Abstract
Objective
This study aimed to investigate the relationship between clinical findings and the trabecular microstructure of the subchondral bone in patients with hip osteoarthritis (OA) due to developmental dysplasia of the hip (DDH) using multidetector row computed tomography (MDCT).
Design
A total of 63 patients (69 hips) with OA due to DDH were retrospectively reviewed, with 12 healthy controls being included for comparison. Clinical evaluation was performed using the Japanese Orthopaedic Association Hip Disease Evaluation Questionnaire (JHEQ). The trabecular bone microstructure was analyzed using MDCT. Regions of interest in the subchondral trabecular bones of the acetabulum and femoral head were defined in the coronal view, and various trabecular microstructural parameters were evaluated.
Results
Bone volume fraction (BV/TV) and trabecular thickness (Tb.Th) exhibited a significant positive correlation with the OA stage, whereas trabecular separation (Tb.Sp) showed a negative correlation. In addition, BV/TV and Tb.Th were negatively correlated with the JHEQ total and pain scores, whereas Tb.Sp was positively correlated with the pain score in all regions.
Conclusions
This is the first study to evaluate the bone microstructure and its relationship with clinical findings in patients with hip OA due to DDH. Our findings suggest that as OA progresses, osteosclerotic changes increase in the acetabulum and femoral head; these changes are associated with worsening clinical symptoms, particularly pain. Targeting the subchondral bone may emerge as a novel treatment strategy for patients with OA due to DDH; nevertheless, further comprehensive studies are required.
Keywords: hip osteoarthritis, subchondral trabecular bone microstructure, multidetector row computed tomography, osteosclerotic changes, hip pain
Introduction
Osteoarthritis (OA) is characterized by cartilage attrition and joint pain, posing considerable challenges in affected individuals. Among the contributing factors to OA pathogenesis, developmental dysplasia of the hip (DDH) is a disabling musculoskeletal condition that disrupts hip joint development. 1 DDH is an anatomical deformity that leads to uneven stress distribution within the joint, ultimately resulting in secondary OA due to alterations in the joint structure. 2 Notably, the subchondral bone in OA exhibits various changes such as trabecular bone thickening, cyst formation, decreased bone mineralization, and increased bone turnover. 3 These alterations in the subchondral trabecular bone microstructure are considered to be related to the pathophysiology of OA. Functionally, the subchondral trabecular bone acts as a shock absorber that protects the articular cartilage; thus, subchondral bone sclerosis is considered to decrease the ability to absorb shock, leading to cartilage damage.4,5 Moreover, high bone turnover increases the release of various cytokines from the subchondral bone, resulting in cartilage degeneration.6,7 As the cartilage lacks nerve supply, bone pain could be one of the primary causes of pain in OA. 8 In OA, abnormalities in the subchondral bone, as identified by histological analysis, are associated with joint pain. 9 Despite extensive research, the relationship between quantitatively evaluated subchondral trabecular bone microstructure and clinical findings in hip OA remains unexplored.
The bone microstructure has been analyzed using micro-computed tomography (CT) or pathological examination, which is limited to an in vitro analysis of extracted bone samples. However, with the remarkable development of clinical medical imaging techniques, particularly multidetector row CT (MDCT), an in vivo analysis of patients’ bone microstructure has become possible. 10 MDCT provides high-resolution images; when combined with dedicated software, it facilitates a detailed analysis of trabecular bone microstructure without using tissue samples.10,11
Recent studies have demonstrated the utility of MDCT in assessing bone microstructure in OA. Chiba et al. 10 examined the subchondral trabecular bone in patients with hip OA and identified significant relationships between bone structural changes and clinical outcomes. Similarly, Oláh et al. 11 evaluated subchondral bone sclerosis and its correlation with OA progression in knee joints using high-resolution MDCT. These findings highlight the role of MDCT in revealing microstructural changes associated with OA, underscoring its importance as a diagnostic tool in clinical practice.
The current study aimed to quantify trabecular bone changes and elucidate their relationship with clinical findings by investigating the subchondral trabecular bone microstructure in the acetabulum and femoral head in patients with hip OA due to DDH using MDCT. The novelty of our study lies in its comprehensive evaluation of this relationship, which has not been thoroughly investigated in previous reports. By focusing on this, our study provides valuable insights into the pathophysiology of hip OA due to DDH and may lead to the development of novel treatment strategies for hip OA.
Methods
Study Participants
This retrospective single-institution study was conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by the Institutional Review Board of our hospital. Informed consent was obtained from all participants.
The research participants were patients with OA due to DDH (defined as a lateral center-edge angle of <20°) who underwent MDCT between February 2016 and March 2020. The exclusion criteria were patients with (1) previous hip injury or hip surgery, (2) osteonecrosis of the femoral head, (3) rheumatoid arthritis, and (4) a history of tumors. In addition, a control group comprising 12 individuals with 12 hip joints (3 men and 9 women; mean age = 56.7 [15-75] years) with no history of hip joint disorders, confirmed through radiographic evaluation, was included for a comparative analysis. Clinical evaluation was performed using the Japanese Orthopaedic Association Hip Disease Evaluation Questionnaire (JHEQ), which is a patient-reported outcome measure (PROM) designed to assess the clinical condition of patients with hip disorders. The total score ranges from 0 point (worst) to 84 points (best). The JHEQ consists of 3 subscales—pain (0-28 points), movement (0-28 points), and mental (0-28 points)—with 0 point representing the worst outcome and 28 points representing the best outcomes for each subscale. 12 Thus, the JHEQ reflects a patient-centered approach, aligning with the concept of PROMs, which emphasizes the patient’s perspective in evaluating health outcomes.
Computed Tomography Scanning
All patients and control-group participants underwent MDCT of the hip joints from the anterior superior iliac spine to the knee joint through the distal femoral condyles using a scanner equipped with 160 detectors (Aquilion Precision; Canon Medical Systems Corp., Tokyo, Japan). Scanning was performed at 120 kV, with an automatic exposure control setting ranging from 50 to 550 mAs, and 0.5-mm slices were obtained. The images were reconstructed with a field of view of 100 mm, matrix 512 × 512 pixels, and pitch factor 0.569. The maximum in-plane resolution was 33-line pairs per centimeter at 2%, according to the manufacturer’s instructions. The average CT dose index-volume and dose length product were 9.7 (range = 5.9-12.9) mGy and 691 (range = 394-1,012) mGy, respectively, which are comparable to those for conventional thoracoabdominal CT scans. 13
Region of Interest Configuration and Subchondral Trabecular Bone Microstructure Analysis
The subchondral trabecular bone microstructure was measured using a bone structure measurement software (TRI/3D-BON-FCS; Ratoc System Engineering Co., Tokyo, Japan). 14 To distinguish the cartilage from the subchondral bone, CT images were reconstructed to form coronal images, and bones with a calcification degree ≥300 mg/cm3 were extracted for structural analysis. Regions of interest (ROIs) were defined in the subchondral trabecular bones of the acetabulum and femoral head based on previous reports.10,13 The method for defining these ROIs was established according to the criteria specified in these reports. The ROI in the femoral head was defined as 5 mm medial, lateral, anterior, and posterior from the top of the femoral head, with a depth of 10 mm. The ROI in the acetabulum was defined as 5 mm medial, lateral, anterior, and posterior from the center of the acetabular weight-bearing portion, with a depth of 10 mm. Both regions were divided into 2 areas of equal width—the shallow and deep layers (femoral shallow layer [FS], femoral deep layer [FD], acetabular shallow layer [AS], and acetabular deep layer [AD]) ( Fig. 1 ). Square columns of 10 × 10 mm² were manually set in each slice of the coronal section, and the cartilage was manually corrected after automatic extraction. In addition, regions without bones, such as bone cysts, were excluded from the ROIs.
Figure 1.
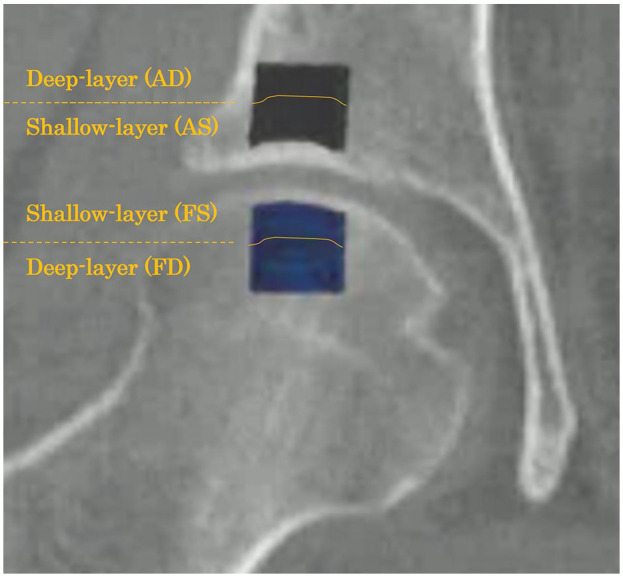
Regions of interest (ROIs) for the trabecular bone microstructure analysis. ROIs were defined in the subchondral trabecular bone structures of the acetabulum and femoral head in the coronal view. Each ROI is the area of the trabecular bone within a 10 × 10 mm2 area. Acetabulum (black area): the subchondral bone region at 5 mm medial, lateral, anterior, and posterior from the center of the acetabular weight-bearing portion, with a depth of 10 mm. Femoral head (blue area): the subchondral bone region at 5 mm medial, lateral, anterior, and posterior from the top of the femoral head, with a depth of 10 mm. Both regions were divided into 2 areas of equal width—the shallow and deep layers (femoral shallow layer [FS], femoral deep layer [FD], acetabular shallow layer [AS], and acetabular deep layer [AD]).
With respect to the trabecular microstructural parameters, the bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular separation (Tb.Sp), 15 and structure model index (SMI) were evaluated. 16 The BV/TV represents the trabecular bone volume contained in the spatial volume of the inner surface of the cortical bone and is the result of a 3-dimensional analysis of bone density. The SMI quantifies the morphology of the trabecular bone from rod-like to plate-like structures; the smaller the value, the more plate-like the structure. Each parameter of the femoral head and acetabular regions was compared between the groups.
Statistical Analyses
Statistical analyses were performed using Statcel, the Useful Addin Forms on Excel, 4th ed. (OMS Publishing, Tokyo, Japan). Four group comparisons of subchondral trabecular bone microstructural parameters were conducted using the Kruskal–Wallis test. Subsequently, within the OA group, further statistical analyses were performed using the Dunn–Bonferroni test to analyze the patients’ background variables. The correlation between subchondral trabecular bone microstructure and both OA stage and JHEQ scores, including pain, was assessed using Spearman’s correlation analysis. Categorical variables were compared using chi-squared test. The results are reported as mean±standard deviation. For all analyses, statistical significance was set at P < 0.05.
Results
The OA group included 63 patients with 69 hip joints (10 men and 53 women; mean age = 59.4 [17-82] years). The OA stage according to the Japanese Orthopaedic Association (JOA) classification was early stage in 27 hips, advanced stage in 20 hips, and end stage in 22 hips ( Table 1 ). 17 No significant differences in patient characteristics, including age, sex, center-edge angle, and acetabular roof angle, were observed in the OA group.
Table 1.
Characteristics of Patients.
| Control | Early stage a | Advanced stage a | End stage a | P | ||
|---|---|---|---|---|---|---|
| Patient | (hips) | 12 | 27 | 20 | 22 | |
| Age | (years) | 56.7 ± 16.9 | 58.0 ± 16.3 | 58.8 ± 11.6 | 62.1 ± 9.8 | ns |
| Sex | (Male/female) (n) | 3–9 | 5–22 | 3–17 | 4–18 | ns |
| Center-edge angle | (degree) | 29.4 ± 4.0 | 14.4 ± 5.2 | 12.4 ± 7.2 | 12.8 ± 4.2 | <0.01 |
| Acetabular roof angle | (degree) | 11.4 ± 3.2 | 28.6 ± 6.8 | 30.2 ± 7.5 | 27.0 ± 6.0 | <0.01 |
| JHEQ-total score | (points) | 60.0 ± 15.5 | 45.5 ± 15.8 | 30.9 ± 17.2 b | 24.8 ± 12.4 b | <0.01 |
| JHEQ-pain score | (points) | 26.9 ± 2.0 | 20.7 ± 7.0 | 16.2 ± 9.8 | 9.7 ± 5.7 b | <0.01 |
| JHEQ-move score | (points) | 17.8 ± 6.5 | 12.7 ± 6.9 | 6.2 ± 5.4 b | 6.0 ± 4.2 b | <0.01 |
| JHEQ-mental score | (points) | 16.9 ± 7.0 | 12.1 ± 7.8 | 9.3 ± 7.1 | 8.3 ± 4.5 | <0.01 |
JHEQ = Japanese Orthopaedic Association Hip Disease Evaluation Questionnaire; ns = not significant.
The Japanese Orthopaedic Association classification.
vs. early stage P < 0.05.
Table 2 presents the results regarding the subchondral bone microstructure of the femoral head and acetabulum. Comparisons among the 4 regions revealed significant differences in the BV/TV, Tb.Th, and Tb.Sp. In addition, the FD region showed a significant difference in the SMI, whereas the AD region exhibited a significant difference in the Tb.N and SMI. In all regions, the BV/TV (end stage vs. control: 53.8 vs. 25.6 [FS], 52.3 vs. 21.7 [FD], 59.0 vs. 28.4 [AS], 52.6 vs. 9.7 [AD]; P < 0.01) and Tb.Th (end stage vs. control: 838.2 vs. 336.1 [FS], 838.0 vs. 324.7 [FD], 934.8 vs. 419.0 [AS], 881.9 vs. 254.6 [AD]; P < 0.01) were significantly higher in the end-stage group compared with the control group, whereas the Tb.Sp was significantly lower (end stage vs. control: 660.2 vs. 1,434.1 [FS], 811.9 vs. 1,601.1 [FD], 668.7 vs. 1,260.1 [AS], 750.7 vs. 3,433.3 [AD]; P < 0.01) in the end-stage group compared with the control group. In addition, these changes were significantly observed in the FD and AD regions in the end-stage group compared with the early-stage group (P < 0.01).
Table 2.
Subchondral Bone Microstructure of Each Group.
| Control | Early stage a | Advanced stage a | End stage a | P | |||
|---|---|---|---|---|---|---|---|
| FS | BV/TV | (%) | 25.6 ± 13.4 | 34.2 ± 18.2 | 47.3 ± 26.5 b | 53.8 ± 15.3c,d | <0.01 |
| Tb.Th | (µm) | 336.1 ± 102.9 | 422.8 ± 171.7 | 643.1 ± 443.4 | 838.2 ± 244.9c,d | <0.01 | |
| Tb.N | (1/mm) | 0.7 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.7 ± 0.1 | ns | |
| Tb.Sp | (µm) | 1,434.1 ± 1,307.2 | 1,010.3 ± 643.3 | 605.2 ± 300.9 c | 660.2 ± 246.2 b | <0.01 | |
| SMI | 1.3 ± 0.5 | 1.0 ± 0.8 | 0.7 ± 1.2 | 1.1 ± 0.8 | ns | ||
| FD | BV/TV | (%) | 21.7 ± 10.4 | 22.2 ± 11.4 | 36.7 ± 21.7 | 52.3 ± 18.4c,d | <0.01 |
| Tb.Th | (µm) | 324.7 ± 83.4 | 331.3 ± 83.9 | 506.8 ± 334.0 | 838.0 ± 369.7c,d | <0.01 | |
| Tb.N | (1/mm) | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.6 ± 0.1 | ns | |
| Tb.Sp | (µm) | 1,601.1 ± 1,355.2 | 1,411.4 ± 654.4 | 986.9 ± 582.8 | 811.9 ± 486.7b,d | <0.01 | |
| SMI | 1.5 ± 0.4 | 1.6 ± 0.5 | 1.0 ± 1.1 | 0.8 ± 0.9b,d | <0.01 | ||
| AS | BV/TV | (%) | 28.4 ± 11.3 | 41.0 ± 16.6 | 58.7 ± 16.0c,d | 59.0 ± 16.2c,d | <0.01 |
| Tb.Th | (µm) | 419.0 ± 90.3 | 604.0 ± 244.7 | 893.7 ± 255.6c,d | 934.8 ± 269.3c,d | <0.01 | |
| Tb.N | (1/mm) | 0.7 ± 0.2 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | ns | |
| Tb.Sp | (µm) | 1,260.1 ± 775.4 | 931.8 ± 436.3 | 644.1 ± 299.1 c | 668.7 ± 335.5 c | <0.01 | |
| SMI | 1.2 ± 0.3 | 1.2 ± 0.4 | 0.8 ± 1.1 | 0.9 ± 0.7 | ns | ||
| AD | BV/TV | (%) | 9.7 ± 8.2 | 15.3 ± 17.6 | 27.3 ± 23.3 | 52.6 ± 21.2c,d | <0.01 |
| Tb.Th | (µm) | 254.6 ± 78.5 | 315.3 ± 153.5 | 449.1 ± 233.9 | 881.9 ± 377.3c,d | <0.01 | |
| Tb.N | (1/mm) | 0.3 ± 0.2 | 0.4 ± 0.3 | 0.5 ± 0.3 | 0.6 ± 0.1c,d | <0.01 | |
| Tb.Sp | (µm) | 3,433.3 ± 1,134.9 | 1,619.7 ± 656.7 c | 1,052.1 ± 554.5 c | 750.7 ± 375.8c,d | <0.01 | |
| SMI | 2.1 ± 0.4 | 2.0 ± 0.7 | 1.6 ± 0.9 | 0.9 ± 0.8c,d | <0.01 |
FS = femoral shallow layer; FD = femoral deep-layer; AS = acetabulum shallow layer; AD = acetabulum deep-layer; BV/TV = bone volume fraction; Tb.Th = trabecular thickness; Tb.N = trabecular number; Tb.Sp = trabecular separation; SMI = structure model index; ns = not significant.
The Japanese Orthopaedic Association classification.
vs. control P < 0.05.
vs. control P < 0.01.
vs. early stage P < 0.01.
Table 3 shows the results for the correlation between the subchondral trabecular bone microstructure and OA stage of the femoral head and acetabulum. In all regions, the BV/TV (r = 0.49 [FS], 0.57 [FD], 0.60 [AS], 0.61 [AD]; P < 0.01) and Tb.Th (r = 0.62 [FS], 0.64 [FD], 0.70 [AS], 0.70 [AD]; P < 0.01) showed a significant positive correlation with the OA stage, whereas the Tb.Sp exhibited a significant negative correlation with the OA stage (r = −0.39 [FS], −0.44 [FD], −0.43 [AS], −0.72 [AD]; P < 0.01).
Table 3.
Interaction Between the Bone Microstructure and Osteoarthritis Stage.
| FS | FD | AS | AD | |
|---|---|---|---|---|
| BV/TV | r = 0.49, P < 0.01 | r = 0.57, P < 0.01 | r = 0.60, P < 0.01 | r = 0.61, P < 0.01 |
| Tb.Th | r = 0.62, P < 0.01 | r = 0.64, P < 0.01 | r = 0.70, P < 0.01 | r = 0.70, P < 0.01 |
| Tb.N | ns | ns | ns | r = 0.44, P < 0.01 |
| Tb.Sp | r = −0.39, P < 0.01 | r = −0.44, P < 0.01 | r = −0.43, P < 0.01 | r = −0.72, P < 0.01 |
| SMI | ns | r = −0.38, P < 0.01 | r = −0.24, P < 0.05 | r = −0.53, P < 0.01 |
FS = femoral shallow layer; FD = femoral deep-layer; AS = acetabulum shallow layer; AD = acetabulum deep-layer; BV/TV = bone volume fraction; Tb.Th = trabecular thickness; Tb.N = trabecular number; Tb.Sp = trabecular separation; SMI = structure model index; ns = not significant.
Table 4 shows the results of the correlation between the subchondral trabecular bone microstructure and JHEQ scores, including pain. In all regions, the BV/TV (r = −0.54 [FS], −0.49 [FD], −0.54 [AS], −0.46 [AD]; P < 0.01) and Tb.Th (r = −0.61 [FS], −0.53 [FD], −0.60 [AS], −0.49 [AD]; P < 0.01) were significantly negatively correlated with the JHEQ total score, whereas Tb.Sp was significantly positively correlated with the JHEQ total score (r = 0.42 [FS], 0.41 [FD], 0.43 [AS], 0.42 [AD]; P < 0.01). In addition, BV/TV (r = −0.62 [FS], −0.61 [FD], −0.65 [AS], −0.57 [AD]; P < 0.01) and Tb.Th (r = −0.67 [FS], −0.63 [FD], −0.67 [AS], −0.58 [AD]; P < 0.01) were significantly negatively correlated with the JHEQ pain score, whereas Tb.Sp was significantly positively correlated with the JHEQ pain score (r = 0.52 [FS], 0.53 [FD], 0.56 [AS], 0.54 [AD]; P < 0.01).
Table 4.
Interaction Between the Bone Microstructure and JHEQ.
| JHEQ-total score |
JHEQ-pain score |
|||||||
|---|---|---|---|---|---|---|---|---|
| FS | FD | AS | AD | FS | FD | AS | AD | |
| BV/TV | r = −0.54, P < 0.01 | r = −0.49, P < 0.01 | r = −0.54, P < 0.01 | r = −0.46, P < 0.01 | r = −0.62, P < 0.01 | r = −0.61, P < 0.01 | r = −0.65, P < 0.01 | r = −0.57, P < 0.01 |
| Tb.Th | r = −0.61, P < 0.01 | r = −0.53, P < 0.01 | r = −0.60, P < 0.01 | r = −0.49, P < 0.01 | r = −0.67, P < 0.01 | r = −0.63, P < 0.01 | r = −0.67, P < 0.01 | r = −0.58, P < 0.01 |
| Tb.N | ns | ns | ns | r = −0.35, P < 0.01 | ns | r = −0.23, P < 0.05 | ns | r = −0.49, P < 0.01 |
| Tb.Sp | r = 0.42, P < 0.01 | r = 0.41, P < 0.01 | r = 0.43, P < 0.01 | r = 0.42, P < 0.01 | r = 0.52, P < 0.01 | r = 0.53, P < 0.01 | r = 0.56, P < 0.01 | r = 0.54, P < 0.01 |
| SMI | r = 0.24, P < 0.05 | r = 0.36, P < 0.01 | r = 0.24, P < 0.05 | r = 0.41, P < 0.01 | r = 0.28, P < 0.05 | r = 0.42, P < 0.01 | r = 0.26, P < 0.05 | r = 0.40, P < 0.01 |
FS = femoral shallow layer; FD = femoral deep-layer; AS = acetabulum shallow layer; AD = acetabulum deep-layer; BV/TV = bone volume fraction; Tb.Th = trabecular thickness; Tb.N = trabecular number; Tb.Sp = trabecular separation; SMI = structure model index; JHEQ = Japanese Orthopaedic Association Hip Disease Evaluation Questionnaire; ns = not significant.
Discussion
To the best of our knowledge, this is the first study to evaluate the bone microstructure and its relationship with clinical findings in patients with secondary hip OA due to DDH. This study revealed that as OA progressed in the hip joint, the osteosclerotic changes in the acetabulum and femoral head of the subchondral bone increased considerably. Furthermore, with the progression of osteosclerosis, increases in BV/TV and Tb.Th were significantly associated with lower JHEQ pain scores. In other words, as the stage of hip OA due to DDH advanced, the patients experienced more intense pain. Conversely, the reduction in Tb.Sp or narrowing of the space between the trabeculae was also associated with increased pain as the stage progressed, resulting in lower JHEQ pain scores. These findings suggest that as the stage of hip OA due to DDH progresses, the bone becomes denser and the trabeculae thicken, contributing to worsened pain.
Imaging modalities such as magnetic resonance imaging (MRI) and radiography have been widely used for OA diagnosis. 18 Unlike conventional radiography, CT enables the quantitative assessment of femoral and acetabular morphology and provides a 3-dimensional visualization of the hip, simplifying the observation of bone morphology. 19 Nonetheless, to date, changes due to hip OA abnormalities in the subchondral trabecular bone microstructure have not been well understood.
Recent advancements in high-resolution imaging and computational techniques have attracted attention in OA research because of their ability to detect early alterations in the bone structure. Although pathological examination and micro-CT are considered useful for analyzing the bone microstructure in detail,20-23 obtaining tissue samples from patients with OA at each stage is difficult. In this study, MDCT was utilized to record detailed images at each stage without obtaining tissue samples.
Clinical MDCT has been used to analyze the human hip joint.10,24 Diederichs et al. 24 investigated the trabecular bone structure using MDCT and suggested the feasibility of an in vivo assessment of bone architecture in clinical practice. Chiba et al. 10 examined the subchondral trabecular bone in patients with OA using MDCT and reported that as the joint space decreased, the BV/TV and Tb.Th increased, whereas the Tb.Sp, Tb.N, and SMI decreased. They considered these changes in the bone trabecular structure to be the pathogenesis of osteosclerosis in OA. In addition, osteosclerotic changes in the subchondral bone microstructure were strongly correlated with OA severity, with sclerotic changes increasing as the disease progressed.11,25 The microstructural changes observed in the subchondral trabecular bone of patients with OA in our study were consistent with the findings of Chiba et al., 10 indicating osteosclerotic changes in both the femoral head and acetabular regions. Furthermore, our results demonstrated a correlation between osteosclerotic changes and OA-severity classification, which is in agreement with previous reports.10,11 In addition, the degree of subchondral bone sclerosis correlated with the clinical symptoms and scores of patients with OA. More severe osteosclerosis was associated with worse clinical outcomes, including increased pain and functional impairment.11,25,26
We found that BV/TV and Tb.Th were significantly negatively correlated with the JHEQ pain score, whereas Tb.Sp was positively correlated with the JHEQ pain score. This suggests that as subchondral bone sclerosis progresses, the ability of the subchondral trabecular bone to absorb shock decreases, which may lead to cartilage damage and increased pain.4,5 Specifically, BV/TV and Tb.Th are the indicators of bone density and thickness, and as the stage of hip OA due to DDH progresses, these values increase, which leads to lower JHEQ pain scores, meaning that the patient experiences more intense pain as the bone sclerosis advances. On the contrary, the decrease in Tb.Sp with disease progression also contributed to increased pain, as reflected by the lower JHEQ pain scores. These results suggest that with the progression of hip OA due to DDH, the trabecular structure becomes denser and more compact, reducing the bone’s ability to absorb mechanical stress, thus exacerbating pain.
The cartilage is aneural and does not contain pain fibers; thus, changes in the cartilage alone are unlikely to be a direct source of pain in mild-to-moderate OA. 27 In contrast, the subchondral bone and osteochondral junction, which contain sensory nerve fibers, are regarded as major sources of pain in OA. 28 Aberrant subchondral bone remodeling, abnormal subchondral bone microarchitecture, and the formation of innervated osteochondral junctions and osteophytes are responsible for pain in OA, especially in the later stages of the disease.27,28 In addition, the degree of subchondral bone sclerosis correlates with clinical symptoms and scores of patients with OA. 29 More severe osteosclerosis is associated with worse clinical outcomes, including increased pain and functional impairment. 30
The subchondral bone plays a crucial role in the pathogenesis and progression of OA. Identifying the causes of structural changes in the subchondral trabecular bone could lead to the development of drugs that may prevent their occurrence and inhibit disease progression.26,30,31 Various drugs targeting the subchondral trabecular bone, such as bisphosphonates, strontium ranelate, and transforming growth factor-β1 inhibitors, have been investigated for their potential disease-modifying effects in OA. However, the results have been inconsistent, with some studies showing benefits and others failing to demonstrate significant effects.6,32 The timing and specific targeting of changes in the subchondral trabecular bone may be vital for the success of these therapies. Further research is necessary to better understand the mechanisms underlying the alterations in the subchondral trabecular bone and to develop more effective drugs capable of preventing or reversing these structural changes in OA.
Our study has several limitations. First, the sample size was relatively small. Although a significant difference was observed in the male-to-female ratio among the groups, no significant differences were identified in all parameters between the sexes. We believe that this factor did not significantly influence our results. A study using a randomly selected sample from a larger population can provide clearer conclusions. However, compared with other studies on the subchondral trabecular bone using MDCT in patients with OA, the number of participants in our study was relatively large, making the results meaningful. Second, our study was cross-sectional, and the accuracy of image analysis was limited. A longitudinal study could potentially reveal earlier changes in the subchondral trabecular bone in OA. The spatial resolution of our method was insufficient for a complete visualization of the trabecular bone, and issues such as the partial volume effect and noise from body movements persisted, as reported in previous studies. 14 As MDCT continues to be developed and refined, inaccuracies are expected to decrease. Nonetheless, the results of this study are consistent with those of previous studies. 9 Therefore, this method was used in the present study.
In summary, osteosclerotic changes in the subchondral trabecular bone are strongly associated with OA severity, and these structural changes correlate with the clinical condition of patients with OA. This underscores the importance of assessing alterations in the subchondral trabecular bone for the evaluation and treatment of OA. Although our study did not fully elucidate the pathophysiology underlying the arthropathic changes, these findings could improve our understanding and management of patients with OA due to DDH. Our findings may also contribute to the development of new treatment strategies targeting the subchondral trabecular bone in OA.
Footnotes
Acknowledgments and Funding: The authors thank Editage (www.editage.com) for English language editing. The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The study protocol was approved by the Institutional Review Board of our hospital.
ORCID iDs: Hiroki Kaneta  https://orcid.org/0009-0005-9697-2748
https://orcid.org/0009-0005-9697-2748
Yuichi Kato  https://orcid.org/0000-0003-1583-3884
https://orcid.org/0000-0003-1583-3884
References
- 1. Patel JH, Moed BR. Instability of the hip joint after posterior acetabular wall fracture: independent risk factors remain elusive. J Bone Joint Surg Am. 2017;99(23):e126. doi: 10.2106/JBJS.16.01427. [DOI] [PubMed] [Google Scholar]
- 2. Xu J, Li D, Ma RF, Barden B, Ding Y. Application of rapid prototyping pelvic model for patients with DDH to facilitate arthroplasty planning: a pilot study. J Arthroplasty. 2015;30(11):1963-70. doi: 10.1016/j.arth.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 3. Chappard C, Peyrin F, Bonnassie A, Lemineur G, Brunet-Imbault B, Lespessailles E, et al. Subchondral bone micro-architectural alterations in osteoarthritis: a synchrotron micro-computed tomography study. Osteoarthritis Cartilage. 2006;14(3):215-23. doi: 10.1016/j.joca.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 4. Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res. 1986(213):34-40. [PubMed] [Google Scholar]
- 5. Chu L, He Z, Qu X, Liu X, Zhang W, Zhang S, et al. Different subchondral trabecular bone microstructure and biomechanical properties between developmental dysplasia of the hip and primary osteoarthritis. J Orthop Translat. 2019;22:50-7. doi: 10.1016/j.jot.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kwan Tat S, Lajeunesse D, Pelletier JP, Martel-Pelletier J. Targeting subchondral bone for treating osteoarthritis: what is the evidence. Best Pract Res Clin Rheumatol. 2010;24(1):51-70. doi: 10.1016/j.berh.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lories RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol. 2011;7(1):43-9. doi: 10.1038/nrrheum.2010.197. [DOI] [PubMed] [Google Scholar]
- 8. Yu D, Xu J, Liu F, Wang X, Mao Y, Zhu Z. Subchondral bone changes and the impacts on joint pain and articular cartilage degeneration in osteoarthritis. Clin Exp Rheumatol. 2016;34(5):929-34. [PubMed] [Google Scholar]
- 9. Felson DT, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56(9):2986-92. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 10. Chiba K, Ito M, Osaki M, Uetani M, Shindo H. In vivo structural analysis of subchondral trabecular bone in osteoarthritis of the hip using multi-detector row CT. Osteoarthritis Cartilage. 2011;19(2):180-5. doi: 10.1016/j.joca.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 11. Oláh T, Cai X, Gao L, Walter F, Pape D, Cucchiarini M, et al. Quantifying the human subchondral trabecular bone microstructure in osteoarthritis with clinical CT. Adv Sci (Weinh). 2022;9(23):e2201692. doi: 10.1002/advs.202201692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsumoto T, Kaneuji A, Hiejima Y, Sugiyama H, Akiyama H, Atsumi T, et al. Japanese orthopaedic association hip disease evaluation questionnaire (JHEQ): a patient-based evaluation tool for hip-joint disease. The subcommittee on hip disease evaluation of the clinical outcome committee of the Japanese orthopaedic association. J Orthop Sci. 2012;17(1):25-38. doi: 10.1007/s00776-011-0166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Inoue T, Shoji T, Kato Y, Fujiwara Y, Sumii J, Shozen H, et al. Investigating the subchondral trabecular bone microstructure in patients with osteonecrosis of the femoral head using multi-detector row computed tomography. Mod Rheumatol. 2023;33(6):1190-6. doi: 10.1093/mr/roac121. [DOI] [PubMed] [Google Scholar]
- 14. Takasu M, Yamagami T, Nakamura Y, Komoto D, Kaichi Y, Tani C, et al. Multidetector computed tomography-based microstructural analysis reveals reduced bone mineral content and trabecular bone changes in the lumbar spine after transarterial chemoembolization therapy for hepatocellular carcinoma. PLoS ONE. 2014;9(10):e110106. doi: 10.1371/journal.pone.0110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hildebrand T, Rüegsegger P. A new method for the model-independent assessment of thickness in three-dimensional images. J Microsc. 1997;185(1):67-75. doi: 10.1046/j.1365-2818.1997.1340694.x. [DOI] [Google Scholar]
- 16. Hildebrand T, Rüegsegger P. Quantification of bone microarchitecture with the structure model index. Comput Methods Biomech Biomed Engin. 1997;1(1):15-23. doi: 10.1080/01495739708936692. [DOI] [PubMed] [Google Scholar]
- 17. Okano K, Enomoto H, Osaki M, Shindo H. Rotational acetabular osteotomy for advanced osteoarthritis secondary to developmental dysplasia of the hip. J Bone Joint Surg Br. 2008;90(1):23-6. doi: 10.1302/0301-620X.90B1.19665. [DOI] [PubMed] [Google Scholar]
- 18. Turmezei TD, Fotiadou A, Lomas DJ, Hopper MA, Poole KE. A new CT grading system for hip osteoarthritis. Osteoarthritis Cartilage. 2014;22(10):1360-6. doi: 10.1016/j.joca.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 19. Dolan MM, Heyworth BE, Bedi A, Duke G, Kelly BT. CT reveals a high incidence of osseous abnormalities in hips with labral tears. Clin Orthop Relat Res. 2011;469(3):831-8. doi: 10.1007/s11999-010-1539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tumminello M, Aste T, Di Matteo T, Mantegna RN. A tool for filtering information in complex systems. Proc Natl Acad Sci U S A. 2005;102(30):10421-6. doi: 10.1073/pnas.0500298102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hayami T, Pickarski M, Wesolowski GA, McLane J, Bone A, Destefano J, et al. The role of subchondral bone remodeling in osteoarthritis: reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum. 2004;50(4):1193-206. doi: 10.1002/art.20124. [DOI] [PubMed] [Google Scholar]
- 22. Kadri A, Funck-Brentano T, Lin H, Ea HK, Hannouche D, Marty C, et al. Inhibition of bone resorption blunts osteoarthritis in mice with high bone remodelling. Ann Rheum Dis. 2010;69(8):1533-8. doi: 10.1136/ard.2009.124586. [DOI] [PubMed] [Google Scholar]
- 23. Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19(6):704-12. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diederichs G, Link T, Marie K, Huber M, Rogalla P, Burghardt A, et al. Feasibility of measuring trabecular bone structure of the proximal femur using 64-slice multidetector computed tomography in a clinical setting. Calcif Tissue Int. 2008;83(5):332-41. doi: 10.1007/s00223-008-9181-y. [DOI] [PubMed] [Google Scholar]
- 25. Barr AJ, Campbell TM, Hopkinson D, Kingsbury SR, Bowes MA, Conaghan PG. A systematic review of the relationship between subchondral bone features, pain and structural pathology in peripheral joint osteoarthritis. Arthritis Res Ther. 2015;17(1):228. doi: 10.1186/s13075-015-0735-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu Y, Chen X, Wang S, Jing Y, Su J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021;9(1):20. doi: 10.1038/s41413-021-00147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O’Neill TW, Felson DT. Mechanisms of osteoarthritis (OA) pain. Curr Osteoporos Rep. 2018;16(5):611-6. doi: 10.1007/s11914-018-0477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sun Q, Li G, Liu D, Xie W, Xiao W, Li Y, et al. Peripheral nerves in the tibial subchondral bone: the role of pain and homeostasis in osteoarthritis. Bone Joint Res. 2022;11(7):439-52. doi: 10.1302/2046-3758.117.BJR-2021-0355.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kato Y, Nakasa T, Sumii J, Kanemitsu M, Ishikawa M, Miyaki S, et al. Changes in the subchondral bone affect pain in the natural course of traumatic articular cartilage defects. Cartilage. 2023;14(2):247-55. doi: 10.1177/19476035231154514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu X, Chan YT, Yung PSH, Tuan RS, Jiang Y. Subchondral bone remodeling: a therapeutic target for osteoarthritis. Front Cell Dev Biol. 2021;8:607764. doi: 10.3389/fcell.2020.607764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li G, Yin J, Gao J, Cheng TS, Pavlos NJ, Zhang C, et al. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. 2013;15(6):223. doi: 10.1186/ar4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hu W, Chen Y, Dou C, Dong S. Microenvironment in subchondral bone: predominant regulator for the treatment of osteoarthritis. Ann Rheum Dis. 2021;80(4):413-22. doi: 10.1136/annrheumdis-2020-218089. [DOI] [PMC free article] [PubMed] [Google Scholar]


