Abstract
This research aims to reassess women's risk of venous thromboembolism (VTE) events. We conducted an in-depth analysis of the environmental risk factors associated with VTE and their interactions with gender while also exploring the genetic underpinnings of the disease. VTE is identified as a multifactorial condition influenced by a combination of genetic, non-predisposing, and predisposing environmental factors. We further investigated the genetic basis of VTE, focusing on the identification and analysis of risk loci, as well as gene interaction networks and genetic analyses, which offer significant insights into the pathogenesis of VTE. Recognizing the critical role of gender in assessing VTE risk and developing prevention strategies, this research underscores the necessity of adopting an integrated perspective that accounts for individual vulnerabilities at both genetic and environmental levels to formulate effective preventive measures.
Keywords: venous thromboembolism, sex difference, VTE risk factors, VTE in women, VTE genetic analysis
Introduction
Venous thromboembolism (VTE) is a multifactorial disease whose pathogenesis is influenced by the interplay of various risk factors. 1 These factors can be categorized as hereditary (eg, genetic mutations), non-predisposing (eg, age, gender, race/ethnicity, body mass index [BMI] and obesity, oral contraceptive [OC] use, hormone therapy, statin use, dietary habits, physical activity vs sedentary behaviors, cigarette smoking, and air pollution), and predisposing environmental factors (eg, cancer, surgery, trauma or fracture, immobilization, pregnancy vs the postpartum period, long-distance travel, hospitalization, catheterization, and acute infections). 2 It is noteworthy that while the overall incidence of VTE is comparable between men and women, certain risk factors are more pronounced in the female population, thereby placing women at a relatively increased lifetime risk of developing VTE. 3 These specific risk factors include but are not limited to gynecologic neoplasms, breast cancer, the use of oral contraceptives, obesity, and physiological changes associated with pregnancy and the puerperium, as well as COVID-19 infection. 4 These risk factors do not operate in isolation; rather, there are complex interactions and synergistic effects among them that further increase the risk of VTE.5,6 This insight not only enhances our understanding of the pathogenesis of VTE but also underscores the importance of adopting an integrative perspective that considers individual vulnerabilities at both the genetic and environmental levels in the development of risk assessment and prevention strategies. This article examines the risk factors associated with VTE by analyzing their interrelationships. By focusing on sex differences in environmental risk factors, genetics, and biomarkers, it offers new perspectives and insights that enhance our understanding of the sex-specific nature of deep vein thrombosis (DVT) risk factors. However, due to the complexity of DVT pathogenesis, while not attempting to encompass all potential influences.
Summary of the Environmental Risk Factors for VTE
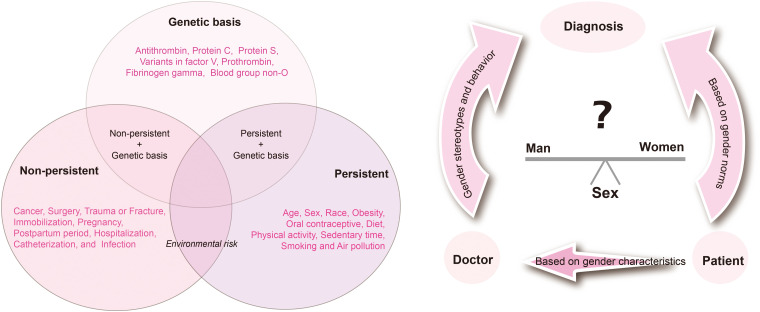
The environmental risk factors for venous thromboembolism (VTE) can be categorized into two broad groups: persistent and non-persistent. As shown in Figure 1, the non-persistent factors encompass a variety of events or conditions that significantly contribute to the development of VTE; conversely, the persistent factors pertain to inherent or cumulative characteristics of the individual. Importantly, gender differences represent a critical dimension that cannot be overlooked in clinical research, and they are challenging to modify through external interventions; these differences profoundly influence the mechanisms and manifestations of VTE. 7 Biologically, males and females exhibit differences not only in the reproductive system but also at the organ and cellular levels, which are evident in anatomical structures, physiological functions, biochemical processes, pathophysiological responses, and even disease outcomes. 8
Figure 1.
Environmental and genetic risk factors for venous thromboembolism.
The incidence of venous thromboembolism (VTE) across the lifespan demonstrates significant gender differences. 9 Although the overall trend indicates a relatively higher incidence in males, there remains an academic debate regarding whether males are inherently more susceptible to VTE than females. Notably, in younger age groups, this pattern is reversed, with females exhibiting a slightly higher annual incidence of VTE. This phenomenon can be attributed to the hormonal environment specific to females of childbearing age, including factors such as pregnancy, the postpartum recovery phase, and the use of birth control pills, all of which influence blood coagulation mechanisms. 10 As individuals reach middle age, the incidence of VTE increases significantly faster in men than in women. This shift may involve a complex interplay of physiological and pathological mechanisms.
The next section focuses on the central variable of gender, with an analysis of its impact on the VTE risk environment. By adopting a gender perspective, we aim to elucidate the unique and common aspects of VTE risk factors, pathogenesis, and prevention strategies across different genders, thereby providing a scientific foundation for the development of more precise and personalized VTE prevention and control measures. (Figure 1)
Environmental Risk Factors, According to Gender
Gender plays a significant role in various health conditions and medical interventions, with complex and multifaceted influences. This review examines the multidimensional impact of gender on the environmental risk factors associated with venous thromboembolism (VTE) and, based on this analysis, categorizes these risk factors into three primary groups. This categorization not only underscores the importance of gender differences in VTE risk assessment but also equips healthcare professionals with a more nuanced perspective, enabling them to consider a patient's gender characteristics more comprehensively when developing treatment plans for improved health management.
Delving into the Nuances of Gender Differences
Cancer
The strong association between cancer and venous thromboembolism (VTE) has long garnered significant attention within the medical community. Compared to the general population, cancer patients face a markedly higher risk of developing VTE, estimated to be approximately four to seven times higher, underscoring the profound impact of cancer on hemodynamics. 11 An analysis conducted by the National Cancer Institute revealed notable gender differences in the association between cancer type and incidence: specific cancer types exhibited a ‘gender preference,’ with breast, cervical, and ovarian cancers being more prevalent among women, whereas prostate cancer is predominantly a male disease. 12 It is essential to recognize that although men and women with cancer experience similar overall incidences of VTE and the risk of bleeding, the risk of VTE and the response to anticoagulant therapy can vary significantly by cancer type. 13 In particular, female-specific cancers, such as ovarian, uterine, and most breast cancers, not only substantially elevate the risk of VTE but also represent a critical women's health issue that necessitates urgent attention due to the high prevalence and poor prognosis. 14 For instance, breast cancer patients are three to four times more likely to develop VTE compared to women without breast cancer, and this complication is strongly correlated with reduced survival rates. 15 The prevalence of venous thromboembolism (VTE) among ovarian cancer patients ranges from 5.2% to 8.1% within the first five years following diagnosis, significantly elevating the risk of mortality. 16 Recent prospective studies indicate that VTE has been identified in as many as 22.7% of patients undergoing venous ultrasound prior to anticancer treatment, with clear cell carcinoma showing a particularly high association with VTE. 17 In contrast, although prostate cancer is associated with a relatively low risk of VTE, the prevention and treatment of VTE in this patient population should not be overlooked, due to the high incidence of prostate cancer and the potential implications for patient survival. 18 Understanding cancer's complex link to VTE requires knowing the specific risks according to the cancer type and crafting tailored prevention/treatment plans. This is vital for cancers highly linked to VTE risk, especially in groups such as female patients, to lower VTE cases and improve survival quality.
Smoking and Air Pollution
Smoking increases the tendency for blood to clot, thereby elevating the incidence of venous thromboembolism (VTE). While both male and female smokers are at risk, the higher prevalence of smoking among men makes it a particularly significant factor in VTE risk for this population. 19 Regarding the relationship between air pollution and VTE, current studies present mixed results. 20 Nonetheless, it is indisputable that air pollution poses potential harm to all populations. Notably, women may be more vulnerable to the effects of air pollution in specific contexts, such as during pregnancy or childhood. Perhaps due to physiological differences and social divisions of labor, women may experience greater exposure to polluted environments in their daily lives, such as through household chores and outdoor work, which could further increase their susceptibility to air pollution. A recent systematic review of 11 studies highlighted that, despite the inter-study heterogeneity, there is overall support for a positive association between air pollution and VTE risk. 21 This heterogeneity likely stems from the variations in the study conditions, geographic locations, pollutant types, and concentration levels. Consequently, when investigating the specific effects of air pollution on VTE risk, it is essential to consider a range of factors to achieve more comprehensive and accurate conclusions.
The Elevated Risks for Females
Oral Contraceptive use
Oral contraceptives (OCs) are a widespread method of birth control currently used by more than 150 million women, and studies have reported an increased risk of thrombotic events among OC users. Excess estrogen increases the risk of venous thromboembolism (VTE). 22 Valeria found that oral contraceptive use was associated with an increased risk of venous thromboembolism in a study of 240 000 women via the UK Biobank, with an OR: 3.09 (95% CL 3.00-3.20). 22 A 2014 systematic evaluation and meta-analysis indicated that oral contraceptives containing ethinylestradiol and levonorgestrel resulted in a 50% to 80% reduction in the risk of venous thromboembolism (VTE), compared to those containing ethinylestradiol with desogestrel, cyproterone acetate, or drospirenone. 23 Similarly, a 2024 systematic evaluation and meta-analysis assessing the risk of VTE associated with oral contraceptives (OCs) using synthetic estrogens, such as ethinylestradiol (EE), versus those utilizing natural estrogens, such as estradiol (E2), involving over 560 000 women, demonstrated that the risk of VTE was significantly reduced, by 33%, in users of natural estrogen-based COCs compared to their synthetic counterparts (OR 0.67, 95% CI 0.51-0.87). 24 In women who are predisposed to a high risk of venous thromboembolism (VTE), the use of oral contraceptives (OC) may significantly increase their already elevated risk of VTE, particularly during the initial two years of treatment. Therefore, it is essential to provide thorough and careful counseling to these women when selecting a contraceptive method, with the goal of comprehensively assessing and exploring more suitable lower-risk contraceptive options.
Pregnancy
Pregnant and postpartum women face a significantly elevated risk of venous thromboembolism (VTE) due to alterations in blood composition, reduced mobility, and the pressure exerted by an enlarged uterus.25,26 The incidence of VTE is 4–5 times higher in pregnant women and those in the postpartum period (up to 12 weeks) compared to non-pregnant women, with an overall incidence of approximately 0%–1%. 27 Women with a history of venous thromboembolism are at an increased risk of recurrence during pregnancy and the postpartum period, with recurrence rates ranging from 2% to 10%. 28 This heightened risk is attributable to the physiological changes during pregnancy that affect all three components of Virchow's triad: hypercoagulability, blood flow stagnation, and endothelial damage. 28 VTE can occur during both the prenatal and postnatal periods, with a relatively even distribution across gestation. Notably, in the peripartum period, 60% of all fatal events occur within the first two weeks following delivery, while 40% occur between the third and sixth weeks post-delivery.29,30 The risk of venous thromboembolism remains a significant concern during both pregnancy and the postpartum period. Cesarean delivery is a crucial risk factor, accounting for over 75% of deaths attributed to puerperal venous thromboembolism. 30 A meta-analysis indicated that the risk of VTE is highest in late pregnancy (43%), followed by mid-gestation (35%) and early pregnancy (25%).31,32 A large population-based study revealed that 50% of venous thromboembolisms occur during pregnancy, while the remaining 50% occur in the puerperium.6,33 However, considering that the duration of pregnancy is approximately 6 to 7 times longer than the puerperium, and that the daily incidence of VTE during the puerperium is significantly higher than during pregnancy, this underscores the notion that the puerperium represents a period of heightened risk for VTE.
Obesity and BMI
Both men and women with a high body mass index (BMI) or obesity face an increased risk of venous thromboembolism (VTE), with the risk more pronounced in women, likely due to the differences in body fat distribution and metabolism.34,35 The MEGA study, which involved 3834 women with their first VTE and 4683 controls, found that a BMI over 30 kg/m² was linked to a 2.4-fold higher risk of thrombosis, compared to those with a normal BMI. 36 In a large cohort of breast cancer patients, BMI was also identified as a key predictor of VTE, with a hazard ratio of 3.0 (95% CI 2.1-4.4).36,37 The CAVECCAS study further confirmed obesity as a significant factor in catheter-related thrombosis (CRT) in breast cancer patients. Women with a BMI of 30 kg/m² or greater, who were also using oral contraceptives, were found to have a 24-fold higher risk of VTE compared to women with a normal BMI, who were not using contraceptives (OR 23.78; 95% CI 13.35-42.34). 38 In another population-based case-control study of 454 patients, women with a BMI greater than 25 kg/m² using oral contraceptives had a tenfold higher risk of VTE. 39
Minimalizing Gender's Influence
The risk for some partial VTEs can be primarily attributed to medical risk factors and lifestyle habits, where gender differences are not a predominant factor. Medical interventions such as hospitalization, surgery, catheterization, and infection significantly elevate the risk of VTE in both men and women by disrupting normal blood circulation patterns and promoting hypercoagulability and venous stasis, which create conditions conducive to VTE occurrence.40,41 Notably, women's risk of VTE is further heightened during physiological phases associated with hormonal changes, such as pregnancy or hormone replacement therapy. 42 Hormonal fluctuations during these periods may directly influence blood clotting mechanisms, thereby increasing the risk of thrombosis. Trauma and fractures, particularly severe injuries to the lower extremities, are significant risk factors for VTE in both genders. The immobilization that follows a fracture results in reduced venous blood flow, creating an environment that favors thrombosis. 43 However, of particular concern is the increased likelihood of fractures among women, especially postmenopausal women, due to a higher incidence of osteoporosis, which indirectly elevates their risk of developing VTE. 44 Lifestyle factors, including dietary habits, sedentary behavior, and levels of physical activity, play a crucial role in the prevention of VTE. While these factors generally affect both sexes, it is also important to explore gender differences. Women may be more likely to adopt a balanced diet with nutritional diversity, which may contribute to maintaining vascular health and reducing the risk of VTE.45,46 Men may prioritize intense physical activity; however, neglecting rest and recovery can exacerbate venous pressure and contribute to the development of venous thromboembolism (VTE). Consequently, individual gender characteristics and lifestyle habits must be thoroughly considered when formulating personalized prevention strategies. While the impact of medical risk factors and unhealthy lifestyle choices on the risk of VTE is evident in both genders, the risk is particularly pronounced in women under specific circumstances, such as during pregnancy, hormone therapy, and following osteoporosis-related fractures. This highlights the necessity of implementing targeted prevention strategies tailored to different genders.
Genetic Evidence: sex Differences in VTE
Genetic Basis
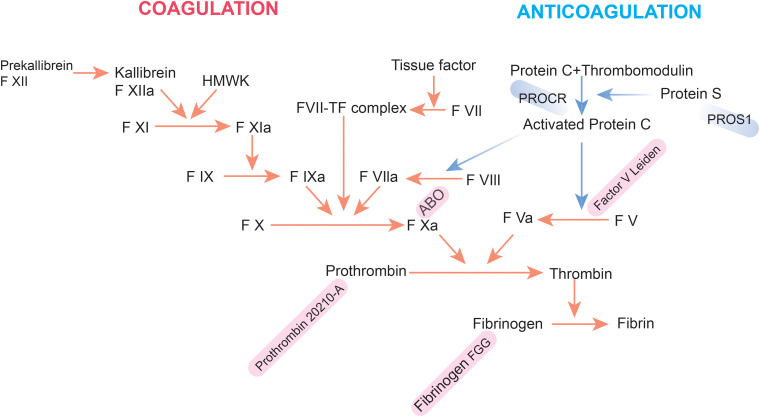
Venous thromboembolism (VTE) is inherently multifactorial, with its etiology differing from that of arterial thrombosis. The risk of VTE arises from a combination of genetic predispositions and environmental factors, with approximately 50%–60% of VTE cases attributable to genetic influences. 47 Heit's study highlights a significant genetic basis for VTE, suggesting either a multifactorial or non-Mendelian model of inheritance, as individuals with siblings who have had a VTE have a 2.5-fold increased risk of VTE compared to the general population. 48 Furthermore, the role of genetic factors in VTE risk is reinforced by Reitsma's research, which found that identical twins exhibit a higher risk of VTE than dizygotic twins. 49 Most of the identified genetic risk factors are associated with mutations in the clotting system (Figure 2). 2 Mutations in genes related to natural anticoagulants—specifically antithrombin, protein C, and protein S—are well-established contributors to an increased risk of venous thromboembolism (VTE). These mutations typically occur in the SERPINC1, PROC, and PROS1 genes. In addition to mutations in natural anticoagulant genes, genetic variations in genes encoding coagulation factors, such as variants in factor V, prothrombin, fibrinogen gamma, and blood group non-O, also play a significant role in influencing VTE risk. Genome-wide association studies (GWAS) have greatly enhanced the identification of genetic loci associated with VTE. A major study involving over 30 000 VTE cases identified 34 independent genetic variations linked to VTE, including 11 novel loci, although some previously identified loci did not achieve statistical significance in this analysis.50,51 Although numerous single nucleotide polymorphisms (SNPs) contribute to an individual's susceptibility to venous thrombosis (VT), research has identified five SNPs that are most strongly associated with this condition. These include rs6025 (Leiden mutation) in the F5 gene, rs1799963 (prothrombin G20210A) in the coagulation factor 2 gene (F2), rs8176719 (non-O blood type) in the ABO gene, rs2036914 in the coagulation factor eleven gene (F11), and rs2066865 in the fibrinogen gamma gene (FGG). Collectively, these SNPs play a significant role in determining the incidence and recurrence of VT in genetically predisposed individuals (Table 1). Among the most well-characterized genetic variants related to VTE is Factor V Leiden (FVL), a mutation in the F5 gene (F5-rs6025, R506Q) that confers resistance on Factor V to degradation by activated protein C. 52 FVL is found in approximately 5% of individuals of European descent, increasing their risk of VTE about threefold. This mutation is part of the intrinsic coagulation pathway and leads to resistance to activated protein C (Figure 2). While the specific genetic variants significantly influence the risk of venous thromboembolism (VTE), it is essential to recognize that VTE is a complex disease characterized by the interplay of multiple factors. Consequently, evaluating an individual's risk of developing VTE necessitates a thorough consideration of both the genetic background and environmental influences. Previous studies have demonstrated that the risk of VTE is highest when genetic predisposition is coupled with environmental risk factors.53–55
Figure 2.
Coagulation–anticoagulation schematic.
Table 1.
Five SNP Mutations Predominant in Venous Thromboembolism.
| Reference | Candidate Gene | Major Mutations | Odds Ratio | 95% CI |
|---|---|---|---|---|
| Horvei 56 | Variants in factor V | rs6025 | 2.38 | 1.63–3.45 |
| Bruzelius 57 | Prothrombin | rs1799963 | 1.86 | 1.27–2.73 |
| Zhang 58 | Fibrinogen gamma | rs2066865 | 1.41 | 1.25–1.58 |
| Louisa 59 | Blood group non-O | rs8176719 | 1.78 | 1.67–1.92 |
| Li 60 | Factor XI | rs2036914 | 1.33 | 1.11–1.59 |
Sex Differences in the Genetic Basis
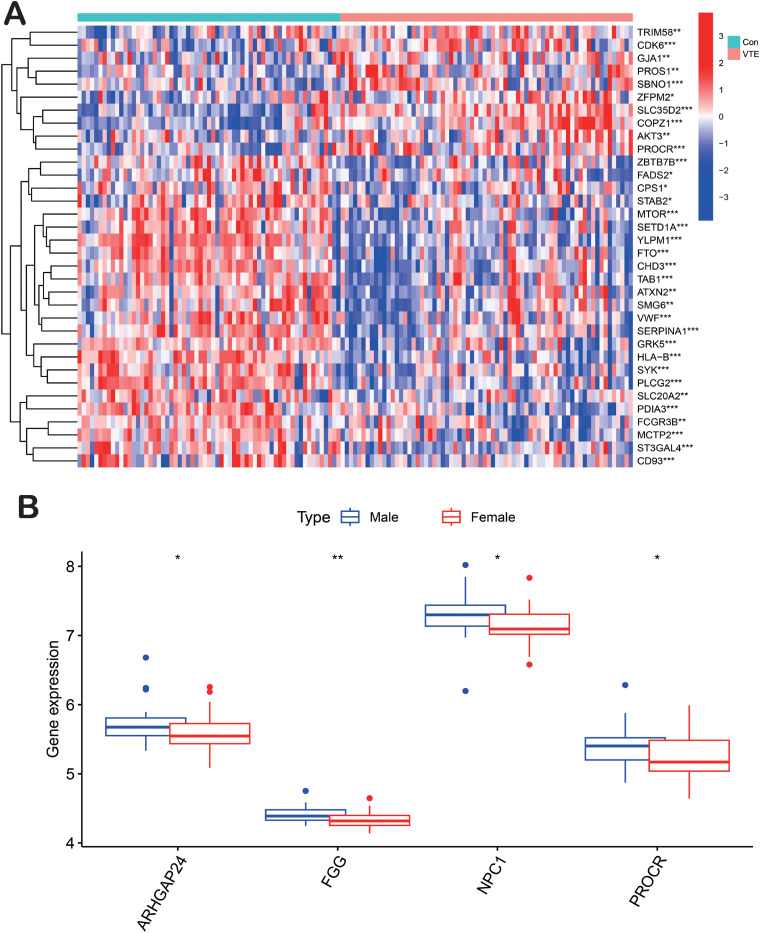
With RNA-sequencing data becoming increasingly publicly available, bioinformatics is increasing in importance in clinical research. Despite the unclear differences in the molecular mechanisms of venous thromboembolism (VTE) between genders, we aimed to identify differentially expressed genes (DEGs) and potential molecular pathways through bioinformatics analysis. The GSE19151 dataset was sourced in 2011 from the public Gene Expression Omnibus (GEO) database at the National Center for Biotechnology Information (NCBI) (www.ncbi.nlm.nih.gov/geo). The microarray data of GSE19151 platform, based on the GPL571 platform ([HG-U133A_2] Affymetrix Human Genome U133A 2.0 Array), and series matrix file(s) were downloaded from the GEO and saved as TXT files. R software (version 4.2.1) was utilized to process the downloaded data. The samples were categorized into two groups: Group 1 comprised 70 adult blood samples from VTE patients and 63 blood samples from healthy controls. Group 2 included 28 blood samples from men with VTE and 44 blood specimens from women with VTE. We selected a genome-wide meta-analysis that identified 93 novel VTE risk loci, 81 190 cases from six cohorts, and 1,419,671 controls of European ancestry. Box and heat map plots were generated to illustrate the up- and down-regulated DEGs between the two cohorts, with adjusted p-values of <.05 and a fold change (logFC) of >1 considered to indicate DEGs. A total of 34 DEGs were identified between the cohort of normal and VTE samples (Figure 3A), consistent with risk-prediction analyses for monogenic venous thromboembolism. In the second group of male and female VTE samples, four DEGs were identified. The functions of these four key genes and the KEGG pathway are shown in Table 2 (Figure 3B). The FGG-encoded protein represents the gamma component of fibrinogen, which is cleaved by thrombin to form fibrin following vascular injury, and it is the most abundant constituent of blood clots.61,62 Additionally, fibrinogen and various cleavage products of fibrin play crucial roles in regulating cell adhesion and spreading, exhibit vasoconstrictor and chemotactic activities, and act as mitogens for several cell types. Mutations in this gene lead to a range of disorders, including abnormal fibrinogenemia, hypofibrinogenemia, and thrombotic tendencies. 63 The protein encoded by the PROCR gene functions as a receptor for activated protein C, a serine protease that is activated during the coagulation pathway. Related pathways encompass hemostatic disorders and responses to elevated platelet cytoplasmic Ca2 + . Mutations in this gene have been linked to venous thromboembolism, myocardial infarction, and fetal miscarriage during late pregnancy.64,65 ARHGAP24 primarily plays a significant role in cancer pathways, with notable associations with predominantly female high-prevalence diseases such as breast cancer and lung adenocarcinoma.66–70 NPC1 protein is essential for cholesterol transport in human cells; mutations in NPC1 can lead to abnormal cholesterol accumulation in lysosomes, potentially resulting in excessive lipid buildup in the liver, kidneys, spleen, and even the brain, causing organ lesions and possibly fatal outcomes.71–73
Figure 3.
(A) heat map plot of the cohort of normal and VTE samples. (B) Box plot of the male and female VTE samples. Heat map: remarkable DEGs according to the adjusted p-value and logFC. Red indicates a higher gene expression, and green indicates a lower gene expression. ** p < .01, and * p < .05.
Table 2.
Functions of Four key Genes and the KEGG Pathway.
| Genes | Location | Function | Super Pathway |
|---|---|---|---|
| FGG | chr4 | Together with fibrinogen alpha (FGA) and fibrinogen beta (FGB), it polymerizes to form an insoluble fibrin matrix. It has a major function in hemostasis, as one of the primary components of blood clots. | Signaling downstream of RAS mutants |
| Toll Like Receptor 3 (TLR3) Cascade | |||
| Response to elevated platelet cytosolic Ca2+ | |||
| MAPK family signaling cascades | |||
| Diseases of the immune system | |||
| PROCR | chr20 | It binds activated protein C and enhances protein C activation via the thrombin–thrombomodulin complex. | Diseases of hemostasis |
| Response to elevated platelet cytosolic Ca2+ | |||
| Cell surface interactions at the vascular wall | |||
| NPC1 | chr18 | It is an intracellular cholesterol transporter, which acts in concert with NPC2 and plays an important role in the egress of cholesterol from the endosomal. | Plasma lipoprotein assembly |
| Cholesterol and sphingolipids transport | |||
| Transport of inorganic cations/anions | |||
| Degradation pathway of sphingolipids | |||
| ARHGAP24 | chr4 | It is an Rho GTPase-activating protein involved in cell polarity, cell morphology, and cytoskeletal organization. | Signaling via Rho GTPases |
| RHOC GTPase cycle | |||
| Signal transduction |
Sex Differences in Biomarkers
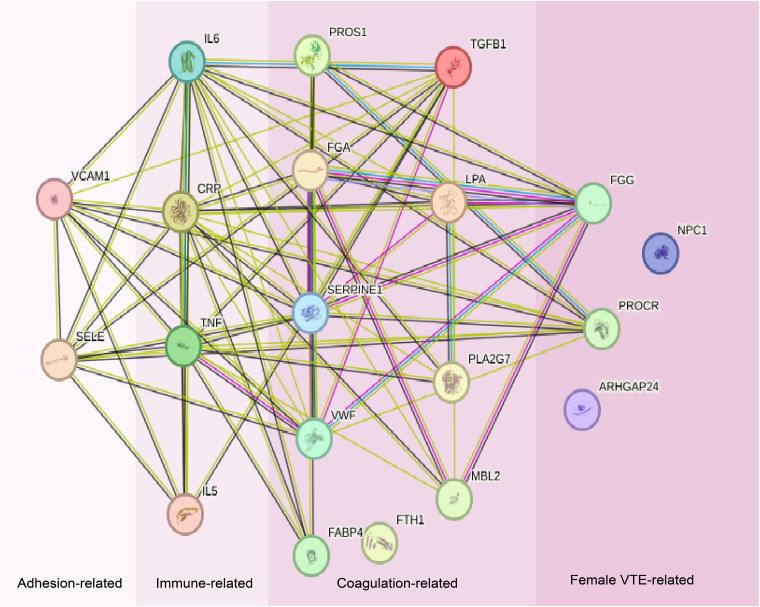
Georg et al searched for and classified blood protein biomarkers into three main categories: inflammation, coagulation and adhesion. 74 These categories were further subdivided into immunity-associated, coagulation-associated, and adhesion-associated categories, following the classification framework established by Lehmann et al 75 The STRING database (https://cn.string-db.org/) is a precomputed worldwide resource for the exploration and analysis of interactions between known and predicted protein–protein interactions. To enhance our understanding of gender differences in venous thromboembolism (VTE), we integrated proteins encoded by genes that were differentially expressed between the male and female VTE patient samples, as analyzed through advanced bioinformatics methods. Utilizing the STRING database, a robust resource, we constructed a functional interaction network that illustrates the intricate connections among these proteins and encompasses the most known protein biomarkers. Within the complex network generated by STRING, we identified several key biological processes crucial for elucidating the sex-specific mechanisms of VTE. These processes include but are not limited to the negative regulation of vascular wound healing, vascular endothelial growth factor (VEGF) production, opsonization, fibrinolysis, positive regulation of heterotypic cell–cell adhesion, and signaling receptor binding, which is a core molecular function. Collectively, these biological processes provide a nuanced understanding of the pathophysiology of VTE (Figure 4). Particularly striking is the differential expression of two proteins, fibrinogen gamma chain (FGG) and prothrombin kinase receptor (PROCR), in female patients with venous thromboembolism (VTE). These proteins are not only significantly involved in several key processes described above, but they also highlight the unique pathogenesis of VTE in women. These findings underscore the complexity and multilayered nature of the physiological responses in women at risk for VTE. In contrast, the risk of VTE in men, while present, is more closely associated with direct factors such as trauma and surgery, which exhibit relatively straightforward mechanisms of influence. In women, VTE risk assessment must consider additional variables, including cyclical fluctuations in hormone levels and the intricate regulatory mechanisms of various genes associated with thrombosis. These factors collectively shape the susceptibility and distinctiveness of women in terms of the development of VTE. Therefore, in the prevention and treatment of VTE, it is crucial to fully account for gender differences and to develop more individualized and refined strategies to achieve optimal clinical outcomes.
Figure 4.
STRING network of the proteins. Using the default parameters of the STRING web interface, the layout considers the node connectivity.
Summary
This study delved into the multifactorial nature of venous thromboembolism (VTE) by examining various hereditary and environmental factors in the context of gender differences. It identified several non-persistent factors (eg, trauma, long-distance travel, pregnancy) and persistent factors (eg, genetic predispositions, obesity), emphasizing that these factors often interacted synergistically, further increasing the risk of VTE. Significant gender differences in risk factors were observed, with a tendency for higher prevalence among women in particular. For instance, while men generally had a higher lifetime incidence, young women displayed a higher risk due to specific factors like pregnancy, oral contraceptives, and certain cancers. In-depth analysis revealed that conditions such as obesity and lifestyle factors like smoking and air pollution presented differential impacts on VTE risk across genders. Genetic analysis underscored a significant hereditary component in VTE, with key genes such as F5, F2, F11, and PROCR contributing to the risk. Additionally, bioinformatics analysis of differentially expressed genes (DEGs) showed gender-specific molecular pathways in VTE, with certain biomarkers (eg, FGG and PROCR) particularly prominent in women, suggesting distinct biological processes contributing to VTE pathogenesis. Biomarkers also highlighted pathways such as inflammation and coagulation, with unique regulatory mechanisms in women linked to hormonal cycles and other physiological characteristics.
In conclusion, VTE risk was influenced by a complex interplay of genetic, environmental, and gender-specific factors, with significant implications for personalized risk assessment and prevention strategies. Gender differences were pronounced, with women experiencing increased VTE risk in the context of hormonal factors, cancer types, and specific environmental exposures.
Limitations
Advances in the field of genetics related to venous thromboembolism (VTE) have generated new optimism regarding the application of genomic predictors in clinical practice. However, similar to other biomarkers, the implementation of genomic predictors in clinical settings encounters several challenges that extend beyond the mere discovery of genetic associations. For genetic tests to effectively replace existing clinical risk predictors in real-world applications, they must significantly enhance their prognostic accuracy and demonstrate clear clinical benefits.
A more integrated approach that combines environmental risk factors, biological data, and multidisciplinary genomic analyses should be considered for future research. In particular, it is essential to emphasize gender differences to better evaluate the risk of women for the development of VTE and the prognostic impact on complication rates. This comprehensive strategy is anticipated to further enhance risk assessment and prevention efforts related to VTE.
Acknowledgment
None
Footnotes
Authors’ Contributions: C.H. and J.C. analyzed and interpreted the raw data and prepared the draft. Y.L., Y.D. and K.X. interpreted the gene analysis data and reviewed this manuscript. C.H. and C.C. were the major contributors in writing the manuscript. All authors reviewed and approved the manuscript.
Availability of Data and Materials: The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics and Patient Consent: We confirm that Ethical Committee approval was sought where necessary and is acknowledged within the text of the submitted manuscript.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China, (grant number NO. 82470776, NO.82000729).
ORCID iDs: Yiqing Li https://orcid.org/0000-0002-7036-717X
Chuanqi Cai https://orcid.org/0000-0001-9182-5478
References
- 1.Guntupalli SR, Spinosa D, Wethington S, Eskander R, Khorana AA. Prevention of venous thromboembolism in patients with cancer. Br Med J. 2023 Jun 1;381:e072715. doi: 10.1136/bmj-2022-072715 [DOI] [PubMed] [Google Scholar]
- 2.Crous-Bou M, Harrington LB, Kabrhel C. Environmental and genetic risk factors associated with venous thromboembolism. Semin Thromb Hemost. 2016;42(08):808–820. doi: 10.1055/s-0036-1592333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keller K, Rappold L, Gerhold-Ay Aet al. Sex-specific differences in pulmonary embolism. Thromb Res. 2019 Jun; 178:173–181. doi: 10.1016/j.thromres.2019.04.020 [DOI] [PubMed] [Google Scholar]
- 4.Cai C, Guo Y, You Y, et al. Deep venous thrombosis in COVID-19 patients: A cohort analysis. Clin Appl Thromb/Hemost. 2020 Jan–Dec;26:1076029620982669. doi: 10.1177/1076029620982669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glise Sandblad K, Hansson P-O, Philipson Jet al. et al. Prevalence of cancer in patients with venous thromboembolism: A retrospective nationwide case-control study in Sweden. Clin Appl Thromb Hemost. 2023 Jan–Dec;29:10760296231158368. doi: 10.1177/10760296231158368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fatokun TB, Swartz SE, Ebeid Aet al. et al. Venous thromboembolism risk factors in women with obesity who undergo cesarean delivery. Clin Appl Thromb Hemost. 2024 Jan–Dec;30:10760296241247203. doi: 10.1177/10760296241247203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bewley S, McCartney M, Meads C, Rogers A. Sex, gender, and medical data. Br Med J. 2021 Mar 19;372:n735. doi: 10.1136/bmj.n735 [DOI] [PubMed] [Google Scholar]
- 8.Schiebinger L. Sex, gender, and intersectional puzzles in health and biomedicine research. Med. 2022;3(5):284–287. 10.1016/j.medj.2022.04.003 [DOI] [PubMed] [Google Scholar]
- 9.Nimjee SM, Akhter AS, Zakeri A, Herson PS. Sex differences in thrombosis as it affects acute ischemic stroke. Neurobiol Dis. 2022. Apr;165:105647. doi: 10.1016/j.nbd.2022.105647 [DOI] [PubMed] [Google Scholar]
- 10.James AH. Pregnancy, contraception and venous thromboembolism (deep vein thrombosis and pulmonary embolism). Vasc Med. 2017;22(2):166–169. doi: 10.1177/1358863x17690601 [DOI] [PubMed] [Google Scholar]
- 11.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712–1723. 10.1182/blood-2013-04-460121 [DOI] [PubMed] [Google Scholar]
- 12.Jackson SS, Marks MA, Katki HAet al. Sex disparities in the incidence of 21 cancer types: Quantification of the contribution of risk factors. Cancer. 2022;128(19):3531–3540. doi: 10.1002/cncr.34390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roach RE, Lijfering WM, Rosendaal FR, Cannegieter SC, le Cessie S. Sex difference in risk of second but not of first venous thrombosis: Paradox explained. Circulation. 2014;129(1):51–56. doi: 10.1161/circulationaha.113.004768 [DOI] [PubMed] [Google Scholar]
- 14.Yasui M, Ikeda M, Miyake M, et al. Comparison of bleeding risks related to venous thromboembolism prophylaxis in laparoscopic vs open colorectal cancer surgery: A multicenter study in Japanese patients. Am J Surg. 2017;213(1):43–49. doi: 10.1016/j.amjsurg.2015.10.019 [DOI] [PubMed] [Google Scholar]
- 15.Walker AJ, Card TR, West J, Crooks C, Grainge MJ. Incidence of venous thromboembolism in patients with cancer - a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49(6):1404–1413. doi: 10.1016/j.ejca.2012.10.021 [DOI] [PubMed] [Google Scholar]
- 16.Matsuo K, Hom MS, Yabuno A, et al. Association of statins, aspirin, and venous thromboembolism in women with endometrial cancer. Gynecol Oncol. 2019;152(3):605–611. doi: 10.1016/j.ygyno.2018.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebina Y, Uchiyama M, Imafuku H, Suzuki K, Miyahara Y, Yamada H. Risk factors for deep venous thrombosis in women with ovarian cancer. Medicine (Baltimore). 2018;97(23):e11009. doi: 10.1097/md.0000000000011009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abreu SC, Tavares V, Carneiro F, Medeiros R. Venous thromboembolism and prostate cancer: What about genetic markers? Pharmacogenomics. 2021;22(6):365–373. doi: 10.2217/pgs-2020-0094 [DOI] [PubMed] [Google Scholar]
- 19.Pastori D, Cormaci VM, Marucci S, et al. A Comprehensive Review of Risk Factors for Venous Thromboembolism: From Epidemiology to Pathophysiology. Int J Mol Sci. 2023. Feb 5;24(4):3169. doi: 10.3390/ijms24043169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pun VC, Hart JE, Kabrhel C, Camargo CA, Jr., Baccarelli AA, Laden F. Prospective study of ambient particulate matter exposure and risk of pulmonary embolism in the Nurses’ health study cohort. Environ Health Perspect. 2015;123(12):1265–1270. doi: 10.1289/ehp.1408927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franchini M, Mengoli C, Cruciani M, Bonfanti C, Mannucci PM. Association between particulate air pollution and venous thromboembolism: A systematic literature review. Eur J Intern Med. 2016 Jan;27:10–13. doi: 10.1016/j.ejim.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 22.Lo Faro V, Johansson T, Johansson Å. The risk of venous thromboembolism in oral contraceptive users: The role of genetic factors-a prospective cohort study of 240,000 women in the UK biobank. Am J Obstet Gynecol. 2024. Mar;230(3):360.e361–360.e313. doi: 10.1016/j.ajog.2023.09.012 [DOI] [PubMed] [Google Scholar]
- 23.de Bastos M, Stegeman BH, Rosendaal FRet al. Combined oral contraceptives: Venous thrombosis. Cochrane Database Syst Rev. 2014. Mar 3;2014(3):Cd010813. doi: 10.1002/14651858.CD010813.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Douxfils J, Raskin L, Didembourg Met al. et al. Are natural estrogens used in contraception at lower risk of venous thromboembolism than synthetic ones? A systematic literature review and meta-analysis. Front Endocrinol (Lausanne). 2024. Aug 16;15:1428597. doi: 10.3389/fendo.2024.1428597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nichols KM, Henkin S, Creager MA. Venous thromboembolism associated with pregnancy: JACC focus seminar. J Am Coll Cardiol. 2020;76(18):2128–2141. doi: 10.1016/j.jacc.2020.06.090 [DOI] [PubMed] [Google Scholar]
- 26.Antic D, Lefkou E, Otasevic V, et al. Position paper on the management of pregnancy-associated superficial venous thrombosis. Balkan working group for prevention and treatment of venous thromboembolism. Clin Appl Thromb Hemost. 2022. Jan–Dec;28:1076029620939181. doi: 10.1177/1076029620939181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pomp ER, Lenselink AM, Rosendaal FR, Doggen CJM. Pregnancy, the postpartum period and prothrombotic defects: Risk of venous thrombosis in the MEGA study. J Thromb Haemostasis. 2008;6(4):632–637. 10.1111/j.1538-7836.2008.02921.x [DOI] [PubMed] [Google Scholar]
- 28.Bourjeily G, Paidas M, Khalil H, Rosene-Montella K, Rodger M. Pulmonary embolism in pregnancy. Lancet. 2010;375(9713):500–512. 10.1016/S0140-6736(09)60996-X [DOI] [PubMed] [Google Scholar]
- 29.Greer IA. Thrombosis in pregnancy: Maternal and fetal issues. Lancet. 1999;353(9160):1258–1265. doi: 10.1016/s0140-6736(98)10265-9 [DOI] [PubMed] [Google Scholar]
- 30.Martinelli I, De Stefano V, Taioli E, Paciaroni K, Rossi E, Mannucci PM. Inherited thrombophilia and first venous thromboembolism during pregnancy and puerperium. Thromb Haemost. 2002. May;87(5):791–795. [PubMed] [Google Scholar]
- 31.James AH, Tapson VF, Goldhaber SZ. Thrombosis during pregnancy and the postpartum period. Am J Obstet Gynecol. 2005;193(1):216–219. doi: 10.1016/j.ajog.2004.11.037 [DOI] [PubMed] [Google Scholar]
- 32.Alsheef MA, Alabbad AM, Albassam RAet al. Predictors of pregnancy-associated venous thromboembolism: A case-control study. Front Cardiovasc Med. 2022. Oct 14;9:920089. doi: 10.3389/fcvm.2022.920089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaya-Bodestyne SL, Lee LH, Tan LK, et al. Risk factors for pregnancy-associated venous thromboembolism in Singapore. J Perinat Med. 2021;49(2):153–158. doi: 10.1515/jpm-2020-0298 [DOI] [PubMed] [Google Scholar]
- 34.Ten Cate V, Koeck T, Prochaska J, et al. A targeted proteomics investigation of the obesity paradox in venous thromboembolism. Blood Adv. 2021;5(14):2909–2918. doi: 10.1182/bloodadvances.2020003800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navti OB, Pavord S. Venous thromboembolism in pregnant obese individuals. Best Pract Res Clin Obstet Gynaecol. 2024. Jun;94:102471. doi: 10.1016/j.bpobgyn.2024.102471 [DOI] [PubMed] [Google Scholar]
- 36.Walker AJ, West J, Card TR, Crooks C, Kirwan CC, Grainge MJ. When are breast cancer patients at highest risk of venous thromboembolism? A cohort study using English health care data. Blood. 2016;127(7):849–857; quiz 953. doi: 10.1182/blood-2015-01-625582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vučković BA, Cannegieter SC, van Hylckama Vlieg A, Rosendaal FR, Lijfering WM. Recurrent venous thrombosis related to overweight and obesity: Results from the MEGA follow-up study. J Thromb Haemost. 2017;15(7):1430–1435. doi: 10.1111/jth.13710 [DOI] [PubMed] [Google Scholar]
- 38.Pomp ER, le Cessie S, Rosendaal FR, Doggen CJ. Risk of venous thrombosis: Obesity and its joint effect with oral contraceptive use and prothrombotic mutations. Br J Haematol. 2007;139(2):289–296. doi: 10.1111/j.1365-2141.2007.06780.x [DOI] [PubMed] [Google Scholar]
- 39.Abdollahi M, Cushman M, Rosendaal FR. Obesity: Risk of venous thrombosis and the interaction with coagulation factor levels and oral contraceptive use. Thromb Haemost. 2003;89(03):493–498. [PubMed] [Google Scholar]
- 40.Neeman E, Liu V, Mishra Pet al. et al. Trends and risk factors for venous thromboembolism among hospitalized medical patients. JAMA Netw Open. 2022;5(11):e2240373. doi: 10.1001/jamanetworkopen.2022.40373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh T, Lavikainen LI, Halme ALE, et al. Timing of symptomatic venous thromboembolism after surgery: Meta-analysis. Br J Surg. 2023;110(5):553–561. doi: 10.1093/bjs/znad035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nichols KM, Henkin S, Creager MA. Venous thromboembolism associated with pregnancy: JACC focus seminar. J Am Coll Cardiol. 2020;76(18):2128–2141. 10.1016/j.jacc.2020.06.090 [DOI] [PubMed] [Google Scholar]
- 43.Ngarmukos S, Kim KI, Wongsak Set al. et al. Asia-Pacific venous thromboembolism consensus in knee and hip arthroplasty and hip fracture surgery: Part 1. Diagnosis and risk factors. Knee Surg Relat Res. 2021;33(1):18. doi: 10.1186/s43019-021-00099-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Breart G, Cooper C, Meyer O, Speirs C, Deltour N, Reginster JY. Osteoporosis and venous thromboembolism: A retrospective cohort study in the UK general practice research database. Osteoporos Int. 2010;21(7):1181–1187. doi: 10.1007/s00198-009-1050-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Łuszczki E, Boakye F, Zielińska Met al. et al. Vegan diet: Nutritional components, implementation, and effects on adults’ health. Front Nutr. 2023. Nov 9;10:1294497. doi: 10.3389/fnut.2023.1294497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun X, Wang R, He P, Liu B. Effects of environmental and nutritional labels on the dietary choices of consumers: Evidence from China. Environ Impact Assess Rev. 2024. Mar;105:107407. 10.1016/j.eiar.2023.107407 [DOI] [Google Scholar]
- 47.Vossen CY, Conard J, Fontcuberta J, et al. Risk of a first venous thrombotic event in carriers of a familial thrombophilic defect. The European prospective cohort on thrombophilia (EPCOT). J Thromb Haemost. 2005;3(3):459–464. doi: 10.1111/j.1538-7836.2005.01197.x [DOI] [PubMed] [Google Scholar]
- 48.Heit JA, Phelps MA, Ward SA, Slusser JP, Petterson TM, De Andrade M. Familial segregation of venous thromboembolism. J Thromb Haemost. 2004;2(5):731–736. doi: 10.1111/j.1538-7933.2004.00660.x [DOI] [PubMed] [Google Scholar]
- 49.Larsen TB, Sørensen HT, Skytthe A, Johnsen SP, Vaupel JW, Christensen K. Major genetic susceptibility for venous thromboembolism in men: A study of danish twins. Epidemiology. 2003;14(3):328–332. [PubMed] [Google Scholar]
- 50.Germain M, Chasman Daniel I, de Haan H, et al. Meta-analysis of 65,734 individuals identifies TSPAN15 and SLC44A2 as two susceptibility loci for venous thromboembolism. Am J Hum Genet. 2015;96(4):532–542. 10.1016/j.ajhg.2015.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindström S, Wang L, Smith EN, et al. Genomic and transcriptomic association studies identify 16 novel susceptibility loci for venous thromboembolism. Blood. 2019;134(19):1645–1657. 10.1182/blood.2019000435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bertina RM, Koeleman BP, Koster Tet al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature. 1994;369(6475):64–67. doi: 10.1038/369064a0 [DOI] [PubMed] [Google Scholar]
- 53.Rosendaal FR. Venous thrombosis: The role of genes, environment, and behavior. Hematol Am Soc Hematol Educ Program. 2005;2002(1):1–12. doi: 10.1182/asheducation-2005.1.1.1 [DOI] [PubMed] [Google Scholar]
- 54.Costa J, Araújo A. The contribution of inherited thrombophilia to venous thromboembolism in cancer patients. Clin Appl Thromb Hemost. 2024. Jan–Dec;30:10760296241232864. doi: 10.1177/10760296241232864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiong W, Cheng Y, Zhao Y. Risk scores in venous thromboembolism guidelines of ESC, ACCP, and ASH: An updated review. Clin Appl Thromb Hemost. 2024. Jan–Dec;30:10760296241263856. doi: 10.1177/10760296241263856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horvei LD, Brækkan SK, Smith ENet al. Joint effects of prothrombotic genotypes and body height on the risk of venous thromboembolism: The tromsø study. J Thromb Haemostasis. 2018;16(1):83–89. 10.1111/jth.13892 [DOI] [PubMed] [Google Scholar]
- 57.Bruzelius M, Bottai M, Sabater-Lleal Met al. Predicting venous thrombosis in women using a combination of genetic markers and clinical risk factors. J Thromb Haemostasis. 2015;13(2):219–227. 10.1111/jth.12808 [DOI] [PubMed] [Google Scholar]
- 58.Zhang Z, Li H, Weng H, et al. Genome-wide association analyses identified novel susceptibility loci for pulmonary embolism among han Chinese population. BMC Med. 2023;21(1):153. doi: 10.1186/s12916-023-02844-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goumidi L, Thibord F, Wiggins KL, et al. Association between ABO haplotypes and the risk of venous thrombosis: Impact on disease risk estimation. Blood. 2021;137(17):2394–2402. doi: 10.1182/blood.2020008997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y, Bezemer ID, Rowland CMet al. Genetic variants associated with deep vein thrombosis: The F11 locus. J Thromb Haemost. 2009;7(11):1802–1808. doi: 10.1111/j.1538-7836.2009.03544.x [DOI] [PubMed] [Google Scholar]
- 61.Paulsen B, Skille H, Smith ENet al. Fibrinogen gamma gene rs2066865 and risk of cancer-related venous thromboembolism. Haematologica. 2020;105(7):1963–1968. doi: 10.3324/haematol.2019.224279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mohsenian S, Palla R, Menegatti M, et al. Congenital fibrinogen disorders: A retrospective clinical and genetic analysis of the prospective rare bleeding disorders database. Blood Adv. 2024;8(6):1392–1404. doi: 10.1182/bloodadvances.2023012186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neerman-Arbez M, Germanos-Haddad M, Tzanidakis Ket al. et al. Expression and analysis of a split premature termination codon in FGG responsible for congenital afibrinogenemia: Escape from RNA surveillance mechanisms in transfected cells. Blood. 2004;104(12):3618–3623. doi: 10.1182/blood-2004-06-2312 [DOI] [PubMed] [Google Scholar]
- 64.Chihara N, Madi A, Kondo T, et al. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature. 2018;558(7710):454–459. doi: 10.1038/s41586-018-0206-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dennis J, Johnson CY, Adediran ASet al. et al. The endothelial protein C receptor (PROCR) Ser219Gly variant and risk of common thrombotic disorders: A HuGE review and meta-analysis of evidence from observational studies. Blood. 2012;119(10):2392–2400. doi: 10.1182/blood-2011-10-383448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin X, Zhang B, Zhang H, Yu H. Smoking-associated upregulation of CBX3 suppresses ARHGAP24 expression to activate Rac1 signaling and promote tumor progression in lung adenocarcinoma. Oncogene. 2022;41(4):538–549. doi: 10.1038/s41388-021-02114-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chao H, Zhang M, Hou H, Zhang Z, Li N. HOTAIRM1 Suppresses cell proliferation and invasion in ovarian cancer through facilitating ARHGAP24 expression by sponging miR-106a-5p. Life Sci. 2020 Feb 15;243:117296. doi: 10.1016/j.lfs.2020.117296 [DOI] [PubMed] [Google Scholar]
- 68.Dai X, Geng F, Dai J, Li M, Liu M. Rho GTPase activating protein 24 (ARHGAP24) regulates the anti-cancer activity of sorafenib against breast cancer MDA-MB-231 cells via the signal transducer and activator of transcription 3 (STAT3) signaling pathway. Med Sci Monit. 2018. Nov 30;24:8669–8677. doi: 10.12659/msm.911394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uehara S, Saito K, Asami H, Ohta Y. Role of ARHGAP24 in ADP ribosylation factor 6 (ARF6)-dependent pseudopod formation in human breast carcinoma cells. Anticancer Res. 2017. Sep;37(9):4837–4844. doi: 10.21873/anticanres.11891 [DOI] [PubMed] [Google Scholar]
- 70.Feng M, Bao Y, Li Z, et al. RASAL2 Activates RAC1 to promote triple-negative breast cancer progression. J Clin Invest. 2014;124(12):5291–5304. doi: 10.1172/jci76711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qian H, Wu X, Du Xet al. et al. Structural basis of low-pH-dependent lysosomal cholesterol egress by NPC1 and NPC2. Cell. 2020;182(1):98–111.e118. doi: 10.1016/j.cell.2020.05.020 [DOI] [PubMed] [Google Scholar]
- 72.Colombo A, Dinkel L, Müller SA, et al. Loss of NPC1 enhances phagocytic uptake and impairs lipid trafficking in microglia. Nat Commun. 2021;12(1):1158. doi: 10.1038/s41467-021-21428-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kunkel TJ, Townsend A, Sullivan KAet al. et al. The cholesterol transporter NPC1 is essential for epigenetic regulation and maturation of oligodendrocyte lineage cells. Nat Commun. 2023;14(1):3964. doi: 10.1038/s41467-023-39733-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fuellen G, Walter U, Henze Let al. Protein biomarkers in blood reflect the interrelationships between stroke outcome, inflammation, coagulation, adhesion, senescence and cancer. Cell Mol Neurobiol. 2023;43(4):1413–1424. doi: 10.1007/s10571-022-01260-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lehmann ALCF, Alfieri DF, de Araújo MCM, et al. Immune-inflammatory, coagulation, adhesion, and imaging biomarkers combined in machine learning models improve the prediction of death 1 year after ischemic stroke. Clin Exp Med. 2022;22(1):111–123. doi: 10.1007/s10238-021-00732-w [DOI] [PubMed] [Google Scholar]