964 Sep;88:745–757. doi: 10.1128/jb.88.3.745-757.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORIHARA K., TSUZUKI H., OKA T., INOUE H., EBATA M. PSEUDOMONAS AERUGINOSA ELASTASE. ISOLATION, CRYSTALLIZATION, AND PRELIMINARY CHARACTERIZATION. J Biol Chem. 1965 Aug;240:3295–3304. [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H. Phosphoramidon as an inhibitor of elastase from Pseudomonas aeruginosa. Jpn J Exp Med. 1978 Feb;48(1):81–84. [PubMed] [Google Scholar]
- Mull J. D., Callahan W. S. The role of the elastase of Pseudomonas aeruginosa in experimental infection. Exp Mol Pathol. 1965 Dec;4(6):567–575. doi: 10.1016/0014-4800(65)90037-7. [DOI] [PubMed] [Google Scholar]
- Nishino N., Powers J. C. Pseudomonas aeruginosa elastase. Development of a new substrate, inhibitors, and an affinity ligand. J Biol Chem. 1980 Apr 25;255(8):3482–3486. [PubMed] [Google Scholar]
- Ohlsson K., Olsson I. The neutral proteases of human granulocytes. Isolation and partial characterization of granulocyte elastases. Eur J Biochem. 1974 Mar 1;42(2):519–527. doi: 10.1111/j.1432-1033.1974.tb03367.x. [DOI] [PubMed] [Google Scholar]
- Powers J. C., Tuhy P. M. Active-site specific inhibitors of elastase. Biochemistry. 1973 Nov 6;12(23):4767–4774. doi: 10.1021/bi00747a032. [DOI] [PubMed] [Google Scholar]
- Redman C. M., Banerjee D., Manning C., Huang C. Y., Green K. In vivo effect of colchicine on hepatic protein synthesis and on the conversion of proalbumin to serum albumin. J Cell Biol. 1978 May;77(2):400–416. doi: 10.1083/jcb.77.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker R. B., Heng-Khoo C. S., Dubick M., Lefevre M., Cross C. E. Partial characterization of a tropoelastin precursor isolated from chick aorta. Biochemistry. 1979 Sep 4;18(18):3854–3859. doi: 10.1021/bi00585a004. [DOI] [PubMed] [Google Scholar]
- Schmidt W., Havemann K. Isolation of elastase-like and chymotrypsin-like neutral proteases from human granulocytes. Hoppe Seylers Z Physiol Chem. 1974 Sep;355(9):1077–1082. doi: 10.1515/bchm2.1974.355.2.1077. [DOI] [PubMed] [Google Scholar]
- Schumacher G. F., Schill W. B. Radial diffusion in gel for micro determination of enzymes. II. Plasminogen activator, elastase, and nonspecific proteases. Anal Biochem. 1972 Jul;48(1):9–26. doi: 10.1016/0003-2697(72)90165-0. [DOI] [PubMed] [Google Scholar]
- Sellers A., Murphy G., Meikle M. C., Reynolds J. J. Rabbit bone collagenase inhibitor blocks the activity of other neutral metalloproteinases. Biochem Biophys Res Commun. 1979 Mar 30;87(2):581–587. doi: 10.1016/0006-291x(79)91834-5. [DOI] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Human lysosomal elastase. Catalytic and immunological properties. Biochem J. 1976 May 1;155(2):265–271. doi: 10.1042/bj1550265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Neutral proteinases of human spleen. Purification and criteria for homogeneity of elastase and cathepsin G. Biochem J. 1976 May 1;155(2):255–263. doi: 10.1042/bj1550255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone P. J., Crombie G., Franzblau C. The use of tritiated elastin for the determination of subnanogram amounts of elastase. Anal Biochem. 1977 Jun;80(2):572–577. doi: 10.1016/0003-2697(77)90680-7. [DOI] [PubMed] [Google Scholar]
- Suda H., Aoyagi T., Takeuchi T., Umezawa H. Letter: A thermolysin inhibitor produced by Actinomycetes: phospholamidon. J Antibiot (Tokyo) 1973 Oct;26(10):621–623. doi: 10.7164/antibiotics.26.621. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Seifter S., Yang F. C. A new radioactive assay for enzymes with elastolytic activity using reduced tritiated elastin. The effect of sodium dodecyl sulfate on elastolysis. Biochim Biophys Acta. 1973 Nov 15;327(1):138–145. doi: 10.1016/0005-2744(73)90111-3. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Gordon S., Reich E. Secretion of plasminogen activator by stimulated macrophages. J Exp Med. 1974 Apr 1;139(4):834–850. doi: 10.1084/jem.139.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C., Tobia A., Ossowski L., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. I. Chick embryo fibroblast cultures transformed by avian RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):85–111. doi: 10.1084/jem.137.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virca G. D., Travis J., Hall P. K., Roberts R. C. Purification of human alpha-2-macroglobulin by chromatography on Cibacron Blue Sepharose. Anal Biochem. 1978 Aug 15;89(1):274–278. doi: 10.1016/0003-2697(78)90750-9. [DOI] [PubMed] [Google Scholar]
- Visser L., Blout E. R. The use of p-nitrophenyl N-tert-butyloxycarbonyl-L-alaninate as substrate for elastase. Biochim Biophys Acta. 1972 Apr 7;268(1):257–260. doi: 10.1016/0005-2744(72)90223-9. [DOI] [PubMed] [Google Scholar]
- Werb Z., Aggeler J. Proteases induce secretion of collagenase and plasminogen activator by fibroblasts. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1839–1843. doi: 10.1073/pnas.75.4.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Dingle J. T., Reynolds J. J., Barrett A. J. Proteoglycan-degrading enzymes of rabbit fibroblasts and granulocytes. Biochem J. 1978 Sep 1;173(3):949–958. doi: 10.1042/bj1730949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Gordon S. Elastase secretion by stimulated macrophages. Characterization and regulation. J Exp Med. 1975 Aug 1;142(2):361–377. doi: 10.1084/jem.142.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Gordon S. Secretion of a specific collagenase by stimulated macrophages. J Exp Med. 1975 Aug 1;142(2):346–360. doi: 10.1084/jem.142.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]



