Abstract
Peri-prosthetic joint infection (PJI) is a significant complication following total knee arthroplasty (TKA). Early identification and management are essential to prevent severe morbidity and mortality in these patients. Long-term complications of PJI include the need for multiple operations, disability, joint stiffness, reduced range of motion, and increased mortality. Clinical signs, inflammatory markers, imaging, tissue sampling, and synovial fluid analysis are required to diagnose PJI. Debridement antibiotics and implant retention (DAIR) is an effective management option, but single- or two-stage exchange arthroplasty may be ultimately required. All cases of PJI in TKA must be discussed in a multi-disciplinary (MDT) meeting. This review incorporates the updated British Orthopaedic Association (BOA) standard and speciality standard to provide an up-to-date guideline on the early identification and management of PJI. We highlight that adhering to the BOA guidelines and adopting an MDT approach are essential for optimal patient outcomes.
Keywords: british orthopaedic association, british orthopaedic association standards, peri-prosthetic joint infection, primary knee replacement, tka (total knee arthoplasty), total knee replacement complications
Introduction and background
Peri-prosthetic joint infection (PJI) is a significant complication in patients who undergo total knee arthroplasty (TKA) [1]. The National Joint Registry (NJR) recorded 226,350 total knee replacements between 2018 and 2020 in England, Wales, Northern Ireland, the Isle of Man, and the States of Guernsey, and these numbers are on the rise [2,3]. Therefore, prompt identification and management of this condition is essential to prevent severe morbidity and mortality, as well as preserve prosthetic function [4,5]. The British Orthopaedic Association (BOA) Standard: Acute Management of Peri-Prosthetic Joint Infection was released in 2023 to identify life-threatening sepsis and immediate recognition of PJI [6]. This was followed by the BOA Specialty Standards (SpecS) Peri-Prosthetic Joint Infection for Definitive Management in 2024 [7], underlying the importance of early identification and management of PJI. Our literature review primarily aims to present up-to-date information on epidemiology, clinical features, investigations, and management options for PJI in TKA based on the recent BOA guidelines. The secondary aim is to consolidate our findings into a flowchart, which, we believe, will assist clinicians in identifying and managing PJI as per the BOA guidelines.
Epidemiology
The incidence of PJI after TKA ranges from 0.5% to 1.8% in primary procedures [8,9]. The risk of developing a PJI is highest in the first two years postoperatively [3,9,10]. In the NJR, recorded between April 2003 and December 2020, 23.5% of TKR revisions were performed due to an infection (n=20,527), second only to aseptic loosening/lysis (33.6%, n=29,387) [3,11]. The NJR’s prosthesis time incident rates (PTIR) estimates for revision due to infection show 0.94 (95% CI: 0.92-0.96) revisions per 1,000 prosthesis years for all cases of TKA, second only to aseptic loosening. This number is 1.95 (1.87-2.02) per 1,000 prosthesis years due to infection in the first year, 0.48 (0.45-0.52) in years five to seven, and 0.28 in years 13-15 post-primary procedure [3].
Review
Aetiology
The presence of a foreign body significantly reduces the concentration of bacteria required to cause an infection and increases the possibility of biofilm formation. The organism is protected from host defences in this state with the extracellular component, composed of polysaccharides and proteins [1,10]. The most commonly isolated organisms in PJI are Staphylococcus aureus and coagulase-negative staphylococci [12], seen in around 50-60% of PJI. Staphylococcus aureus is most likely to present early in the postoperative period in primary arthroplasty, defined as a PJI within three months postoperatively [13]. The study by Tai et al. shows that a positive culture for enterococcus, corynebacterium species, fungi, or mycobacteria may suggest a polymicrobial infection [13].
Pathogens can infect prosthetic joints through three different identified mechanisms:
Direct Inoculation
This accounts for the majority of PJI that occurs within the first postoperative year. This occurs during the prosthetic joint implantation phase of surgery, through contamination of the prosthesis or peri-prosthetic tissue from direct contact or air-borne contamination [1].
Contiguous Spread
This occurs when pathogens spread to the prosthetic joint from adjacent infected tissue. Incomplete healing of the superficial and deep fascial planes postoperatively can lead to surgical site infections (SSI), which may subsequently lead to PJI [1,10]. Contiguous spread can cause delayed PJI secondary to trauma or surgery in the surrounding tissue area [1].
Haematogenous Spread
This occurs when pathogens spread via the blood from other body sites to the prosthetic joint. Although rare, there is an estimated risk of 30-40% of haematogenous spread with staphylococcus aureus bacteraemia [14]. Late infections (after 12 months) generally arise from haematogenous spread [1,12]. Bacteraemia due to less virulent organisms such as enterococci and coagulase-negative staphylococci usually presents from 3-12 months [15]. Streptococci, associated with delayed and late presentation, and enterococci, are associated with 10% of PJI infections combined [10].
Risk factors
Risk factors associated with PJI include malnutrition [16], immunosuppression, and underlying rheumatological conditions [1]. Diabetes mellitus, hyperglycaemia, alcohol use, and malignancy are also implicated in PJI, as is revision surgery following the primary implantation [10]. Perioperative infections of distant sites, such as urinary or pulmonary infections, are associated with PJI in TKA [10]. Infection risk is around 1.8 times after primary knee TKA in both current and former smokers [17].
Long-term complications
PJI significantly impacts patient health and quality of life due to long-term complications. PJI can lead to suboptimal outcomes, multiple operations, long periods of disability, and an increased risk of mortality. Mortality is reportedly higher in the elderly or if the isolated species is either enterococci or methicillin-resistant Staphylococcus aureus (MRSA) [18].
As a result of repeated surgeries or prolonged infection, patients may present with joint stiffness and reduced range of motion, which severely limits daily activities and mobility [19]. Treatment of prosthetic joint infection requires long-term antibiotics, debridement and retention of implants, or revisional surgery performed in one-stage or two-stage [12]. This process is expensive and prolonged, and its impact on patients and their families is substantial [2].
Clinical features
Acute PJI presentations vary depending on the mode of infection, the affected joint and the soft tissue around the joint [10]. Acute PJI after TKA is characterised by the onset of arthralgia, swelling, erythema, and warmth. Systemic signs, including a fever, may be present. Wound drainage, or a sinus tract, may accompany it [19,20]. Chronic PJI after TKA may present with more subtle clinical features. Patients often report discomfort, persistent arthralgia, stiffness, and joint instability. Sinus tract formation can also be associated with chronic infection [10].
Earlier classification systems defined infections within three months of arthroplasty as early, those presenting between 3 and 24 months as delayed, and those after two years as late [20]. The current suggested classifications are based partly on timing but incorporate mode of infection, haematogenous spread, bone and soft tissue defects responsible pathogen, and the systemic host status - which may correspond to factors such as age, neutropenia or other risk factors [20,21].
Investigation and examination
A thorough history and examination are essential in patients presenting with suspected PJI following TKA, with interval observations of vital signs. Prosthesis surgery date, implant brand and size, operation notes, and any postoperative complications should be documented. All subsequent infections should be reported, including recent antibiotic use. The orthopaedic surgeon must investigate other sources of infection, including endocarditis [6]. Clinicians should not start antibiotics until an orthopaedic surgeon is consulted unless the patient is haemodynamically unstable from sepsis [7].
Initial investigations in acute PJI without evidence of sepsis include C-reactive protein (CRP), full blood count (FBC), renal function, and plain radiographs of the affected joint [6]. PJI should not be ruled out with normal inflammatory markers if clinical suspicion remains high, especially in the presence of immunosuppression [6]. An orthopaedic consultant should assess the patient within 48 hours in a stable or systemically well patient. Empirical antibiotics should only be commenced after peri-prosthetic tissue and fluid sampling [6,7,22].
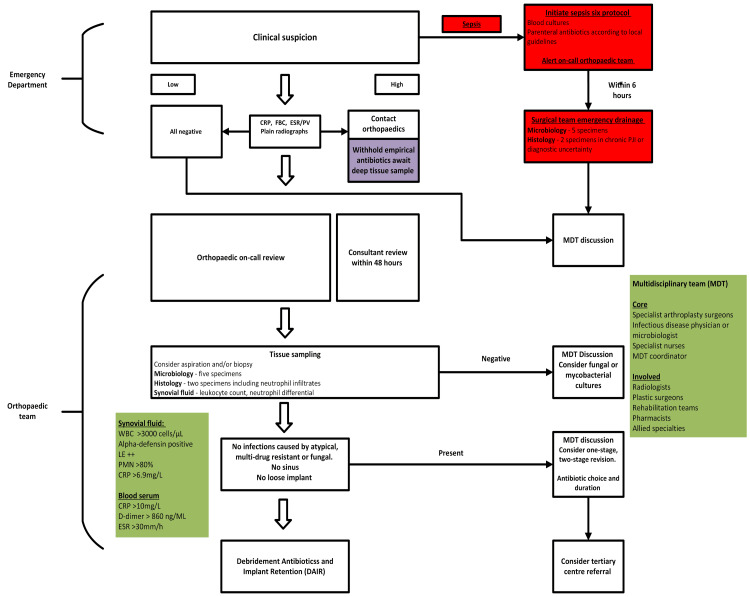
In the presence of sepsis, BOA Standards for Trauma and Orthopaedics (BOAST) guidelines highlight the importance of initiating the "Sepsis Six" protocol immediately and alerting the on-call orthopaedic team. The sepsis protocol [23] must include parenteral antibiotics, according to local guidelines, and patients should have blood cultures taken. The orthopaedic clinician must aspirate the affected joint, preferably within six hours [6]. If debridement is indicated, five microbiological and two histological samples should be taken. A suggested management plan in the emergency department is summarised in Figure 1 based on the BOAST acute PJI guideline from 2023 [6].
Figure 1. Suggested treatment algorithm for managing PJI in TKA.
Initial management follows the British Orthopaedic Association (BOA) Acute Management of PJI guidelines, in the emergency department. Immediate recognition and resuscitation are essential. The BOA speciality standards highlight the importance of the orthopaedic speciality roles. The synovial fluid and blood serum markers highlight the definition agreed upon by the International Consensus Meeting
CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; FBC: full blood count; LE: leukocyte esterase; MDT: multi-disciplinary team; PJI: peri-prosthetic joint infection; PMN: polymorphonuclear; PV: plasma viscosity; TKA: total knee arthroplasty; WBC: white blood cells
Image Credit: Sizar Doski
Following the initial management, the orthopaedic team must ensure multidisciplinary input throughout the treatment process; these include consultant radiologists, microbiologists, physiotherapists and district nurses. Mandatory investigations include FBC, renal function, CRP, and serological analysis, including erythrocyte sedimentation rate (ESR) or plasma viscosity (PV), with plain radiographs. A CT and MRI may help with evaluation and discussion in MDT [26].
Plain radiographs may show displacement of the prosthesis, the components, and osteolysis. However, it can't differentiate between septic and aseptic osteolysis [1]. CT scans may demonstrate infection with fluid collection or periosteal reaction, with good diagnostic performance [26]. MRI is particularly accurate in detecting purulent infection and peri-prosthetic osteolysis [1,26]. The SpecS guidelines do not recommend a bone scan routinely; however, it suggests that newer nuclear imaging techniques may be helpful in diagnostic uncertainty [7]. Tissue sampling should be undertaken with five separate microbiological and two histological samples. A synovial fluid leucocyte count, neutrophil differential, or additional markers such as leucocyte esterase should also be conducted [7]. The suggested definitive management technique is summarised in Figure 1.
Parvizi et al. have proposed a scoring-based definition for PJI, an update of the International Consensus Meeting (ICM) for the definition and diagnosis of PJI in 2013, which was formalised in ICM 2018 [24,25,27]. This incorporates the major criteria for PJI diagnosis defined by the Musculoskeletal Infection Society (MSIS). Major criteria for diagnosis of PJI include one of the following: either a sinus tract formation communicating with the joint or two cultures with the same isolated pathogen. In the minor and additional criteria, the scoring system incorporates blood serum, synovial, and histological analysis to aid the diagnosis of PJI [24]. The BOA speciality standard for investigating and managing PJI in TKA recommends diagnosis using standardised criteria of the ICM 2013 [22,25].
Differential diagnosis
Differentiating aseptic complications of a TKA is the primary challenge encountered when diagnosing PJI. Common symptoms in the immediate postoperative phase such as erythema, swelling, pain, and joint stiffness can resemble PJI [28]. Aseptic cases of TKA dysfunction may also include osteolysis, increased wear or debonding of cement. However, it is essential to rule out PJI since approximately 12% of aseptic joint dysfunctions have an underlying PJI [29,30]. Continuous pain is associated with PJI, whereas pain on mobilising or weight bearing is associated with aseptic failure [1].
Synovial fluid analysis may help differentiate between hemarthrosis or other crystal arthropathies, such as gout and calcium pyrophosphate deposition [28]. Spinal radiculopathy, vascular claudication, tendinopathy or local bursitis, and systemic conditions, such as autoimmune disease, may result in joint pain that resembles PJI [28].
Management
Debridement antibiotics and implant retention (DAIR) can help effectively manage PJI and may treat around 60% of cases [5]. However, DAIR is contraindicated in the presence of a sinus, atypical, fungal, or multidrug-resistant organisms. The infected joint must not show signs of loosening. If the patient is immunocompromised, the performing surgeon must take caution with DAIR as it may not be indicated [7]. Single- or two-stage exchange arthroplasty is necessary following failed management with DAIR. This is a decision that should be taken by an MDT. Arthroscopic washout and debridement should only be used in an emergency, i.e., if mortality is an immediate risk; they have no place in the definitive management of PJI [7,22].
Complex cases involving previous revision for a PJI, multi-drug resistant bacteria, or fungal infections should be discussed in the MDT and referred to a tertiary centre [22]. The referring team may use the Revision Knee Complexity Classification (RKCC) system to evaluate complex cases to determine if a referral is indicated [31].
Conclusions
Prompt identification and management of acute PJI is critical to preserve joint function and prevent morbidity and mortality. It should be considered in any patient who presents with a painful, hot, swollen knee with or without drainage after primary or revision TKA. Key clinical features include acute pain, erythema, and discharge; patients may also show systemic signs of illness. Prompt identification and immediate resuscitation are essential in the presence of sepsis. Blood inflammatory markers and plain radiographs are necessary for initial investigations. This should be followed by a comprehensive assessment by the orthopaedic team and deep tissue sampling. DAIR is an effective definitive management if indicated, whilst other surgical interventions may include single-stage or two-stage revision surgery. Of note, acute PJI management should always involve a coordinated MDT.
Disclosures
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Sizar Doski, Alexandra Sebastiao, Prashant Thayaparan
Acquisition, analysis, or interpretation of data: Sizar Doski, Alexandra Sebastiao, Prashant Thayaparan
Drafting of the manuscript: Sizar Doski, Alexandra Sebastiao, Prashant Thayaparan
Critical review of the manuscript for important intellectual content: Sizar Doski, Alexandra Sebastiao, Prashant Thayaparan
References
- 1.Periprosthetic joint infection. Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Lancet. 2016;387:386–394. doi: 10.1016/S0140-6736(14)61798-0. [DOI] [PubMed] [Google Scholar]
- 2.Risk factors associated with revision for prosthetic joint infection following knee replacement: an observational cohort study from England and Wales. Lenguerrand E, Whitehouse MR, Beswick AD, Kunutsor SK, Foguet P, Porter M, Blom AW. Lancet Infect Dis. 2019;19:589–600. doi: 10.1016/S1473-3099(18)30755-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Shlomo Y, Blom A, Boulton C, et al. London, UK: The National Joint Registry; 2021. The National Joint Registry 18th Annual Report 2021. [PubMed] [Google Scholar]
- 4.2022 American Association of Hip and Knee Surgeons Symposium: periprosthetic joint infection. Tarabichi S, Chen AF, Higuera CA, Parvizi J, Polkowski GG. J Arthroplasty. 2023;38:0–9. doi: 10.1016/j.arth.2023.01.045. [DOI] [PubMed] [Google Scholar]
- 5.Debridement, antibiotics and implant retention for periprosthetic joint infections: a systematic review and meta-analysis of treatment outcomes. Kunutsor SK, Beswick AD, Whitehouse MR, Wylde V, Blom AW. J Infect. 2018;77:479–488. doi: 10.1016/j.jinf.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 6.British Orthopaedic Association - acute management of peri-prosthetic joint infection. Joint Infection. [ Nov; 2024 ]. 2023. https://www.boa.ac.uk/resource/boast-acute-management-of-peri-prosthetic-joint-infection.html https://www.boa.ac.uk/resource/boast-acute-management-of-peri-prosthetic-joint-infection.html
- 7.British Orthopaedic Association: Speciality Standards (SpecS) - peri-prosthetic joint infection. British Orthopaedic Association: Speciality Standards (SpecS) Peri-prosthetic Joint Infection. [ Nov; 2024 ]. 2024. https://www.boa.ac.uk/resource/speciality-standards-specs-peri-prosthetic-joint-infection.html https://www.boa.ac.uk/resource/speciality-standards-specs-peri-prosthetic-joint-infection.html
- 8.Risk factors associated with deep surgical site infections after primary total knee arthroplasty: an analysis of 56,216 knees. Namba RS, Inacio MC, Paxton EW. J Bone Joint Surg Am. 2013;95:775–782. doi: 10.2106/JBJS.L.00211. [DOI] [PubMed] [Google Scholar]
- 9.Prosthetic joint infection risk after TKA in the Medicare population. Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Clin Orthop Relat Res. 2010;468:52–56. doi: 10.1007/s11999-009-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prosthetic joint infection. Tande AJ, Patel R. Clin Microbiol Rev. 2014;27:302–345. doi: 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mortality and re-revision following single-stage and two-stage revision surgery for the management of infected primary knee arthroplasty in England and Wales: evidence from the National Joint Registry. Lenguerrand E, Whitehouse MR, Kunutsor SK, et al. Bone Joint Res. 2022;11:690–699. doi: 10.1302/2046-3758.1110.BJR-2021-0555.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Periprosthetic joint infection: the incidence, timing, and predisposing factors. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Clin Orthop Relat Res. 2008;466:1710–1715. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Microbiology of hip and knee periprosthetic joint infections: a database study. Tai DB, Patel R, Abdel MP, Berbari EF, Tande AJ. Clin Microbiol Infect. 2022;28:255–259. doi: 10.1016/j.cmi.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Periprosthetic joint infection following Staphylococcus aureus bacteremia. Sendi P, Banderet F, Graber P, Zimmerli W. J Infect. 2011;63:17–22. doi: 10.1016/j.jinf.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Clinical practice. Infection associated with prosthetic joints. Del Pozo JL, Patel R. N Engl J Med. 2009;361:787–794. doi: 10.1056/NEJMcp0905029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Procrastination of wound drainage and malnutrition affect the outcome of joint arthroplasty. Jaberi FM, Parvizi J, Haytmanek CT, Joshi A, Purtill J. Clin Orthop Relat Res. 2008;466:1368–1371. doi: 10.1007/s11999-008-0214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Is there an association between smoking status and prosthetic joint infection after primary total joint arthroplasty? Gonzalez AI, Luime JJ, Uçkay I, Hannouche D, Hoffmeyer P, Lübbeke A. J Arthroplasty. 2018;33:2218–2224. doi: 10.1016/j.arth.2018.02.069. [DOI] [PubMed] [Google Scholar]
- 18.Prosthetic-joint infections: mortality over the last 10 years. Fischbacher A, Borens O. J Bone Jt Infect. 2019;4:198–202. doi: 10.7150/jbji.35428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Osmon DR, Berbari EF, Berendt AR, et al. Clin Infect Dis. 2013;56:0. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 20.The clinical presentation of prosthetic joint infection. Barrett L, Atkins B. J Antimicrob Chemother. 2014;69:0–7. doi: 10.1093/jac/dku250. [DOI] [PubMed] [Google Scholar]
- 21.Bone and joint infections in adults: a comprehensive classification proposal. Romanò CL, Romanò D, Logoluso N, Drago L. Eur Orthop Traumatol. 2011;1:207–217. doi: 10.1007/s12570-011-0056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.British Orthopaedic Association: BOA Specialty Standard - investigation and management of prosthetic joint infection in knee replacement. [ Nov; 2024 ]. 2020. https://www.boa.ac.uk/resource/boast-investigation-and-management-of-prosthetic-joint-infection-in-knee-replacement.html https://www.boa.ac.uk/resource/boast-investigation-and-management-of-prosthetic-joint-infection-in-knee-replacement.html
- 23.Sepsistrust: Sepsis Screening Tool Acute Assessment 16+ [ Nov; 2024 ]. 2024. https://sepsistrust.org/wp-content/uploads/2024/08/SEPSIS-Trust-Screening-Acute-Tool-Kits-16-2024.pdf https://sepsistrust.org/wp-content/uploads/2024/08/SEPSIS-Trust-Screening-Acute-Tool-Kits-16-2024.pdf
- 24.The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, Shohat N. J Arthroplasty. 2018;33:1309–1314. doi: 10.1016/j.arth.2018.02.078. [DOI] [PubMed] [Google Scholar]
- 25.Proceedings of the International Consensus on Periprosthetic Joint Infection. Parvizi J, Gehrke T, Chen AF. Bone Joint J. 2013;95-B:1450–1452. doi: 10.1302/0301-620X.95B11.33135. [DOI] [PubMed] [Google Scholar]
- 26.The role of imaging techniques to define a peri-prosthetic hip and knee joint infection: multidisciplinary consensus statements. Romanò CL, Petrosillo N, Argento G, et al. J Clin Med. 2020;9:16–18. doi: 10.3390/jcm9082548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Parvizi J, Zmistowski B, Berbari EF, et al. Clin Orthop Relat Res. 2011;469:2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Small I, Budhiparama NC, Shohat N. Infection in Knee Replacement. Vol. 2. Cham, Switzerland: Springer International Publishing; 2022. Differential diagnosis of periprosthetic joint infection; pp. 14–16. [Google Scholar]
- 29.Why do revision total knee arthroplasties fail? A single-center review of 1632 revision total knees comparing historic and modern cohorts. Geary MB, Macknet DM, Ransone MP, Odum SD, Springer BD. J Arthroplasty. 2020;35:2938–2943. doi: 10.1016/j.arth.2020.05.050. [DOI] [PubMed] [Google Scholar]
- 30.Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. Sloan M, Premkumar A, Sheth NP. J Bone Joint Surg Am. 2018;100:1455–1460. doi: 10.2106/JBJS.17.01617. [DOI] [PubMed] [Google Scholar]
- 31.Revision knee complexity classification-RKCC: a common-sense guide for surgeons to support regional clinical networking in revision knee surgery. Phillips JR, Al-Mouazzen L, Morgan-Jones R, Murray JR, Porteous AJ, Toms AD. Knee Surg Sports Traumatol Arthrosc. 2019;27:1011–1017. doi: 10.1007/s00167-019-05462-x. [DOI] [PubMed] [Google Scholar]