Abstract
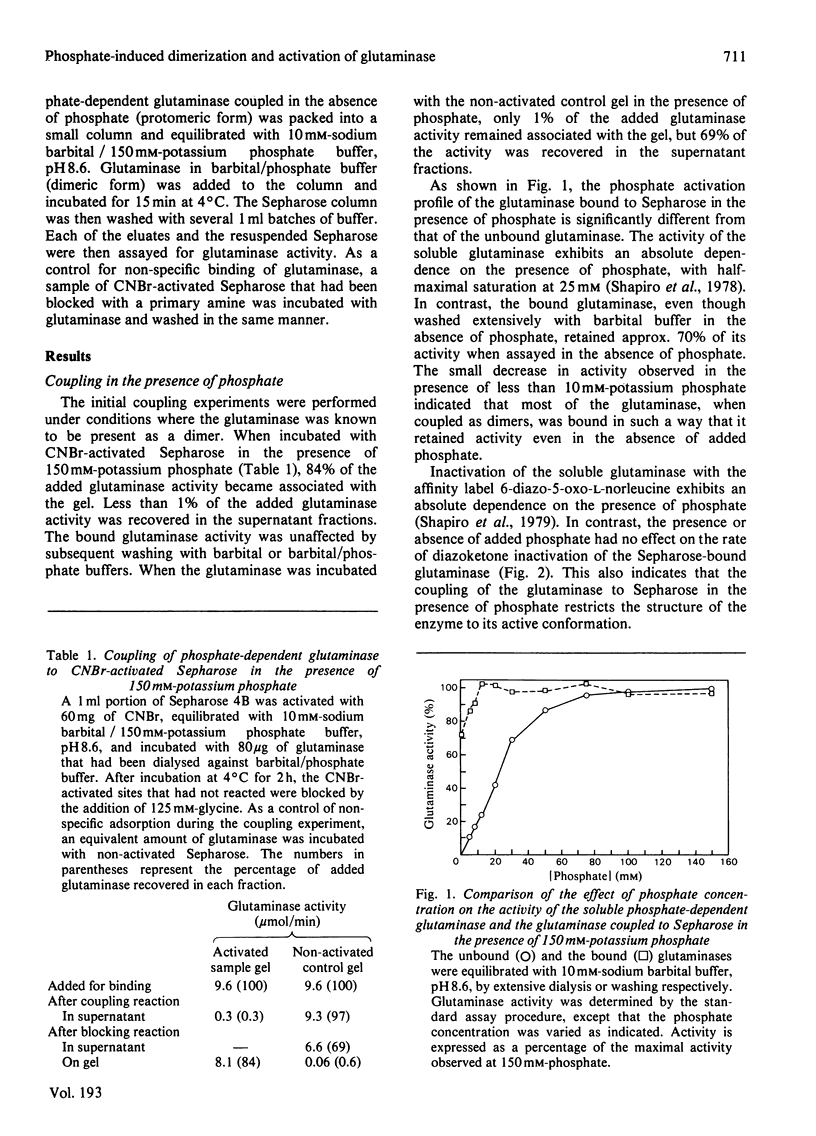
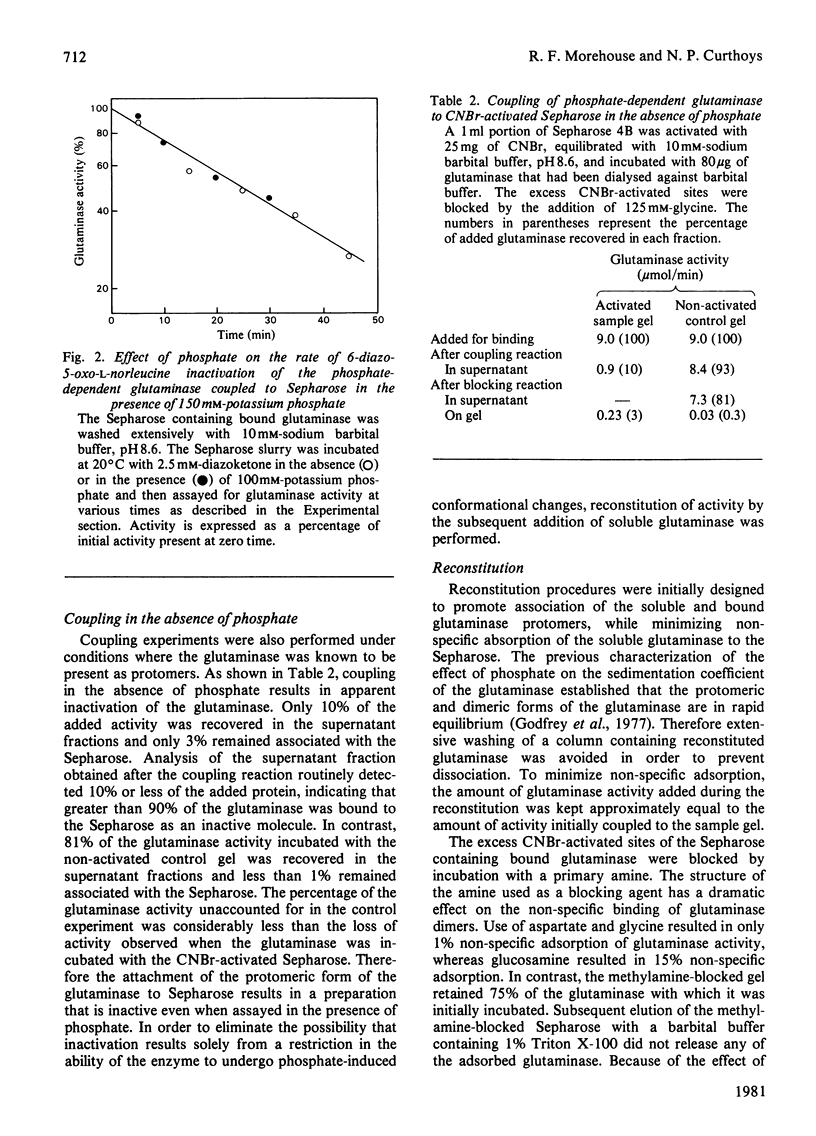
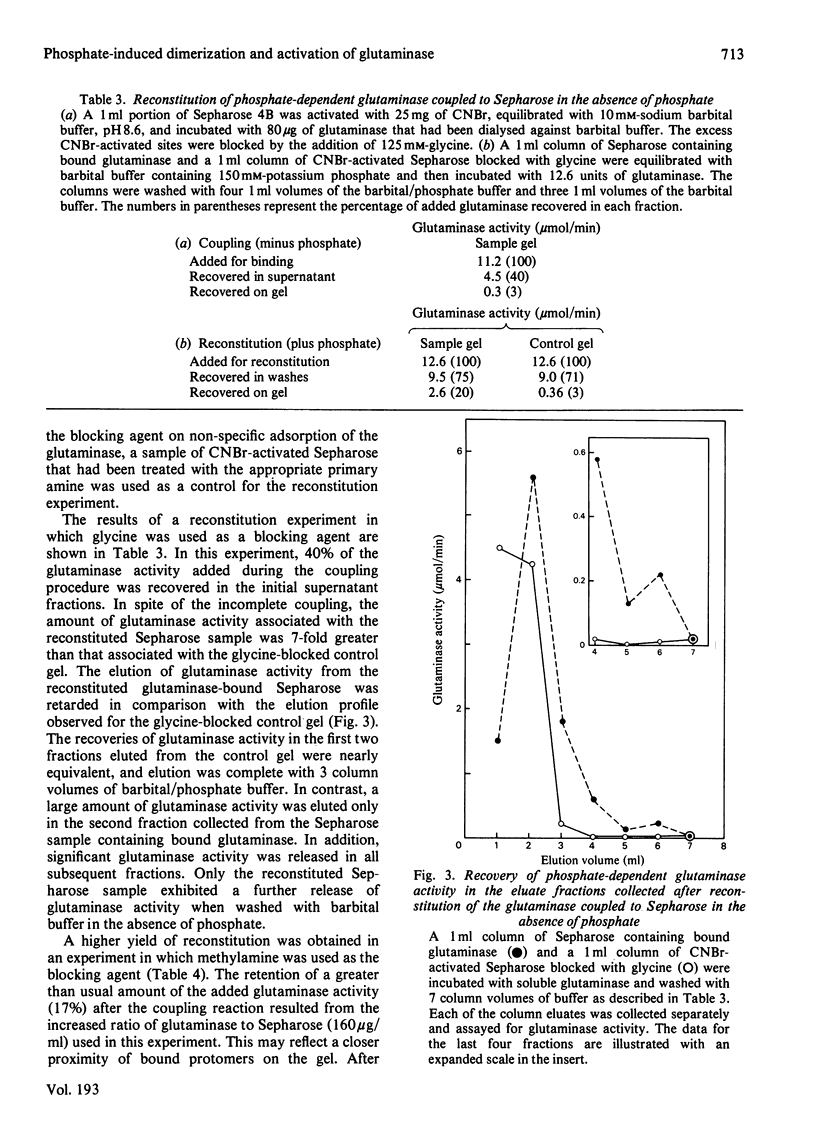
In the absence of phosphate, purified rat renal phosphate-dependent glutaminase exists as a catalytically inactive protomer. The addition of phosphate results in both dimerization and activation of the glutaminase. Covalent attachment of the dimeric form of the glutaminase to CNBr-activated Sepharose was achieved with 84% retention of activity. At least 70% of the bound glutaminase activity was expressed even in the absence of added phosphate. In addition, 6-diazo-5-oxo-L-norleucine, which interacts only with the catalytically active form of the glutaminase, inactivates the bound dimeric form of glutaminase at the same rate in either the absence or the presence of added phosphate. Therefore retention of dimeric structure is apparently sufficient to maintain glutaminase activity. In contrast, the coupling of the protomeric form of the enzyme to Sepharose resulted in retention of only 3% of the phosphate-induced glutaminase activity. However, up to 48% of this activity could be reconstituted by addition of soluble glutaminase under conditions that promote dimerization. These results indicate that the monomeric form of the glutaminase has minimal inherent activity and that dimerization is an essential step in the phosphate-induced activation of the glutaminase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M. Evidence that the catalytic and regulatory functions of carbamylphosphate synthetase from Escherichia coli are not dependent on oligomer formation. Biochemistry. 1977 Feb 22;16(4):583–586. doi: 10.1021/bi00623a004. [DOI] [PubMed] [Google Scholar]
- Boyd T. A., Goldstein L. Kidney metabolite levels and ammonia production in acute acid-base alterations in the rat. Am J Physiol. 1979 Mar;236(3):E289–E295. doi: 10.1152/ajpendo.1979.236.3.E289. [DOI] [PubMed] [Google Scholar]
- Chan W. W., Mosbach K. Effects of subunit interactions on the activity of lactate dehydrogenase studied in immobilized enzyme systems. Biochemistry. 1976 Sep 21;15(19):4215–4222. doi: 10.1021/bi00664a013. [DOI] [PubMed] [Google Scholar]
- Clark V. M., Curthoys N. P. Cause of subunit heterogeneity in purified rat renal phosphate-dependent glutaminase. J Biol Chem. 1979 Jun 25;254(12):4939–4941. [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Curthoys N. P., Lowry O. H. The distribution of glutaminase isoenzymes in the various structures of the nephron in normal, acidotic, and alkalotic rat kidney. J Biol Chem. 1973 Jan 10;248(1):162–168. [PubMed] [Google Scholar]
- Curthoys N. P., Weiss R. F. Regulation of renal ammoniagenesis. Subcellular localization of rat kidney glutaminase isoenzymes. J Biol Chem. 1974 May 25;249(10):3261–3266. [PubMed] [Google Scholar]
- FISHER H. F., CROSS D. G., McGREGOR L. L. Catalytic activity of sub-units of glutamic dehydrogenase. Nature. 1962 Dec 1;196:895–896. doi: 10.1038/196895b0. [DOI] [PubMed] [Google Scholar]
- FRIEDEN C. Glutamic dehydrogenase. I. The effect of coenzyme on the sedimentation velocity and kinetic behavior. J Biol Chem. 1959 Apr;234(4):809–814. [PubMed] [Google Scholar]
- Godfrey S., Kuhlenschmidt T., Curthoys P. Correlation between activation and dimer formation of rat renal phosphate-dependent glutaminase. J Biol Chem. 1977 Mar 25;252(6):1927–1931. [PubMed] [Google Scholar]
- Goldstein L. Pathways of glutamine deamination and their control in the rat kidney. Am J Physiol. 1967 Oct;213(4):983–989. doi: 10.1152/ajplegacy.1967.213.4.983. [DOI] [PubMed] [Google Scholar]
- Goldstein L. Relation of glutamate to ammonia production in the rat kidney. Am J Physiol. 1966 Mar;210(3):661–666. doi: 10.1152/ajplegacy.1966.210.3.661. [DOI] [PubMed] [Google Scholar]
- Holcenberg J. S., Ericsson L., Roberts J. Amino acid sequence of the diazooxonorleucine binding site of Acinetobacter and Pseudomonas 7A glutaminase--asparaginase enzymes. Biochemistry. 1978 Feb 7;17(3):411–417. doi: 10.1021/bi00596a005. [DOI] [PubMed] [Google Scholar]
- Kovacević Z. Importance of the flux of phosphate across the inner membrane of kidney mitochondria for the activation of glutaminase and the transport of glutamine. Biochim Biophys Acta. 1976 Jun 8;430(3):399–412. doi: 10.1016/0005-2728(76)90015-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Regulation of renal ammoniagenesis. Purification and characterization of phosphate-dependent glutaminase from rat kidney. Arch Biochem Biophys. 1976 May;174(1):82–89. [PubMed] [Google Scholar]
- Shapiro R. A., Clark V. M., Curthoys N. P. Covalent interaction of L-2-amino-4-oxo-5-chloropentanoic acid with rat renal phosphate-dependent glutaminase. Evidence for a specific glutamate binding site and of subunit heterogeneity. J Biol Chem. 1978 Oct 10;253(19):7086–7090. [PubMed] [Google Scholar]
- Shapiro R. A., Clark V. M., Curthoys N. P. Inactivation of rat renal phosphate-dependent glutaminase with 6-diazo-5-oxo-L-norleucine. Evidence for interaction at the glutamine binding site. J Biol Chem. 1979 Apr 25;254(8):2835–2838. [PubMed] [Google Scholar]
- Squires E. J., Hall D. E., Brosnan J. T. Arteriovenous differences for amino acids and lactate across kidneys of normal and acidotic rats. Biochem J. 1976 Oct 15;160(1):125–128. doi: 10.1042/bj1600125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannen R. L. Ammonia metabolism. Am J Physiol. 1978 Oct;235(4):F265–F277. doi: 10.1152/ajprenal.1978.235.4.F265. [DOI] [PubMed] [Google Scholar]
- Tannen R. L., Kunin A. S. Effect of pH on ammonia production by renal mitochondria. Am J Physiol. 1976 Dec;231(6):1631–1637. doi: 10.1152/ajplegacy.1976.231.6.1631. [DOI] [PubMed] [Google Scholar]
- Tannen R. L., Ross B. D. Ammoniagenesis by the isolated perfused rat kidney: the critical role of urinary acidification. Clin Sci (Lond) 1979 Apr;56(4):353–364. doi: 10.1042/cs0560353. [DOI] [PubMed] [Google Scholar]
- Vinay P., Allignet E., Pichette C., Watford M., Lemieux G., Gougoux A. Changes in renal metabolite profile and ammoniagenesis during acute and chronic metabolic acidosis in dog and rat. Kidney Int. 1980 Mar;17(3):312–325. doi: 10.1038/ki.1980.37. [DOI] [PubMed] [Google Scholar]


