Abstract
Background
The aim of this study was to quantify mediators of neutrophilic inflammation within airway extracellular vesicles (EVs) of children treated for a cystic fibrosis (CF) pulmonary exacerbation (PEx).
Methods
EVs were isolated from stored sputum samples collected before and after antibiotic therapy for PEx between 2011 and 2013, and characterised by nanoparticle tracking analysis (NTA) and transmission electron microscopy (TEM). Western blot analysis of EV protein extracts was used for EV canonical protein markers CD63, CD9 and flotillin-1 (FLOT1), as well as neutrophil elastase (NE), myeloperoxidase (MPO) and interleukin-8. The EV content of NE and MPO were expressed as ratios of NE/FLOT1 and MPO/FLOT1 protein band densities.
Results
Sputum samples from 21 children aged 13.3 (range 8.0–17.0) years were analysed. NTA showed high concentrations of particles at the size of small EVs (50–200 nm), and typical EV morphology was confirmed by TEM. CD63, CD9 and FLOT1 were detectable in all samples. Median (interquartile range (IQR)) NE/FLOT1 increased from 2.46 (1.68–5.25) before to 6.83 (3.89–8.89, p<0.001) after PEx therapy, and median (IQR) MPO/FLOT1 increased from 2.30 (1.38–4.44) before to 5.76 (3.45–6.94, p<0.01) after, while EV size remained unchanged. Improvement in lung function (percent predicted forced expiratory volume in 1 s (ppFEV1)) with PEx therapy correlated with NE EV content (r=0.657, p=0.001).
Conclusions
Airways of children with CF contain EVs that carry NE and MPO as cargo. The lower NE and MPO content at the time of PEx, compared with after therapy, and the correlation with pulmonary function suggest both a functional role of EVs in CF airway inflammation and the potential of EVs as a biomarker to monitor CF lung disease.
Shareable abstract
Extracellular vesicles (EVs) in cystic fibrosis airways contain neutrophil elastase (NE) and myeloperoxidase (MPO). NE and MPO EV cargo increase with therapy for pulmonary exacerbations and changes in EV cargo correlate with measures of pulmonary function. https://bit.ly/460Gx92
Introduction
Cystic fibrosis (CF) is an inherited multiple-organ disease that causes chronic pulmonary function decline over time, leading to respiratory failure. CF lung disease is associated with neutrophilic inflammation, recurrent or chronic bacterial infection of the airways and acute pulmonary exacerbations (PEx) [1]. Neutrophil elastase (NE) and myeloperoxidase (MPO) are mediators of neutrophilic inflammation that have been intensively studied in the context of CF lung disease. Both are elevated in CF airways during PEx and inversely correlate with pulmonary function [2–7]. Increased NE activity is a key risk factor for the onset and progression of bronchiectasis and lung function decline in people with CF (pwCF) [2–4, 8]. MPO is the product of neutrophil degranulation or cell death and contributes to damage of the CF lung by producing hypochlorous acid [9–11]. Multiple mechanisms and pathways are involved in CF airway inflammation and there is an ongoing need for informative biomarkers of CF PEx [12].
Extracellular vesicles (EVs) are nano-sized (50–1000 nm) lipid-membrane-bound structures that can be released by all living cells [13, 14]. The cargo of EVs largely consists of nucleic acids, proteins and lipid molecules, which can be transferred to recipient cells, and thus are involved in intercellular communication and signalling [15, 16]. EVs have been shown to play numerous roles in both physiological and pathological processes [13–15], and there is increasing evidence of an involvement of EVs in the pathogenesis of lung diseases [16–18]. For example, differences in proteomic content were found between EVs isolated from bronchoalveolar lavage (BAL) fluid of pwCF, people with primary ciliary dyskinesia and those with asthma [19]. EVs isolated from BAL fluid of pwCF express unique protein fingerprints and pathways, and differences in EV number and protein content were seen between pwCF and healthy controls [20]. Studies have shown that EVs isolated from expectorated CF sputum samples are of predominantly neutrophilic origin [21], and can activate naïve neutrophils, inducing the exocytosis of primary granule and promoting inflammation in CF airways [22]. However, while previous studies in CF included airway specimens from clinically stable pwCF and CF patients experiencing PEx [20, 23], there is currently no study on changes in EV content with PEx therapy.
The aims of this study were to characterise airway EVs in the sputum of children with CF, and to quantify EV NE and MPO protein content before and after therapy for a CF PEx.
Methods
Biobanked sputum samples, collected as part of previously published studies conducted at the Hospital for Sick Children between 2011 and 2013 [24–27], were used for this study. The studies were approved by the Research Ethics Board (REB) at the Hospital for Sick Children and collective approval for the analyses performed was obtained (REB# 1000064004). Informed written consent was obtained from all participants or their parents or legal guardians.
We used sputum from CF children aged 6–18 years that was expectorated immediately before and at the end of PEx therapy with either 14 days of intravenous (IV) (inpatients) [24–26] or 3 weeks of oral (outpatients) [27] antibiotics. Microbiology testing was performed as per clinical routine [26], and lung function was assessed immediately before and at the end of PEx therapy by spirometry in accordance with the American Thoracic Society (ATS) criteria, with forced expiratory volume in 1 s (FEV1) calculated and expressed as percent predicted (ppFEV1) using the Global Lung Function Initiative (GLI) reference equations [28]. A nitrogen multiple breath washout (MBW) test was performed in the IV-treated subset using the Exhalyzer D (Eco Medics) device; the results were analysed by Spiroware 3.3.2 software in accordance with the ATS and European Respiratory Society consensus statement, and the lung clearance index (LCI) calculated, as previously reported [29]. A PEx was defined as new or worsening respiratory symptoms for a minimum of 3 days, or a decrease in pulmonary function (FEV1) of ≥10%, as well as a physician decision to treat with antibiotic therapy.
Sputum processing
Spontaneously expectorated sputum was collected in sterile cups during routine clinic visits or hospital admissions, processed within 1 h and immediately frozen after processing, in accordance with standardised protocols developed by the Therapeutics Development Network (TDN) [30]. In brief, mucus plugs were separated from sputum samples and were dissolved by 10% Sputolysin (dithiothreitol). Samples were then filtered by nylon mesh and two-step centrifugation (at 400 g and 1700 g) was performed to produce sputum supernatant. In addition to preparation of sputum supernatant, as previously reported [27], isolated mucus plugs were also incubated in an equal volume of 1% Triton X-100 containing freshly added protease inhibitors. The mix was then homogenised by vortexing for 5 min (sputum homogenate), and all samples stored at −80 °C for later analysis.
Isolation of EVs
EVs were isolated from processed sputum samples by differential centrifugation. Sputum homogenate samples were first filtered with 0.2 μm microfilter (Cat. #431219, Corning, NY), followed by centrifugation at 1700×g for 20 min. Supernatants of filtered homogenate samples were then centrifuged at 100 000×g for 14 h (XL-70 ultracentrifuge, Beckman Coulter, Villepinte, France). EV pellets were resuspended in 500 μL of phosphate-buffered saline and stored at −80 °C.
Characterisation of EVs
Sputum EVs were characterised in accordance with the latest minimal information for studies of extracellular vesicles (MISEV) guidelines of the International Society for Extracellular Vesicles [15]. Details on nanoparticle tracking analysis (NTA) for EV quantification, on transmission electron microscopy (TEM) to assess EV morphology and on Western blot analyses of canonical EV markers (CD63, CD9 and flotillin-1 (FLOT1)) and of proteins involved in CF airway inflammation (NE, MPO and interleukin-8 (IL-8)) in EV protein extracts are given in the supplementary material.
Quantification of IL-8 and NE in sputum
Single-plex ELISA analysis was used to measure the concentrations of NE and IL-8 in sputum supernatant, as previously described [27].
Statistical analysis
All statistical analyses were performed in GraphPad Prism 9 (GraphPad Software, San Diego, CA). Continuous data were expressed as mean and standard deviation (sd) or as median and interquartile range (IQR), depending on data distribution. The paired t-test or Wilcoxon test was used for group comparisons and the Pearson or Spearman correlation was used for correlations, where appropriate. Significance was defined as a value of p<0.05.
Results
42 sputum samples from 21 children with CF (13 females) with a mean age of 13.3 (range 8.0–17.0) years, collected between 2011 and 2013, were analysed; 12 received IV antibiotic therapy, while nine were treated with oral antibiotics. None of the participants was treated with cystic fibrosis transmembrane conductance regulator (CFTR) modulators at the time. Demographics of the study participants by treatment are given in table 1.
TABLE 1.
Demographics of study participants
| All participants (n=21) | IV ABx (n=12) | Oral ABx (n=9) | p-value# | |
|---|---|---|---|---|
| Age, years, mean (range) | 13.3 (8.0–17.0) | 13.8 (8.0–17.0) | 12.5 (8.7–16.7) | 0.33 |
| Male, n (%) | 8 (38) | 3 (25) | 5 (55) | 0.26 |
| Pancreatic insufficiency, n (%) | 18 (85) | 11 (91) | 7 (78) | 0.73 |
| ppFEV1, mean (sd) | ||||
| Before ABx | 64.6 (14.9) | 60.2 (8.9) | 70.4 (19.6) | 0.04 |
| After ABx | 77.6 (13.8) | 72.2 (10.8) | 84.8 (14.8) | 0.03 |
| Microbiology , n | ||||
| Staphylococcus aureus | 9 | 4 | 5 | |
| Staphylococcus aureus and Haemophilus influenzae | 6 | 2 | 4 | |
| Staphylococcus aureus and Pseudomonas aeruginosa | 3 | 3 | 0 | |
| Pseudomonas aeruginosa | 3 | 3 | 0 | |
#: p-values represent comparison between intravenous (IV) antibiotic (ABx) and oral ABx therapy groups. An unpaired t-test or Wilcoxon test was used for group comparisons. ppFEV1: precent predicted forced expiratory volume in 1 s.
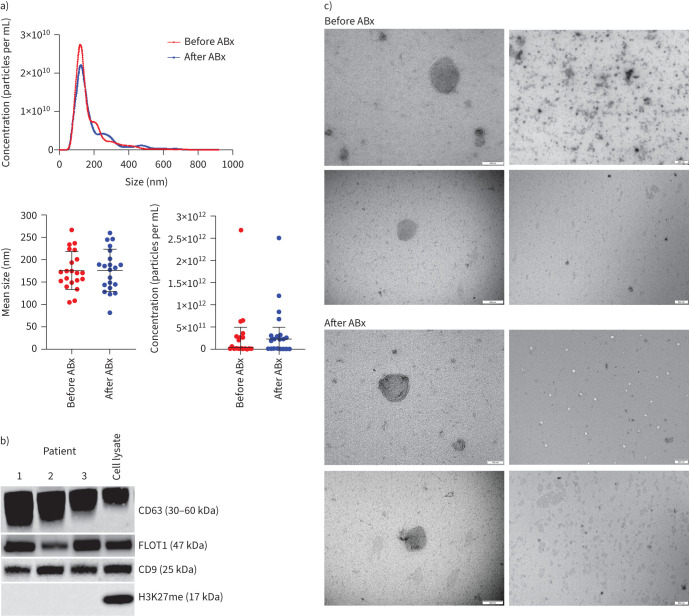
All sputum homogenate samples were positive for the three EV markers CD63, CD9 and FLOT1 in Western blotting analysis (figure 1b), but not the histone marker H3K27me3. NTA of sputum homogenates showed an abundance of particles at the expected size of small EVs (50–200 nm) (figure 1a), and the presence of EVs was confirmed by TEM, which detected nano-sized vesicles with characteristic EV morphology (figure 1c). In contrast, EV protein markers were not detectable by Western blotting in sputum supernatant. Subsequent EV analyses were therefore performed in sputum homogenate samples only.
FIGURE 1.
Characterisation of extracellular vesicles (EVs) in sputum lysates: a) size distribution analysis of EVs before antibiotics (ABx) (red) and after ABx (blue) by nanoparticle tracking analysis (NTA); b) Western blot with anti-CD63, anti-CD9 and anti-FLOT1 antibodies, used as EV markers. CD63 core protein is a 26-kDa protein, the antibody detects the various glycosylated forms ranging from 30 to 60 kDa. Western blot with H3K27me (a tri-methylation of lysine 27 on histone H3 protein) was used as a control, to rule out cell debris in EV extracts; and c) transmission electron microscopy (TEM) for the morphologic characterisation of EVs in sputum homogenate. The two different magnifications highlight the morphology of individual EVs at narrow fields (left column, scale bars=100 nm (top) and 200 nm (bottom)) and a population of EVs at wide fields (right column, scale bar=500 nm).
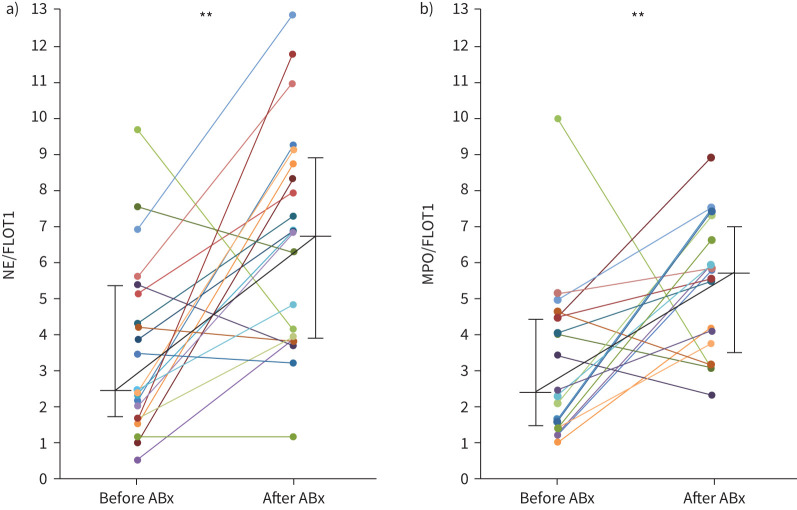
Both the concentration and the size of the EVs isolated from sputum homogenate were similar before and after therapy for PEx (table 2). Quantification of EV protein content by Western blot analysis revealed an increase in NE cargo (NE/FLOT1 ratio) from a median of 2.46 (IQR 1.68–5.25) before to 6.83 (IQR 3.89–8.89) after therapy (p<0.001). Similarly, the MPO cargo (MPO/FLOT1 ratio) increased from a median of 2.30 (IQR 1.38–4.44) before to 5.76 (IQR 3.45–6.94) after (p<0.01). IL-8 was not detectable in EV protein extracts by Western blotting. Concentrations of NE and IL-8 in sputum supernatant before and after PEx therapy are shown in table 2.
TABLE 2.
Extracellular vesicle (EV) characteristics, protein cargo and sputum biomarker concentrations in cystic fibrosis (CF) pulmonary exacerbation (PEx)
| All participants (n=21) | IV ABx therapy (n=12) | Oral ABx therapy (n=9) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before ABx | After ABx | p-value | Before ABx | After ABx | p-value | Before ABx | After ABx | p-value | |
| Sputum EV size (nm)# | 175.9 (42.4) | 176.2 (47.2) | 0.97 | 151.2 (26.6) | 145.8 (31.8) | 0.47 | 208.9 (37.2) | 216.7 (30.6) | 0.65 |
| Particles (1×1011 per mL) | 0.53 (0.07–4.93) | 2.2 (0.05–4.94) | 0.76 | 2.64 (0.28–15.2) | 2.21 (0.94–3.04) | 0.88 | 0.07 (0.07–3.2) | 0.18 (0.05–7.57) | 0.30 |
| EV cargo NE/FLOT1 | 2.46 (1.68–5.25) | 6.83 (3.89–8.89) | <0.001 | 2.96 (1.77–5.11) | 5.81 (3.04–10.5) | 0.016 | 2.32 (1.27–6.32) | 6.87 (5.21–8.51) | 0.07 |
| MPO/FLOT1 | 2.30 (1.38–4.44) | 5.76 (3.45–6.94) | <0.01 | 2.93 (2.13–4.57) | 5.66 (4.18–7.1) | 0.012 | 1.39 (1.13–4.22) | 4.18 (3.07–6.69) | 0.16 |
|
Sputum supernatant
NE (ng·mL−1) |
65.6 (25.4–205.9) | 61.9 (11.2–133.6) | 0.25 | 60.8 (25.9–521) | 50.6 (7.8–157) | 0.054 | 65.6 (24.7–79.7) | 62.4 (25.8–130) | 0.65 |
| IL-8 (ng·mL−1) | 48.4 (23.3–158) | 40.0 (14.6–117) | 0.216 | 55.0 (24.3–234) | 23.8 (8.8–47.6) | 0.007 | 41.4 (8.5–128) | 111.9 (39.4–170) | 0.57 |
Results are presented as mean (sd) or median (interquartile range (IQR)). p-values represent comparison between before and after therapy. #: significant difference between treatment groups (p<0.001). IV: intravenous; ABx: antibiotic; NE: neutrophil elastase; FLOT1: flotillin-1; MPO: myeloperoxidase; IL-8: interleukin 8. Bold indicates statistically significant p-values.
Subgroup analysis by antibiotic treatment (IV versus oral) was largely consistent with the pooled analysis (table 2 and figure 2). EV size at PEx was greater in the oral group than in the IV group (208.9 versus 151.2 nm, p<0.001), but did not change with therapy in either group. EV concentration and protein cargo appeared to be similar between groups. However, changes in MPO/FLOT1 and NE/FLOT1 with therapy, while numerically similar between groups, did not reach statistical significance in the orally treated participants (table 2).
FIGURE 2.
Neutrophil elastase (NE) and myeloperoxidase (MPO) extracellular vesicle (EV) content. Changes in EV cargo expressed as: a) NE/flotillin-1 (FLOT1) and b) MPO/FLOT1 ratios with therapy for PEx. Black horizontal lines are medians and vertical lines are interquartile ranges. **: p<0.01, Mann–Whitney test.
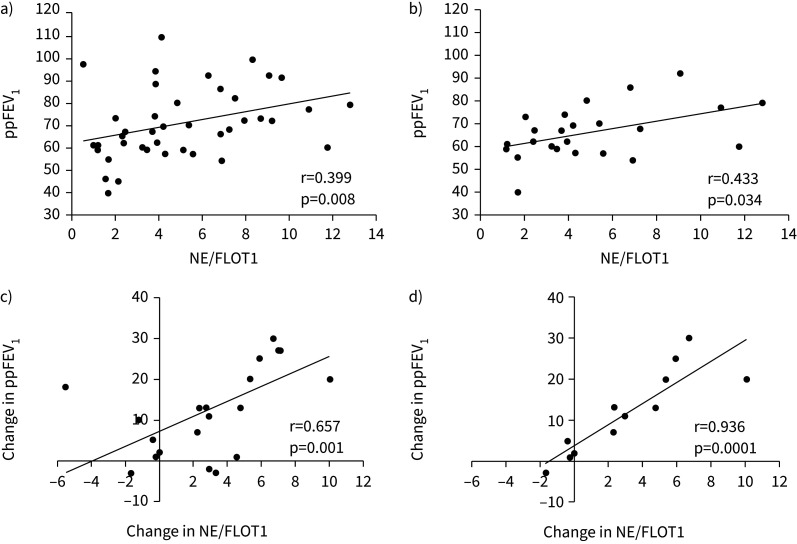
Correlations of EV cargo with pulmonary function
Lung function (ppFEV1) correlated with NE/FLOT1 in all study participants at all time points, as well as in the IV group at all time points and at PEx (pre-treatment) (figure 3). Improvement in ppFEV1 with therapy correlated with the changes in NE/FLOT1 in all patients and in the IV group (figure 3). MPO/FLOT1 also correlated with ppFEV1 in the IV group at all time points and at PEx, but not with changes in ppFEV1. However, changes in LCI correlated with changes in MPO/FLOT1 in the 10 patients who performed MBW (r=0.683, p=0.05), but not with changes in NE/FLOT1 (table 3).
FIGURE 3.
Correlations of extracellular vesicle (EV) protease cargo (neutrophil elastase (NE)/flotillin-1 (FLOT1)) with pulmonary function (percent predicted forced expiratory volume in 1 s (ppFEV1)): a) correlation between NE/FLOT1 and ppFEV1 in all study participants at all time points, b) correlation between NE/FLOT1 and ppFEV1 in the group treated with intravenous antibiotics at all time points, c) the changes in NE/FLOT1 and changes in ppFEV1 with antibiotic therapy for all study participants and d) the changes in NE/FLOT1 and changes in ppFEV1 with antibiotic therapy for those treated with intravenous antibiotics. R-value/p-value, Pearson correlation.
TABLE 3.
Correlation analysis between extracellular vesicle (EV) cargo proteins, soluble inflammatory markers and measures of pulmonary function
| NE/FLOT1 | MPO/FLOT1 | |||||
|---|---|---|---|---|---|---|
| All PEx | IV ABx | Oral ABx | All PEx | IV ABx | Oral ABx | |
| NE sputum | ||||||
| All time points | −0.138/0.383 | −0.253/0.233 | 0.054/0.829 | −0.006/0.967 | −0.065/0.762 | −0.011/0.964 |
| Before ABx | 0.030/0.895 | −0.157/0.623 | 0.350/0.358 | −0.046/0.840 | −0.042/0.904 | −0.400/0.291 |
| After ABx | −0.233/0.307 | −0.167/0.604 | −0.466/ 0.212 | 0.202/0.378 | 0.098/0.761 | 0.350/0.358 |
| Change | 0.082/0.722 | 0.161/0.619 | −0.116/0.775 | −0.058/0.801 | −0.133/0.683 | −0.083/0.843 |
| IL-8 sputum | ||||||
| All time points | 0.017/0.913 | −0.243/0.251 | 0.329/0.182 | −0.185/0.239 | −0.443/0.030 | −0.036/0.887 |
| Before ABx | 0.275/0.227 | 0.154/0.630 | 0.466/0.212 | −0.087/0.707 | 0.112/0.733 | −0.633/0.076 |
| After ABx | −0.165/0.475 | −0.328/0.297 | −0.066/0.880 | −0.167/0.469 | −0.402/0.194 | −0.183/0.643 |
| Change | −0.015/0.949 | 0.077/0.817 | −0.383/0.312 | 0.165/0.475 | 0.217/0.499 | −0.083/0.843 |
| ppFEV1 | ||||||
| All time points | 0.399/0.008 | 0.433/0.034 | 0.223/0.373 | 0.255/0.102 | 0.450/0.027 | 0.287/0.248 |
| Before ABx | 0.197/0.392 | 0.702/0.023 | 0.166/0.677 | 0.185/0.421 | 0.661/0.012 | 0.416/0.269 |
| After ABx | 0.129/0.576 | 0.417/0.178 | −0.527/0.149 | −0.061/0.791 | 0.193/0.543 | −0.175/0.652 |
| Change | 0.657/0.001 | 0.936/0.0001 | 0.477/0.197 | 0.140/0.543 | 0.382/0.219 | −0.100/0.800 |
| LCI | ||||||
| All time points | – | −0.295/0.193 | – | – | 0.124/0.592 | – |
| Before ABx | – | −0.182/0.613 | – | – | −0.018/0.973 | – |
| After ABx | – | −0.354/0.286 | – | – | 0.332/0.315 | – |
| Change | – | 0.283/0.463 | – | – | 0.683/0.050 | – |
Values are R-value/p-value. NE: neutrophil elastase; FLOT1: flotillin-1; PEx: pulmonary exacerbation; IV: intravenous; ABx: antibiotic; MPO: myeloperoxidase; IL-8: interleukin 8; ppFEV1: precent predicted forced expiratory volume in 1 s; LCI: lung clearance index. Bold indicates statistically significant p-values.
Discussion
We show here that EVs isolated from expectorated sputum samples of children with CF contain neutrophil-derived inflammatory proteins. The quantities of the EV cargo proteins NE and MPO, enzymes that are both known to contribute to CF lung disease progression, were significantly lower before treatment of PEx than after, while EV abundance and size did not change. Furthermore, the amount of NE and MPO in the sputum EVs correlated with measures of pulmonary function (ppFEV1) at the time of PEx, and changes in NE cargo with IV antibiotic therapy for PEx positively correlated with the improvement in ppFEV1.
The presence of EVs and their potential role in CF lung disease has been previously studied using airway epithelial and monocyte-derived macrophage cell cultures [22, 31–33], as well as BAL fluid [19, 20] and sputum samples from pwCF [21–23, 31, 32]. EVs isolated from CF airway cell cultures and BAL fluid demonstrated higher levels of proteins involved in leukocyte chemotaxis in pwCF, compared with controls [19, 20], and EVs isolated from human CF sputum elicited a strong neutrophil response when instilled into murine lung [34]. Further evidence for a functional role of CF EVs was suggested by the observation that neutrophil-derived CF EVs promote primary granule exocytosis by naïve neutrophils and activate CF inflammatory pathways [22]. A pathogenic role of neutrophil-derived EVs has also been proposed for COPD [18, 35]. As NE and MPO are myeloid-derived enzymes, our findings are in keeping with previous reports that EVs isolated from CF sputum are of predominantly granulocyte origin [21]. The chemokine IL-8, which is produced by macrophages and airway epithelium and is an established marker of CF airway inflammation [11, 30], was not detectable in sputum EVs in our study.
To our knowledge, this is the first study that describes changes in EV protein content of pwCF receiving treatment for PEx. Our findings may suggest a functional role of EV cargo in CF PEx, as we observed an increase in EV protease cargo with PEx therapy, while EV quantity and size remained unchanged. This is in contrast to a recent cross-sectional study in paediatric CF patients that found no differences in microRNA expression profiles of EVs isolated from sputum comparing clinically stable CF patients with CF patients with PEx [23]. One study described differences in the quantity of EV cargo proteins including NE, comparing stable paediatric CF patients of different age groups with adult pwCF with PEx by proteomics analysis; however, it was unclear whether the observed differences in that study could be explained by patient age alone, bacterial colonisation or PEx [20]. One possible explanation for the increase in EV protein content with PEx therapy in our study is that NE and MPO release by EVs may be accelerated in the earlier phase of PEx, rather than in the later phase, while the increase in EV cargo with therapy may result from reduced release or retention of these proteins. Alternatively, maturation of intracellular EVs and time to exocytosis may also be affected by PEx. While EV size did not change with PEx therapy in either treatment group in our study, EVs were significantly larger in those receiving oral antibiotics than in those receiving IV antibiotics, both before and after therapy for PEx. We can only speculate that this was potentially related to less advanced lung disease in the oral treatment group, as evidenced by ppFEV1 that was, on average, approximately 10% better for the oral group than for the IV group; there were no other demographic differences. However, the concentrations of IL-8 and NE in sputum supernatant, which are both established markers of CF airway inflammation, and the magnitude of the changes in EV content with therapy were similar between groups.
The observed positive correlations of NE and MPO EV content with pulmonary function (ppFEV1) and between the changes in ppFEV1 and changes in EV content with therapy for PEx add to multiple studies on NE as a biomarker for CF lung disease severity and progression. Those studies, however, generally focused on concentrations or activity of free NE in airway secretions [7, 36], or surface-bound elastase activity on neutrophils [37], whereas the potential role of EV NE cargo as a biomarker has not been studied in CF yet, to our knowledge. Longitudinal studies that include samples from stable CF patients and from periods of clinical deterioration are needed to help understand whether EV NE content could serve as a biomarker of CF PEx.
To accurately confirm the existence of EVs in sputum, we characterised our EV preparations by a variety of methods, in accordance with the MISEV2023 guidelines [15]. These included confirmation of particle shape typical for EV by TEM, and the global particle size determined by NTA-matched expected EV size. Western blot analysis of protein extract of EV preparations confirmed strong expression of the canonical EV markers CD63, CD9 and FLOT1; the histone marker H3K27me3 was not detectable, confirming that the EV isolates were not composed of apoptotic blebs or cellular debris [15]. However, to date there are no standardised protocols for processing sputum samples for the best EV isolation and analysis, and in most previous studies sputum EVs were isolated from sputum supernatant, not the mucus plugs [21–23, 31, 32]. We initially tested supernatant of lysed mucus plugs processed in accordance with a standardised protocol for sputum processing of soluble biomarkers developed by TDN [30], but this did not result in a quantifiable number of EVs in the liquid phase. This finding deviates from previous reports using sputum supernatant for EV detection; however, the protocols used for sputum processing in previous studies were different from ours [21–23, 31, 32]. The liquid phase of sputum, or supernatant, is likely to contain a fraction of the EV portion, released from the originating cells and available to interact with other cells or molecules in the inflamed airway environment. By contrast, using mucus plug homogenates allowed for isolation of the total EV content in airway secretions and did not distinguish between intracellular (stored) and extracellular (released) EVs, as, by following the aforementioned TDN protocol [30], sputum plugs were homogenised by dithiothreitol (Sputolysin) prior to EV isolation, similar to the protocol used by Stachowiak et al. in their microRNA profile study [23]. However, samples were then treated with Triton X-100, a nonionic surfactant, which most likely resulted in passive release of intracellular EVs from lysed cells. Exposure to Triton X-100 may also affect the integrity of EVs, which could explain why, in our preparations, EVs were not detectable in sputum supernatant. Despite the probable effect of Triton on EV lipid membranes at the concentration used, we were nevertheless able to visualise intact vesicles with characteristic morphology of small EVs by TEM. Moreover, the high concentration of these particles at the characteristic size also suggests that, despite the potential lysis effect of Triton X-100, EV integrity was largely unaffected. The measured EV concentrations in our samples were within a 1011 order of magnitude per mL of sputum, while previous reports had found EVs in BAL fluid and in sputum supernatant within a 106 order of magnitude per mL [20, 32].
Our study has limitations, including the small number of children treated with oral antibiotic therapy for PEx. The smaller sample size may be responsible for the lack of statistical significance in the changes of EV content with treatment in this group, as numerical changes in NE/FLOT1 and MPO/FLOT1 ratios were similar to the changes in the IV treatment group. The small sample size may also, at least in part, explain the lack of correlations between biomarkers in some of the other subgroups. Furthermore, we analysed the EV protein expression of only three biomarker candidates (NE, MPO and IL-8), and expression of IL-8 could not be demonstrated in EVs.
The absence of a strong correlation between NE and MPO EV content is interesting and calls for an unbiased, more versatile characterisation of EV cargo with, for instance, proteomics approaches, as CF airway EVs may contain additional proteins with biomarker potential for monitoring CF lung disease severity. As MPO, unlike NE, is also expressed in monocytes and macrophages, the MPO-expressing EVs may represent a different subpopulation than those EVs carrying NE. Biomarker studies utilising biobanked sputum samples have limitations in the current era, and with the reduced number of pwCF able to provide a sputum sample following the introduction of effective CFTR therapies, future EV studies should focus on alternative biomaterials including blood and urine, as EVs have been identified in those body fluids as well [38, 39].
In conclusion, using a protocol for processing of sputum homogenates allows for isolation and characterisation of CF airway EVs. The observed increases in the neutrophil-derived enzymes MPO and NE within the EVs with therapy for PEx and the strong correlation of improvement in lung function and NE EV content with therapy for CF PEx suggest that EV cargo has the potential to serve as a biomarker to monitor CF lung disease severity and treatment responses.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00547-2024.SUPPLEMENT (127.3KB, pdf)
Supplementary figure 00547-2024.SUPPLEMENT (43.1KB, jpg)
Footnotes
Provenance: Submitted article, peer reviewed.
Ethics statement: The studies were approved by the Research Ethics Board (REB) at the Hospital for Sick Children and collective approval for the analyses performed was obtained (REB# 1000064004).
Conflict of interest: F. Ratjen reports receiving consulting fees from Vertex Pharmaceuticals outside the submitted work.
Conflict of interest: The remaining authors have nothing to disclose.
Support statement: Funded by Cystic Fibrosis Canada. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Grasemann H, Ratjen F. Cystic fibrosis. N Engl J Med 2023; 389: 1693–1707. doi: 10.1056/NEJMra2216474 [DOI] [PubMed] [Google Scholar]
- 2.Sly PD, Brennan S, Gangell C, et al. Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med 2009; 180: 146–152. doi: 10.1164/rccm.200901-0069OC [DOI] [PubMed] [Google Scholar]
- 3.Sagel SD, Wagner BD, Anthony MM, et al. Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am J Respir Crit Care Med 2012; 186: 857–865. doi: 10.1164/rccm.201203-0507OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pham CT. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol 2006; 6: 541–550. doi: 10.1038/nri1841 [DOI] [PubMed] [Google Scholar]
- 5.Kim JS, Okamoto K, Rubin BK. Pulmonary function is negatively correlated with sputum inflammatory markers and cough clearability in subjects with cystic fibrosis but not those with chronic bronchitis. Chest 2006; 129: 1148–1154. doi: 10.1378/chest.129.5.1148 [DOI] [PubMed] [Google Scholar]
- 6.Hamblett N, Aitken ML, Accurso FJ, et al. Association between pulmonary function and sputum biomarkers in cystic fibrosis. Am J Respir Crit Care Med 2007; 175: 822–828. doi: 10.1164/rccm.200609-1354OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waters VJ, Stanojevic S, Sonneveld N, et al. Factors associated with response to treatment of pulmonary exacerbations in cystic fibrosis patients. J Cyst Fibros 2015; 14: 755–762. doi: 10.1016/j.jcf.2015.01.007 [DOI] [PubMed] [Google Scholar]
- 8.Witko-Sarsat V, Delacourt C, Rabier D, et al. Neutrophil-derived long-lived oxidants in cystic fibrosis sputum. Am J Respir Crit Care Med 1995; 152: 1910–1916. doi: 10.1164/ajrccm.152.6.8520754 [DOI] [PubMed] [Google Scholar]
- 9.Van Der Vliet A, Nguyen MN, Shigenaga MK, et al. Myeloperoxidase and protein oxidation in cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 2000; 279: L537–L546. doi: 10.1152/ajplung.2000.279.3.L537 [DOI] [PubMed] [Google Scholar]
- 10.Grasemann H, Klingel M, Avolio J, et al. Long-term effect of CFTR modulator therapy on airway nitric oxide. Eur Respir J 2020; 55: 1901113. doi: 10.1183/13993003.01113-2019 [DOI] [PubMed] [Google Scholar]
- 11.Sloane AJ, Lindner RA, Prasad SS, et al. Proteomic analysis of sputum from adults and children with cystic fibrosis and from control subjects. Am J Respir Crit Care Med 2005; 172: 1416–1426. doi: 10.1164/rccm.200409-1215OC [DOI] [PubMed] [Google Scholar]
- 12.Lepissier A, Addy C, Hayes K, et al. Inflammation biomarkers in sputum for clinical trials in cystic fibrosis: current understanding and gaps in knowledge. J Cyst Fibros 2022; 21: 691–706. doi: 10.1016/j.jcf.2021.10.009 [DOI] [PubMed] [Google Scholar]
- 13.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 2020; 367: eaau6977. doi: 10.1126/science.aau6977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 2018; 19: 213–228. doi: 10.1038/nrm.2017.125 [DOI] [PubMed] [Google Scholar]
- 15.Welsh JA, Goberdhan DCI, O'Driscoll L, et al. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J Extracell Vesicles 2024; 13: e12404. doi: 10.1002/jev2.12404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trappe A, Donnelly SC, McNally P, et al. Role of extracellular vesicles in chronic lung disease. Thorax 2021; 76: 1047–1056. doi: 10.1136/thoraxjnl-2020-216370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alhamwe BA, Potaczek DP, Miethe S, et al. Extracellular vesicles and asthma-more than just a co-existence. Int J Mol Sci 2021; 22: 4984. doi: 10.3390/ijms22094984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genschmer KR, Russell DW, Lal C, et al. Activated PMN exosomes: pathogenic entities causing matrix destruction and disease in the lung. Cell 2019; 176: 113–126. doi: 10.1016/j.cell.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rollet-Cohen V, Bourderioux M, Lipecka J, et al. Comparative proteomics of respiratory exosomes in cystic fibrosis, primary ciliary dyskinesia and asthma. J Proteomics 2018; 185: 1–7. doi: 10.1016/j.jprot.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 20.Useckaite Z, Ward MP, Trappe A, et al. Increased extracellular vesicles mediate inflammatory signalling in cystic fibrosis. Thorax 2020; 75: 449–458. doi: 10.1136/thoraxjnl-2019-214027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porro C, Lepore S, Trotta T, et al. Isolation and characterization of microparticles in sputum from cystic fibrosis patients. Respir Res 2010; 11: 94. doi: 10.1186/1465-9921-11-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forrest OA, Dobosh B, Ingersoll SA, et al. Neutrophil-derived extracellular vesicles promote feed-forward inflammasome signaling in cystic fibrosis airways. J Leukoc Biol 2022; 112: 707–716. doi: 10.1002/JLB.3AB0321-149R [DOI] [PubMed] [Google Scholar]
- 23.Stachowiak Z, Wojsyk-Banaszak I, Jończyk-Potoczna K, et al. Extracellular vesicles-derived miRNAs as mediators of pulmonary exacerbation in pediatric cystic fibrosis. J Breath Res 2023; 17: 026005. doi: 10.1088/1752-7163/acb792 [DOI] [PubMed] [Google Scholar]
- 24.Sonneveld N, Stanojevic S, Amin R, et al. Lung clearance index in cystic fibrosis subjects treated for pulmonary exacerbations. Eur Respir J 2015; 46: 1055–1064. doi: 10.1183/09031936.00211914 [DOI] [PubMed] [Google Scholar]
- 25.Rayment JH, Couch MJ, McDonald N, et al. Hyperpolarised 129Xe magnetic resonance imaging to monitor treatment response in children with cystic fibrosis. Eur Respir J 2019; 53: 1802188. doi: 10.1183/13993003.02188-2018 [DOI] [PubMed] [Google Scholar]
- 26.Grasemann H, Ciet P, Amin R, et al. Changes in magnetic resonance imaging scores and ventilation inhomogeneity in children with cystic fibrosis pulmonary exacerbations. Eur Respir J 2017; 50: 1700244. doi: 10.1183/13993003.00244-2017 [DOI] [PubMed] [Google Scholar]
- 27.Ratjen F, Waters V, Klingel M, et al. Changes in airway inflammation during pulmonary exacerbations in patients with cystic fibrosis and primary ciliary dyskinesia. Eur Respir J 2016; 47: 829–836. doi: 10.1183/13993003.01390-2015 [DOI] [PubMed] [Google Scholar]
- 28.Quanjer PH, Brazzale DJ, Boros PW, et al. Implications of adopting the Global Lungs Initiative 2012 all-age reference equations for spirometry. Eur Respir J 2013; 42: 1046–1054. doi: 10.1183/09031936.00195512 [DOI] [PubMed] [Google Scholar]
- 29.Perrem L, Stanojevic S, Shaw M, et al. Lung clearance index to track acute respiratory events in school-age children with cystic fibrosis. Am J Respir Crit Care Med 2021; 203: 977–986. doi: 10.1164/rccm.202006-2433OC [DOI] [PubMed] [Google Scholar]
- 30.Sagel SD, Kapsner R, Osberg I, et al. Airway inflammation in children with cystic fibrosis and healthy children assessed by sputum induction. Am J Respir Crit Care Med 2001; 164: 1425–1431. doi: 10.1164/ajrccm.164.8.2104075 [DOI] [PubMed] [Google Scholar]
- 31.Koeppen K, Nymon A, Barnaby R, et al. CF monocyte-derived macrophages have an attenuated response to extracellular vesicles secreted by airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2021; 320: L530–L544. doi: 10.1152/ajplung.00621.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szul T, Bratcher PE, Fraser KB, et al. Toll-like receptor 4 engagement mediates prolyl endopeptidase release from airway epithelia via exosomes. Am J Respir Cell Mol Biol 2016; 54: 359–369. doi: 10.1165/rcmb.2015-0108OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zulueta A, Peli V, Dei Cas M, et al. Inflammatory role of extracellular sphingolipids in cystic fibrosis. Int J Biochem Cell Biol 2019; 116: 105622. doi: 10.1016/j.biocel.2019.105622 [DOI] [PubMed] [Google Scholar]
- 34.Porro C, Di Gioia S, Trotta T, et al. Pro-inflammatory effect of cystic fibrosis sputum microparticles in the murine lung. J Cyst Fibros 2013; 12: 721–728. doi: 10.1016/j.jcf.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 35.Madison MC, Margaroli C, Genschmer KR, et al. Protease-armed, pathogenic extracellular vesicles link smoking and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2023; 208: 1115–1125. doi: 10.1164/rccm.202303-0471OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ordonez CL, Henig NR, Mayer-Hamblett N, et al. Inflammatory and microbiologic markers in induced sputum after intravenous antibiotics in cystic fibrosis. Am J Respir Crit Care Med 2003; 168: 1471–1475. doi: 10.1164/rccm.200306-731OC [DOI] [PubMed] [Google Scholar]
- 37.Hagner M, Frey DL, Guerra M, et al. New method for rapid and dynamic quantification of elastase activity on sputum neutrophils from patients with cystic fibrosis using flow cytometry. Eur Respir J 2020; 55: 1902355. doi: 10.1183/13993003.02355-2019 [DOI] [PubMed] [Google Scholar]
- 38.Gauthier S, Pranke I, Jung V, et al. Urinary exosomes of patients with cystic fibrosis unravel CFTR-related renal disease. Int J Mol Sci 2020; 21: 6625. doi: 10.3390/ijms21186625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trappe A, Lakkappa N, Carter S, et al. Investigating serum extracellular vesicles in cystic fibrosis. J Cyst Fibros 2023; 22: 674–679. doi: 10.1016/j.jcf.2023.02.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00547-2024.SUPPLEMENT (127.3KB, pdf)
Supplementary figure 00547-2024.SUPPLEMENT (43.1KB, jpg)