Abstract
Management of abdominal aortic aneurysm includes reducing the incidence of endoleaks and promoting sac regression. Sac embolization has been shown to promote regression but alone may not adequately address type II endoleak risk. We present three cases with challenging anatomy and follow-up data through 12 months after treatment. Patients were treated with endografts, and shape memory polymer embolization plugs were used to embolize the residual lumen volume outside of the endograft during the procedure. Follow-up imaging indicates that this procedure was used successfully in these patients. None of the patients developed sac expansion or developed type II endoleaks.
Keywords: Endovascular aneurysm repair, Endoleak prevention, Sac shrinkage
Type II endoleaks are a consideration when managing abdominal aortic aneurysms (AAAs) via endovascular aneurysm repair (EVAR). These complications contribute to aneurysm failure to regress, which is linked to lower long-term survival.1 Risk factors include presence of large, patent sac feeding vessels, including inferior mesenteric artery (IMA), lumbar arteries (LAs), and accessory renal or sacral arteries.2,3 Outcomes vary in reports of IMA and sac embolization with coils and fibrin glue.4, 5, 6
Anatomical challenges like angulated and/or short necks and narrow common iliac arteries (CIAs) preclude many patients, especially women, from EVAR eligibility.7,8 Lower-profile devices expand treatment options but are associated with higher type II endoleak rates than standard stent grafts.9, 10, 11
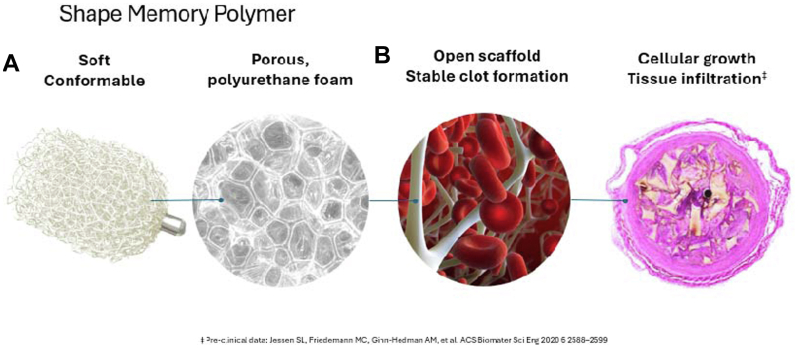
Shape memory polymer (SMP) is a novel vessel embolization technology12, 13, 14, 15, 16 with several properties amenable to AAA sac management. IMPEDE-FX Embolization Plugs (Shape Memory Medical) incorporate SMP, a porous, thrombotic structure when expanded in a vessel. These devices have been shown in preclinical studies to promote cellular growth as the polymer gradually degrades with low incidence of recanalization (Fig 1).14,17
Fig 1.
Shape memory polymer (SMP) embolization device used in all cases. (A) The device is crimped for catheter delivery; (B) The device self-expands on contact with blood to form a porous scaffold. Each device occupies approximately 1.25 mL when fully expanded. The radiopaque marker facilitates delivery and tracking in the sac and on follow-up imaging. The SMP itself is radiolucent. Image provided courtesy of Shape Memory Medical, Inc.
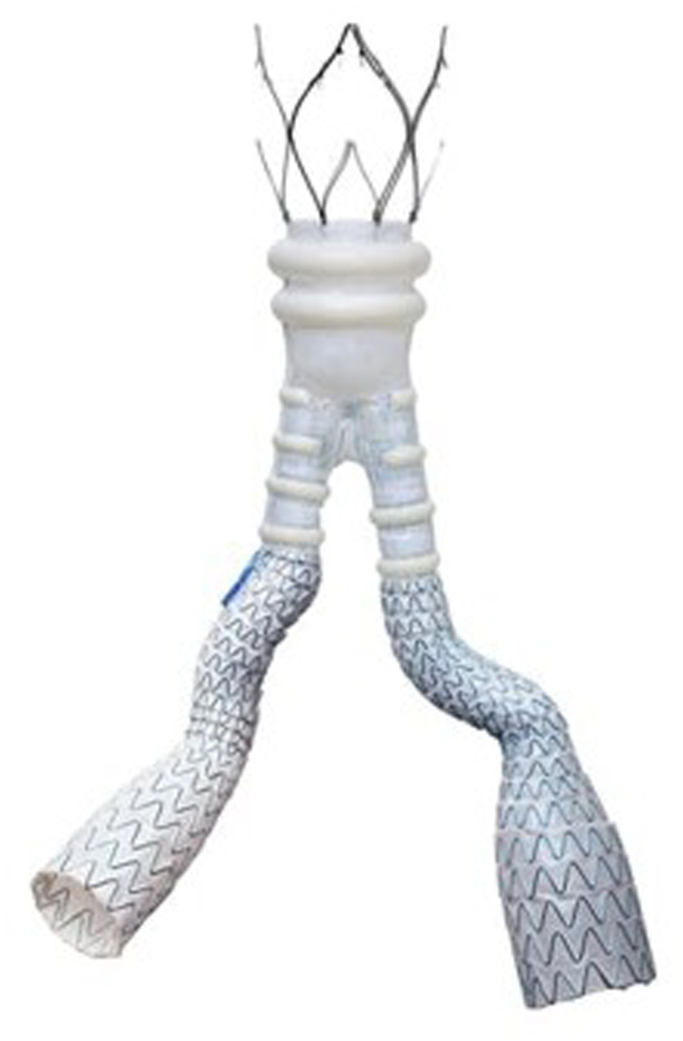
We present three AAA sac management cases with SMP and the low-profile ALTO abdominal stent graft system (Endologix) without complication in patients with challenging anatomy, who provided consent for publication. Featuring an aortic body with a network of polymer-filled sealing rings for aneurysm exclusion and flexible limbs, the ALTO system is suited for challenging femoral artery access and iliac artery tortuosity10 (Fig 2). These patients were also at increased risk of type II endoleak due to presence of large, patent LAs and IMAs. We embolized the AAA sac with SMP devices immediately following ALTO placement. The SMP devices were deployed within the aneurysm lumen, around the endograft, to promote sac thrombosis and feeding vessel occlusion using previously described methods.13 Fill volume were estimated using preoperative computed tomography angiogram (CTA) when available, or intraoperative sacograms, with each SMP device filling 1.25 ml. Following sac embolization, no residual lumbar artery filling was observed upon completion angiography. We evaluated sac regression and endoleak metrics through 12 months.
Fig 2.
Endologix Alto abdominal stent graft.
Patient and preprocedural aneurysm characteristics are listed in Table. Standard procedures are described in the Supplementary Material (online only).
Table.
Case characteristics
| Characteristic | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Sex | Female | Female | Male |
| Age, years | 73 | 62 | 82 |
| AAA diameter | 55 | 51 | 70 |
| IMA diameter, mm | 3 | Occluded | Occluded |
| Patent lumbar arteries | Yes | Yes | Yes |
| Aberrant right renal artery | No | No | No |
| Iliac artery occlusion | Yes | No | Yes, right |
| Infrarenal neck length, mm | 46.5 | 22 | 47.3 |
| Neck angle, ° | 48.2 | 17 | 60 |
| Estimated aneurysm volume, mLa | 86.8 | 88.9 | 196.6 |
| Endograft dimensions | |||
| Main body, mmb | 26 | 20 | 29 |
| Left limb, mmc | 14 × 120 | 10 × 100 | 14 × 160 |
| Right limbc | 14 × 120 | 12 × 120 | 14 × 160 |
| SMP devicesd | |||
| Number of devices, No. | 30 | 50 | 36 |
| Fully expanded volume, mLe | 37.5 | 62.5 | 45 |
AAA, Abdominal aortic aneurysm; IMA, inferior mesenteric artery; SMP, shape memory polymer.
Based on preprocedural computed tomography angiography.
Diameter. ALTO abdominal stent graft system (Endologix).
Diameter x length. Ovation IX limb (Endologix).
12-mm diameter IMPEDE-FX Embolization Plug (Shape Memory Medical).
Each SMP device self-expands to occupy a volume of ∼1.25 mL.14
Case reports
Case 1 procedure
Due to small iliac access vessels, the ALTO endograft was chosen. It was positioned at the right renal artery lower border and anchored by top crown deployment. The endograft crossover technique for wire and sheath placement was employed in preparation for contralateral limb deployment, as described previously.13
With the sac isolated, 15 SMP devices were delivered into the residual flow lumen, concentrated near the large LA ostia in the upper sac region (Fig 3, A-C). An additional 15 SMP devices were placed in the capacious lower sac region for a total filling volume of 37.5 ml. Angiography after SMP delivery demonstrated thrombosis with minimal, non-pulsatile residual flow from the delivery sheath. The guiding sheath was removed and both limbs were balloon-sealed using a kissing technique, particularly at the flow divider and distal seal zones.
Fig 3.
Delivery of shape memory polymer (SMP) devices. (A) Intraoperative radiograph showing endograft main body placement with guidewire access (arrow) into the aneurysm sac for Impede-FX plug placement; (B) Delivery of Impede-FX plug into aneurysm sac via vascular sheath and pushing guidewire; (C) Proximal markers of Impede-FX plugs in aneurysm sac.
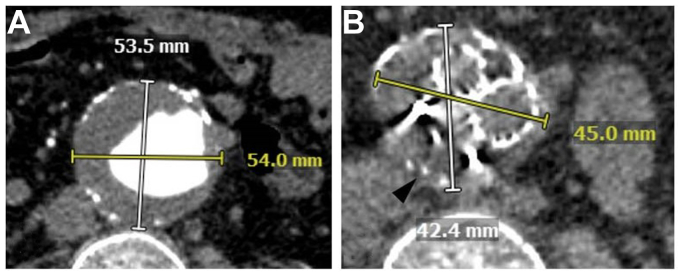
Angiography showed total aneurysm exclusion with no endoleak. The patient was procedurally anti-coagulated, potentially contributing to observation of transgraft contrast blush. One-year follow-up CTA showed no endoleak evidence and continued sac diameter reduction (Fig 4, A-B).
Fig 4.
Pre-procedural and post-procedural imaging for Case 1. (A) Pre-procedural computed tomography angiography (CTA) showing 5.4 cm infrarenal abdominal aortic aneurysm; (B) One-year follow up CT shows continued sac diameter reduction. Metal artifacts from plug markers are seen.
Case 2 procedure
The patient had an area of slight outpouching in the proximal neck below the left renal artery. Due to the type IA endoleak risk, the ALTO endograft was chosen. The endograft was deployed at the left renal artery lower border, with proximal seal confirmed via angiography. The endograft crossover wire technique snared the wire from left to right, and a limb was deployed extending proximally between the third and fourth ring of the aortic body and distally in the left CIA. A guidewire was placed in the sac, the endograft limb was deployed, and the SMP delivery sheath was advanced into the sac over the jailed wire to deliver the SMP plugs.13 Fifty devices, providing 62.5 ml of fill volume were delivered into the residual flow lumen within the lower region of the sac, close to the narrow aortic bifurcation. The delivery sheath was reoriented, and additional devices were advanced toward the lumbar arteries, eliminating outflow as shown via angiography. The guiding sheath was removed and the endograft limbs re-ballooned, confirming distal seal.
Angiography revealed good bilateral filling of external and internal iliac arteries and total aneurysm exclusion with no evidence of endoleak post-procedurally or at six-month CTA. At 1-year follow-up, color-enhanced DUS showed a 1.0 cm diameter regression to 4.1 cm from 5.1 cm and no endoleak.
Case 3 procedure
This patient had a large infrarenal AAA and bilateral iliac artery aneurysms, complicated by extremely tortuous iliac arteries, challenging access, and chronic renal insufficiency limiting contrast use. Following standard access, the patient briefly experienced hypotension as the 11 French sheath was placed on the right side. Angiogram through the sheath side arm revealed extravasation from the distal external iliac artery. The common and external iliac areas were lined with Gore Viabahn and VBX stents (Gore Medical, Inc), extravasation absence was confirmed by angiography, and the patient remained hemodynamically stable. The main body was placed at the right renal artery lower border, with 035 Bentson wire placed in the sac followed by the Iliac limb, placed prior to SMP delivery sheath insertion.13
Thirty-six SMP devices (45 ml fill volume) were delivered using bilateral access13 for complete sac thrombosis. The iliac limbs were re-ballooned in a kissing fashion from the flow divider through both limbs with particular attention to the distal landing zones. Completion angiography showed aneurysm exclusion with no evidence of endoleak. Twelve-month CEUS showed no endoleak and sac regression.
Discussion
These cases highlight the use of a low-profile endograft in patients with challenging AAA neck and iliac anatomy and type II endoleak risk. They also demonstrate the introduction of a novel sac management technology, delivered via vascular sheath in the sac contralaterally (Cases 1-2) and bilaterally (Case 3) without the addition of clinically significant time or substantial cost.18,19 Early in our experience, we used contralateral access, but we have evolved to using a bilateral approach, and think it is an improvement. Currently, we place two 6 French destination sheaths deep into the aneurysm sac over Benston wires and deploy embolization plugs via both sheaths as the sheaths are withdrawn from caudal to cranial orientation within the aneurysm sac.
Coil embolization for both surgical and endovascular AAA repair has been described with some success in preventing type II endoleaks.5,6,20,21 The volume-filling properties of SMP in sac exclusion are intriguing, particularly the material’s radiolucency with minimal imaging artifact when monitoring for endoleaks. The feasibility of sac management using this novel technology has been reported with clear sac regression up to 1-year post-procedure.12,15 Our experience complements these findings and emphasizes the need for adequately filling the residual flow lumen volume, particularly in large aneurysms. EVAR with SMP sac management, rather than EVAR alone, may yield superior results going forward.
Conclusion
In these three cases, the use of an ultra-low profile endograft in combination with SMP devices enabled treatment of patients with complex anatomy, and preemptively addressed type II endoleak risk. Larger studies with longer-term follow-up will establish the efficacy of this approach for sac regression and endoleak prevention.
Acknowledgments
The authors thank Melissa A. Lirag, MSPA, PA-C for data collection; and Dana R. Fletcher, PhD for writing and editing assistance.
Footnotes
Additional material for this article may be found online at www.jvscit.org.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Appendix
Additional material for this article may be found online at www.jvscit.org.
Appendix (online only)
References
- 1.O'Donnell T.F.X., Deery S.E., Boitano L.T., et al. Aneurysm sac failure to regress after endovascular aneurysm repair is associated with lower long-term survival. J Vasc Surg. 2019;69:414–422. doi: 10.1016/j.jvs.2018.04.050. [DOI] [PubMed] [Google Scholar]
- 2.Abularrage C.J., Crawford R.S., Conrad M.F., et al. Preoperative variables predict persistent type 2 endoleak after endovascular aneurysm repair. J Vasc Surg. 2010;52:19–24. doi: 10.1016/j.jvs.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Marchiori A., von Ristow A., Guimaraes M., Schonholz C., Uflacker R. Predictive factors for the development of type II endoleaks. J Endovasc Ther. 2011;18:299–305. doi: 10.1583/10-3116.1. [DOI] [PubMed] [Google Scholar]
- 4.Samura M., Morikage N., Otsuka R., et al. Endovascular aneurysm repair with inferior mesenteric artery embolization for preventing type II endoleak: a prospective randomized controlled trial. Ann Surg. 2020;271:238–244. doi: 10.1097/SLA.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 5.Piazza M., Squizzato F., Zavatta M., et al. Outcomes of endovascular aneurysm repair with contemporary volume-dependent sac embolization in patients at risk for type II endoleak. J Vasc Surg. 2016;63:32–38. doi: 10.1016/j.jvs.2015.08.049. [DOI] [PubMed] [Google Scholar]
- 6.Fabre D., Mougin J., Mitilian D., et al. Prospective, randomised two centre trial of endovascular repair of abdominal aortic aneurysm with or without sac embolisation. Eur J Vasc Endovasc Surg. 2021;61:201–209. doi: 10.1016/j.ejvs.2020.11.028. [DOI] [PubMed] [Google Scholar]
- 7.Ulug P., Sweeting M.J., von Allmen R.S., Thompson S.G., Powell J.T., SwanCollaborators Morphological suitability for endovascular repair, non-intervention rates, and operative mortality in women and men assessed for intact abdominal aortic aneurysm repair: systematic reviews with meta-analysis. Lancet. 2017;389:2482–2491. doi: 10.1016/S0140-6736(17)30639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sweet M.P., Fillinger M.F., Morrison T.M., Abel D. The influence of gender and aortic aneurysm size on eligibility for endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2011;54:931–937. doi: 10.1016/j.jvs.2011.02.054. [DOI] [PubMed] [Google Scholar]
- 9.Mehta M., Valdés F.E., Nolte T., et al. One-year outcomes from an international study of the ovation abdominal stent graft system for endovascular aneurysm repair. J Vasc Surg. 2014;59:65–73.e1-3. doi: 10.1016/j.jvs.2013.06.065. [DOI] [PubMed] [Google Scholar]
- 10.Lyden S.P., Metzger D.C., Henao S., et al. One-year safety and effectiveness of the Alto abdominal stent graft in the ELEVATE IDE trial. J Vasc Surg. 2023;77:446–453.e3. doi: 10.1016/j.jvs.2022.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Iantorno M., Buchanan K.D., Bernardo N.L., Torguson R., Waksman R. Overview of the 2018 US food and drug administration circulatory system devices panel meeting on the INCRAFT AAA stent graft system. Cardiovasc Revasc Med. 2019;20:403–408. doi: 10.1016/j.carrev.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 12.Massmann A., Fries P., Shayesteh-Kheslat R., Buecker A., Berg P., Frenzel F. Feasibility of aortic aneurysm sac embolization using a novel shape memory polymer embolic device. Eur Radiol Exp. 2023;7:12. doi: 10.1186/s41747-023-00328-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holden A., Hill A.A., Khashram M., et al. Active aortic aneurysm sac treatment with shape memory polymer during endovascular aneurysm repair. J Vasc Surg Cases Innov Tech. 2023;9 doi: 10.1016/j.jvscit.2023.101241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holden A., Hill A.A., Buckley B.T. Shape memory polymer technology in peripheral vascular embolization. Vascular. 2023;32:1137–1142. doi: 10.1177/17085381231177851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holden A., Hill A.A., Khashram M., et al. One-year follow-up after active aortic aneurysm sac treatment with shape memory polymer devices during endovascular aneurysm repair. J Vasc Surg. 2024;79:1090–1100.e4. doi: 10.1016/j.jvs.2023.12.045. [DOI] [PubMed] [Google Scholar]
- 16.Morgan R.A., Loftus I., Ratnam L., et al. Clinical experience with a shape memory polymer peripheral vascular embolisation plug: a case series. CVIR Endovasc. 2021;4:29. doi: 10.1186/s42155-021-00214-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jessen S.L., Friedemann M.C., Ginn-Hedman A.M., et al. Microscopic assessment of healing and effectiveness of a foam-based peripheral occlusion device. ACS Biomater Sci Eng. 2020;6:2588–2599. doi: 10.1021/acsbiomaterials.9b00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Columbo J.A., Goodney P.P., Gladders B.H., et al. Medicare costs for endovascular abdominal aortic aneurysm treatment in the Vascular Quality Initiative. J Vasc Surg. 2021;73:1056–1061. doi: 10.1016/j.jvs.2020.06.109. [DOI] [PubMed] [Google Scholar]
- 19.Li C., Deery S.E., Eisenstein E.L., et al. IP059. Early and late costs of endovascular abdominal aortic aneurysm repair from the Endurant stent graft system post approval study (ENGAGE PAS) J Vasc Surg. 2018;67:E105. doi: 10.1016/j.jvs.2019.11.028. [DOI] [PubMed] [Google Scholar]
- 20.Huber K.L., Joseph A., Mukherjee D. Extra-anatomic arterial reconstruction with ligation of common iliac arteries and embolization of the aneurysm for the treatment of abdominal aortic aneurysms in high-risk patients. J Vasc Surg. 2001;33:745–751. doi: 10.1067/mva.2001.112319. [DOI] [PubMed] [Google Scholar]
- 21.Coleman D., Chambers T., Mukherjee D. Axillobifemoral bypass and aortic embolization for the treatment of two patients with ruptured infrarenal aortic aneurysms. J Vasc Surg. 2005;41:340–347. doi: 10.1016/j.jvs.2004.11.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.