Dear Editor
Poliovirus, once eradicated from French Guiana, has re-emerged recently. This marks the first detection of circulating vaccine-derived poliovirus type 3 (cVDPV3) in the region since 1983 when French Guiana was declared polio-free [1,2]. The virus was identified in a wastewater sample collected on 26 June 2024 in Cayenne as part of a research project by the French research agency for emerging infectious diseases (ANRS-MIE). On 2nd August 2024, the Global Specialized Lab (GSL) of the Global Polio Lab Network (GPLN) at the Institut Pasteur in Paris notified Santé Publique France and other French health authorities of the VDPV3 detection [1].
Poliovirus belongs to the enterovirus genus in the Picornaviridae family. It is a small, non-enveloped virus with a single-stranded positive RNA, resistant to acidic conditions. Wild poliovirus (WPV) has 3 serotypes - WPV1, WPV2 and WPV3 distinguished by a slightly different capsid protein [3]. VDPVs can emerge when oral polio vaccine (OPV) viruses proliferate in populations with low herd immunity (cVDPVs) or when they persist in immunocompromised individuals (iVDPVs). Through genetic changes such as recombination with other enteroviruses or reversion of OPV strains, these VDPVs can regain the neurovirulence and transmissibility of WPV. This genetic divergence from the attenuated OPV indicates prolonged replication and circulation, raising significant public health concerns [3,4]. The recent detection of cVDPV3 in French Guiana, which exhibits 15 mutations in the poliovirus protein 1 (VP1), highlights this threat. These mutations represent a significant genetic divergence from previously identified strains, enhancing the virulence and lead to paralysis, particularly in populations with insufficient immunisation [1].
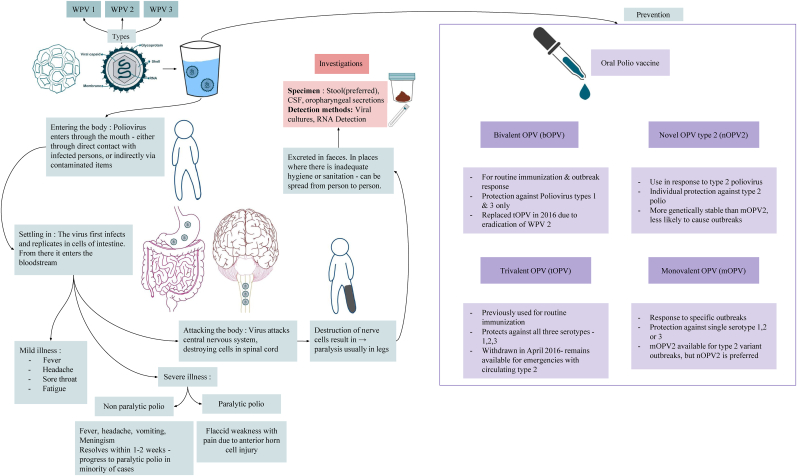
Polio is highly infectious and transmitted mainly through the faecal-oral route. After entry, the virus replicates in the lymphatic tissues of the oropharynx and gut, causing asymptomatic viremia, followed by spread to the reticuloendothelial system [3]. In rare cases poliovirus crosses the blood brain barrier or travels via peripheral nerves to the central nervous system, causing non paralytic aseptic meningitis in up to 5 % of infections depending on the virus type and the invasion site [3] (Fig. 1.).
Fig. 1.
Poliovirus types, pathogenesis, and prevention.
Poliovirus infections are mainly asymptomatic, however, 1–2% may develop severe illness, often manifesting as meningitis. This severe form can be classified into non-paralytic and paralytic polio, the latter causing acute flaccid due to anterior horn cell injury. Paralytic polio affecting 0.5–0.05 % of those infected, can cause significant muscle weakness and, in severe cases, respiratory failure. Diagnosis is made through stool samples, or cerebrospinal fluid with tests like culture, and electrodiagnostic studies, to differentiate poliomyelitis from other conditions that cause acute flaccid paralysis, such as non-polio enteroviruses. Management focuses on supportive care, as no antiviral therapies are currently approved for poliomyelitis. Prevention relies on vaccination and strict infection control measures [4].
The OPV is a safe and highly effective tool in the global fight against polio and it has helped to reduce polio cases by over 99 % since 1988. It is available in different types - monovalent (mOPV), bivalent (bOPV) and novel type 2(nOPV) (Fig. 1.). Unlike the inactivated polio vaccine (IPV), OPV stops person to person transmission, and it is easier to administer. However, it carries a rare risk of vaccine associated paralytic poliomyelitis (VAPP) and can lead to variant poliovirus in under immunised communities as seen in this incident. The bOPV, used for routine immunisation, protects against stable version, addresses the risk of type 2 variant outbreak, providing a sustainable solution in preventing new cases of VDPV [5].
In response to the recent detection of VDPV3 in French Guiana local health authorities have strengthened poliovirus monitoring by setting up a year-long environmental surveillance protocol, informed healthcare workers about vaccination status, and launched a catch-up vaccination campaign for school-aged children. The PAHO/WHO emphasises the need for continuous efforts to maintain high vaccination coverage (at least 95 % for 3 doses of polio vaccine) and strong epidemiological surveillance, including the detection and reporting of acute flaccid paralysis (AFP) cases. Surveillance systems must be sensitive enough to detect at least one case of AFP per 100,00 children, with stool samples collected for laboratory analysis to confirm presence of poliovirus. Additionally, countries are urged to have updated outbreak response plans aligned with latest WHO guidelines to ensure a swift reaction to any polio events [1].
CRediT authorship contribution statement
Nalira Yaugoob: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. Kannan Subbaram: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing. Razana Faiz: Data curation, Investigation, Resources, Supervision, Writing – review & editing. Zeba Un Naher: Formal analysis, Investigation, Methodology, Supervision, Writing – review & editing. Sheeza Ali: Formal analysis, Investigation, Supervision, Visualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Patricia Schlagenhauf
Contributor Information
Nalira Yaugoob, Email: nalirayaugoob@gmail.com.
Kannan Subbaram, Email: kannan.subbaram@mnu.edu.mv.
Razana Faiz, Email: razana.faiz@mnu.edu.mv.
Zeba Un Naher, Email: zeba.naher@mnu.edu.mv.
Sheeza Ali, Email: sheeza.ali@mnu.edu.mv.
References
- 1.Epidemiological Alert - Detection of Poliovirus (cVDPV3) in wastewater . 2024. Considerations for the region of the Americas. [Google Scholar]
- 2.GPEI-polio-free countries. Polioeradication.org. Retrieved October 18, 2024, (n.d.). from https://polioeradication.org/about-polio/polio-free-countries/.
- 3.Wolbert J.G., Rajnik M., Swinkels H.M., Higginbotham K. StatPearls. StatPearls Publishing; 2024. Poliomyelitis. [PubMed] [Google Scholar]
- 4.UpToDate. Uptodate.com. (n.d.). Retrieved October 18, 2024, from https://www.uptodate.com/contents/poliomyelitis-and-post-polio-syndrome?search=Polio%20virus&source=search_result&selectedTitle=%7E145&usage_type=default&display_rank=1.
- 5.Gpei-opv. Polioeradication.org. Retrieved October 18, 2024, (n.d.). from https://www.archive.polioeradication.org/polio-today/polio-prevention/the-vaccines/opv/.