Abstract
Background
The aim of this study was to build and validate a risk prediction model for aspiration in severe acute pancreatitis patients receiving early enteral nutrition (EN) by identifying risk factors for aspiration in these patients.
Methods
The risk factors for aspiration were analyzed to build a prediction model based on the data collected from 339 patients receiving enteral nutrition. Subsequently, we used six machine learning algorithms and the model was validated by the area under the curve.
Results
In this study, the collected data were divided into two groups: a training cohort and a validation cohort. The results showed that 28.31 % (77) of patients had aspiration and 71.69 % (195) of patients had non-aspiration in training cohort. Moreover, age, consciousness, mechanical ventilation, aspiration history, nutritional risk and number of comorbidities were included as predictive factors for aspiration in patients receiving EN. The XGBoost model is the best of all machine learning models, with an AUROC of 0.992 and an F1 value of 0.902. The specificity and accuracy of XGBoost are higher than those of traditional logistic regression.
Conclusion
In accordance with the predictive factors, XGBoost model, characterized by excellent discrimination and high accuracy, can be used to clinically identify severe acute pancreatitis patients with a high risk of enteral nutrition aspiration.
Relevance to clinical practice
This study contributed to the development of a predictive model for early enteral nutrition aspiration in severe acute pancreatitis patients during hospitalization that can be shared with medical staff and patients in the future.
No patient or public contribution
This is a retrospective cohort study, and no patient or public contribution was required to design or undertake this research.
Keywords: Aspiration, Enteral nutrition, Severe acute pancreatitis, Predictive model, Risk factors
1. Introduction
Severe acute pancreatitis (SAP) has a rapid onset and progression with complex clinicopathologic changes. Systemic inflammatory response syndrome and multiple organ dysfunction syndrome (MODS) can occur at an early stage, with a mortality rate of 20%–30 % [1]. SAP patients are in a state of hyper decomposition and hypermetabolism, often accompanied by severe metabolic disorders, and prone to malnutrition [2]. Early enteral nutrition (EEN) (≤48h) can reduce the incidence of complications in SAP patients [3]. Enteral nutrition (EN) could stimulate intestinal metabolic activity, which helps to maintain intestinal function and protect the intestinal mucosal barrier. Intestinal integrity plays a crucial role in regulating the imbalance of intestinal flora [4]. A study had shown that EN had fewer complications than parenteral nutrition, shorter hospital stays and a positive impact on patient outcomes [5]. However, due to complications of EN, there is still a proportion of patients who are unable to reach their energy goals [6]. Aspiration is one of the serious complications of EN, which increases the risk of aspiration pneumonia by 12 times, which results in acute respiratory distress syndrome (ARDS), with a case fatality rate of up to 70 % [7,8], and may also prolong the patient's hospital stay and increase hospitalization costs [9,10].
Aspiration can be categorized into overt and micromisaspiration according to the symptoms [11]. Clinical symptoms such as choking, coughing and shortness of breath immediately after aspiration are characterized as overt aspiration. Microaspiration occurring without any noticeable external signs is a subtle yet critical condition to be aware of. The incidence of overt aspiration in ICU patients ranges from 8.80 % to 22.45 % [11,12], and the incidence of microaspiration reaches 88.00 % [13,14]. Microaspiration is an independent risk factor for ventilator-associated pneumonia and a major source of bacteria [15]. There are several methods for detecting aspiration, but all have their drawbacks, such as the radioactivity of technetium 99 [16], the complexity of methylene blue [17], and the inaccuracy of alpha amylase [18]. Videofluoroscopic swallowing study (VFSS) can comprehensively and visually assess the swallowing function of patients, which are the gold standard for diagnosing swallowing dysfunction [19]. However, there are high requirements for operators, operating environment and patient cooperation, and the cost is expensive, so they are less used in clinical practice. In contrast, pepsin detection is easy to perform. Therefore, it is recommended to use pepsin detection as a marker for microaspiration [17,18]. There is a need for clinical nurses to be fully aware of aspiration procedure, especially, microaspiration [20]. It is recommended that medical staff first screen patients with EAT-10 questionnaire, and further test pepsin for positive patients to determine whether there is aspiration.
Machine learning (ML) algorithms are good at defining data attributes, using computers to mine the laws present in the data, and applying different classifiers to predict the occurrence of diseases [21]. ML has now become a new data analysis paradigm competing with multivariate linear regression in medical research and is mainly applied to clinically assisted medical decision making [22]. In particular, ML models for early detection of diseases had appeared in various fields such as coronary heart disease [23], diabetes [24], kidney disease [25] and lung diseases [26], and the results showed that ML predictive models were comparable to existing screening tools. In addition, ML algorithms can capture nonlinear relationships in data and complexity among multiple predictor variables, accurately identify risk factors for diseases, and make early predictions and diagnosis [27]. Despite the rapidly expanding field of research on ML algorithms, there are no ML-based models for predicting EEN aspiration in patients with SAP.
In a comprehensive review of the literature, we have identified a significant gap in the application of Machine Learning (ML) techniques for predicting the risk of aspiration in patients undergoing early enteral nutrition (EEN) for severe acute pancreatitis (SAP). There were studies indicating that machine learning techniques can be used to predict complications of acute pancreatitis. A retrospective cohort study [28] developed three machine learning models and a traditional logistic regression model for the prediction of AKI in patients with acute pancreatitis,the results showed that compared to the traditional modeling approach, the machine learning method demonstrated superior performance, including net reclassification and net benefit.
A study had constructed a prediction model for the severity of acute pancreatitis based on machine learning algorithms, three models were developed using logistic regression, neural networks, and XGBoost, the results showed that machine learning might be able to improve the accuracy of AP risk strategizing methods and allow for more timely treatment and initiation of interventions [29]. This was of great significance for predicting and evaluating the severity and making clinical decisions. In addition, there were studies [30,31] that integrated the research progress of machine learning in predicting the severity, complications, and mortality of acute pancreatitis, providing theoretical basis and new ideas for the clinical treatment of acute pancreatitis assisted by artificial intelligence.
Although previous studies had shown the potential of machine learning in predicting complications related to acute pancreatitis, there was no study systematically or specifically employed ML techniques to predict the risk of aspiration in patients undergoing EEN for SAP. There was indeed a gap in current knowledge. The ability to predict aspiration risk could revolutionize patient management, reduce complications, and improve outcomes for these patients. Given the critical nature of enteral nutrition support in these patients, and the potential complications of aspiration, our study aims to develop ML models that accurately predicts the risk of aspiration in SAP patients undergoing EEN. Extreme gradient boosting (XGBoost), Logistic Regression (LR), Random Forest (RF), Support Vector Machine (SVM), K-Nearest Neighbor (KNN) and Stochastic Gradient Descent (SGD) were selected to mine target data sets and establish risk prediction models for aspiration. Then the prediction efficiency of the models under different algorithms is evaluated in order to construct the optimal prediction model. The aim is to bridge this gap, enhance risk perception ability and provide new ideas for clinical decision-making and patient outcomes.
What does this paper contribute to the wider global community?
-
•
This study identified a better predictive model for early enteral nutrition aspiration in SAP patients and its association with patient measures for clinical risks.
-
•
The results can be used by clinicians and nurse managers to early intercept patient with aspiration, to implement tailored interventions and improve patient care ability.
2. Methods
2.1. Study design and data collection
A total of 339 patients receiving EN in two hospitals from October 2019 to October 2023 were enrolled and their clinical data collected for this retrospective cohort study. Inclusion criteria: ①Age≥18 years old; ②Meet the Atlanta diagnostic criteria for SAP [32]; ③EN was given for 24–48 h after admission, and the duration was 1 week; ④Patients and their families gave informed consent to the treatment. Exclusion criteria: ①malignant tumor patients; ②pregnancy; ③terminal state; ④incomplete data. For patients with multiple admissions take their first data.
2.2. Data management
The formulas of the unmatched case–control study was used to calculate the sample size [33]. The patients were randomized into the training and validation cohorts with a ratio of 8:2. Considering that a larger sample size would yield better performances of the prediction models, 339 patients were randomly selected for inclusion in the study, with 272 patients in the training group and 67 patients in the validation group.
2.3. Independent variables
The outcome of this study was whether aspiration occurred during EN. The following data were collected for modeling. (1) Demographic characteristics: gender, age (years), length of stay, aspiration history, number of comorbidities, consciousness, nutritional risk; (2) EN related index: intubation days, intubation depth, food type; (3) Nursing measures: mechanical ventilation; (4) medication use: gastric stimulants, antacids; (5) Laboratory test indicators: red blood cells (RBC) counts, haemoglobin (Hb) level, platelet (PLT) counts, white blood cell (WBC) counts, lymphocyte (LY) counts, neutrophil (NEUT) counts. All variables were screened, missing values greater than 25 % were excluded, and patients with more than 5 % missing personal data were excluded, and the remaining missing values were filled in according to multiple interpolation. Table S2 showed the missing values. Finally, a total of 20 variables were included, as shown in Table 1.
Table 1.
Training cohort baseline.
| Characteristic | Non-Aspiration [cases (%)] | Aspiration [cases (%)] | P value |
|---|---|---|---|
| Total | 195 | 77 | |
| Age | 56.00 [51.00, 66.00] | 65.00 [59.00, 69.00] | <0.001 |
| Gender | 0.456 | ||
| Male | 135 (69.2) | 49 (63.6) | |
| Female | 60 (30.8) | 28 (36.4) | |
| Food type | 0.341 | ||
| Commercial nutrition | 161 (82.6) | 59 (76.6) | |
| Home-made meals | 34 (17.4) | 18 (23.4) | |
| Consciousness | <0.001 | ||
| Conscious | 120 (61.5) | 12 (15.6) | |
| Sleepiness | 38 (19.5) | 31 (40.3) | |
| Lethargy | 17 (8.7) | 12 (15.6) | |
| Coma | 20 (10.3) | 22 (28.6) | |
| Mechanical ventilation | <0.001 | ||
| NO | 163 (83.6) | 19 (24.7) | |
| YES | 32 (16.4) | 58 (75.3) | |
| Aspiration history | <0.001 | ||
| NO | 154 (79.0) | 33 (42.9) | |
| YES | 41 (21.0) | 44 (57.1) | |
| Nutritional risk | <0.001 | ||
| NO | 156 (80.0) | 37 (48.1) | |
| YES | 39 (20.0) | 40 (51.9) | |
| Length of Stay (d),median (IQR) | 15.00 [11.00, 22.50] | 15.00 [10.00, 26.00] | 0.735 |
| Intubation depth (cm),median (IQR) | 100.00 [95.00, 105.00] | 100.00 [95.00, 105.00] | 0.073 |
| Intubation days (d),median (IQR) | 13.00 [10.00, 20.00] | 14.00 [9.00, 20.00] | 0.356 |
| Number of comorbidities | <0.001 | ||
| 0 | 125 (64.1) | 4 (5.2) | |
| 1 | 40 (20.5) | 15 (19.5) | |
| 2 | 19 (9.7) | 31 (40.3) | |
| 3 | 8 (4.1) | 18 (23.4) | |
| 4 | 3 (1.5) | 9 (11.7) | |
| Antacids | 0.435 | ||
| NO | 31 (15.9) | 16 (20.8) | |
| YES | 164 (84.1) | 61 (79.2) | |
| Gastricstimulants | 0.935 | ||
| NO | 61 (31.3) | 23 (29.9) | |
| YES | 134 (68.7) | 54 (70.1) | |
| RBC (1012/L),median (IQR) | 3.75 [3.20, 4.50] | 3.55 [3.12, 4.15] | 0.047 |
| Hb (g/L), mean ± SD | 116.00 [111.0, 130.0] | 115.00 [110.0, 129.0] | 0.356 |
| PLT (109/L),median (IQR) | 208.00 [148.5, 307.0] | 190.00 [122.0, 284.0] | 0.056 |
| WBC(109/L),median (IQR) | 11.54 [8.30, 15.47] | 10.94 [7.90, 14.25] | 0.345 |
| Lym(%),median (IQR) | 0.92 [0.66, 1.31] | 0.80 [0.62, 1.14] | 0.176 |
| NEUT(%),median (IQR) | 9.70 [6.32, 13.53] | 9.18 [6.41, 12.53] | 0.572 |
| K + (mEq/L) | 4.00 [3.55, 4.43] | 3.96 [3.66, 4.32] | 0.469 |
Note: Number of comorbidities included the total diseases of neurological complications, hypertension, respiratory complications and heart disease.
Age is presented by mean and interquartile range (IQR).
Hb: Hemoglobin; WBC: white blood cell; RBC: red blood cell; PLT: platelet; Lym: lymphocyte; NEUT: neutrophil.
Abbreviations: IQR, interquartile range.
2.4. Predictive model development and validation
Univariate analysis was performed on the final complete dataset to screen out the factors associated with aspiration in patients with EEN. Subsequently, multivariate logistic regression analysis was performed to obtain the patient's independent risk factors. Based on ML, six prediction models were established using the data of the training and the validation cohort, which were as follows: (1) extreme gradient boosting (XGBoost); (2) Logistic Regression (LR); (3) Random Forest (RF); (4) Support Vector Machine (SVM); (5) K-Nearest Neighbor (KNN); (6) Stochastic Gradient Descent (SGD). Select 10-fold cross-validation for the selection and adjustment of predictive model hyperparameters. The discriminant discrimination ability of the model is measured by the area under the receiver operating characteristic curve (AUROC).
The medical records of SAP patients who received EN from a hospital in Shandong Province were used as an external validation dataset. After converting the optimal predictive model into a web-based calculator, the dataset was used as the basis for external validation to evaluate the accuracy and extrapolation power of the model.
2.5. Ethics statement
The study was approved by the Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong Universityy (No. XJTU1AF2023LSK-507). The requirement for individual informed consent was waived by the ethics committee of the First Affiliated Hospital of Xi'an Jiaotong University because of the retrospective nature of the study. This study was conducted in accordance with the principles of the Declaration of Helsinki.
2.6. Statistical analysis
R statistical software (version 4.0.1) and IBM SPSS26.0 statistical software were used for statistical analysis. The Shapiro-Wilk test was used to detect whether the continuous variables conform to a normal distribution. Categorical variables are expressed as frequency (proportion). The chi-squared (trend) test was used for comparative analysis between the two groups. The area under the receiver operating characteristic curve (AUROC) was analyzed using the “Epi” package in the R software. The sensitivity and specificity were calculated: Sensitivity = TP/(TP + FN); Specificity = TN/(TN + FP). TP denotes true positives, TN denotes true negatives, FP denotes false positives, and FN denotes false negatives. Factors with significant differences are used in regression analysis. p < .05 is considered to have a significant difference.
3. Results
3.1. Characteristics of patients
A total of 339 patients receiving EEN who were randomly selected for inclusion in the study. They were randomly divided into training and validation cohorts by a ratio of 8:2. The comparison of demographic and clinical characteristics between the training cohort and validation cohort is summarized in Supplementary Table S1. The detailed baseline characteristics in the training cohort are displayed in Table 1. There were 77 aspiration patients and 195 non-aspiration patients in the training cohort. A total of 20 variables were adopted in this study, of which 7 variables were statistically significant (p < .05). Compared with non-aspiration patients, the age of patients with aspiration is significantly older (average age, 56 vs. 65, p < .001). Other basic characteristics such as number of comorbidities, nutritional risk, aspiration history and consciousness were also found to be statistically significant (p < .05). There were no significant differences in EN related indicators and medication use. Significant differences were found in mechanical ventilation among the nursing measures (p < .05). RBC counts showed better effects on the latest blood indexes of EN compared with those of PLT counts, Hb levels, WBC counts, lymphocyte count, neutrophil counts and K+.
3.2. Risk factor screening and model construction
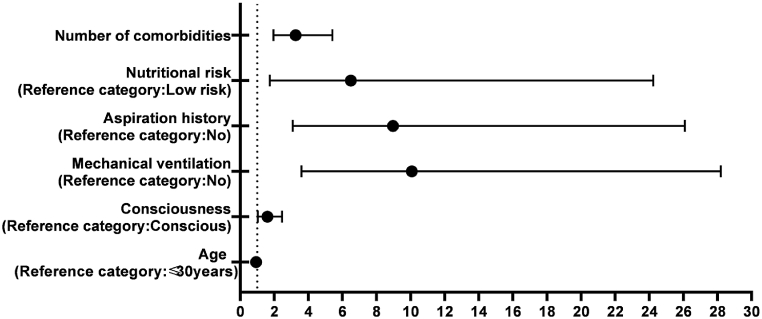
Seven risk factors associated with EEN aspiration in SAP patients were taken as independent variables in univariate analysis, and the dependent variable was whether aspiration occurred. Logistic regression analysis was performed. The final results showed that age, consciousness, aspiration history, mechanical ventilation, nutritional risk and number of comorbidities were independent risk factors. The specific analysis results are shown in Fig. 1 and Table 2.
Fig. 1.
Odds ratio for risk factors of aspiration.
Table 2.
Logistic regression of risk factors for aspiration.
| Variables | Beta coeff. | SE | Z | p | Odds ratio | 95 % CI |
|---|---|---|---|---|---|---|
| Intercept | −2.849 | 3.376 | −0.84 | |||
| Age | −0.068 | 0.026 | −2.66 | 0.008 | 0.93 | 0.89–0.98 |
| Consciousness | 0.466 | 0.222 | 2.10 | 0.036 | 1.59 | 1.03–2.46 |
| Mechanical ventilation | 2.309 | 0.525 | 4.39 | <0.001 | 10.07 | 3.59–28.20 |
| Aspiration history | 2.192 | 0.545 | 4.02 | <0.001 | 8.96 | 3.08–26.09 |
| Nutritional risk | 1.870 | 0.672 | 2.78 | 0.005 | 6.49 | 1.74–24.23 |
| Number of comorbidities | 1.177 | 0.261 | 4.52 | <0.001 | 3.25 | 1.95–5.41 |
Abbreviations: Beta coeff., beta coefficient; SE, standard error.
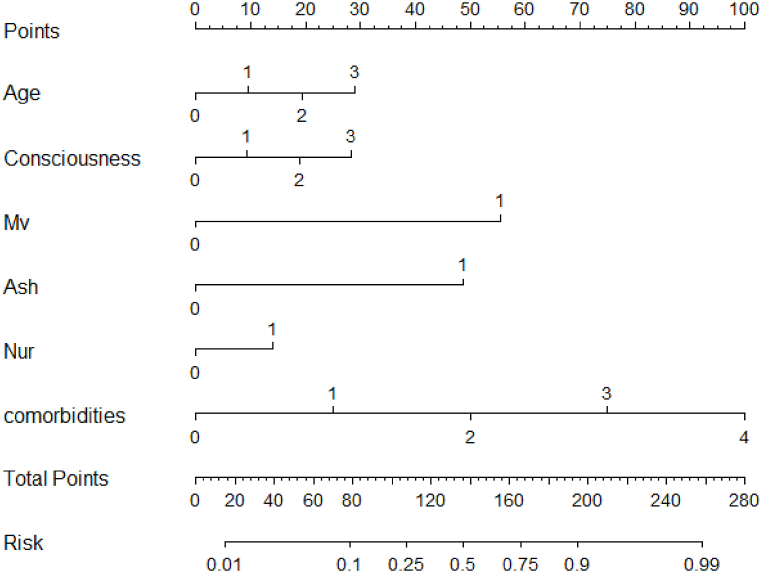
A nomogram was developed and visualized with the six significant factors based on the final regression analysis (Fig. 2). A score was assigned to the value of each variable on a point-scale axis. Predicted probabilities were converted into points on the coordinate axis from 0 to 100. Each evaluation criterion corresponded to a variable. Among the six factors, age had the highest effect (points:100). Patients with number of comorbidities and mechanical ventilation were respectively allocated 85 points and 45 points. Patients with aspiration history were assigned higher points than those without aspiration history (points:38 vs.0). Patients with nutritional risk were only slightly higher than patients without nutritional risk. The risk of aspiration signified the aspiration probability ranging from 0.01 to 0.99. The constructed risk prediction nomogram of aspiration is shown in Fig. 2.
Fig. 2.
Nomogram model for aspiration in patients receiving enteral nutrition.
Note: Mv: Mechanical ventilation; Ash: Aspiration history; Nur: Nutritional risk; comorbidities: Number of comorbidities.
3.3. Model verification
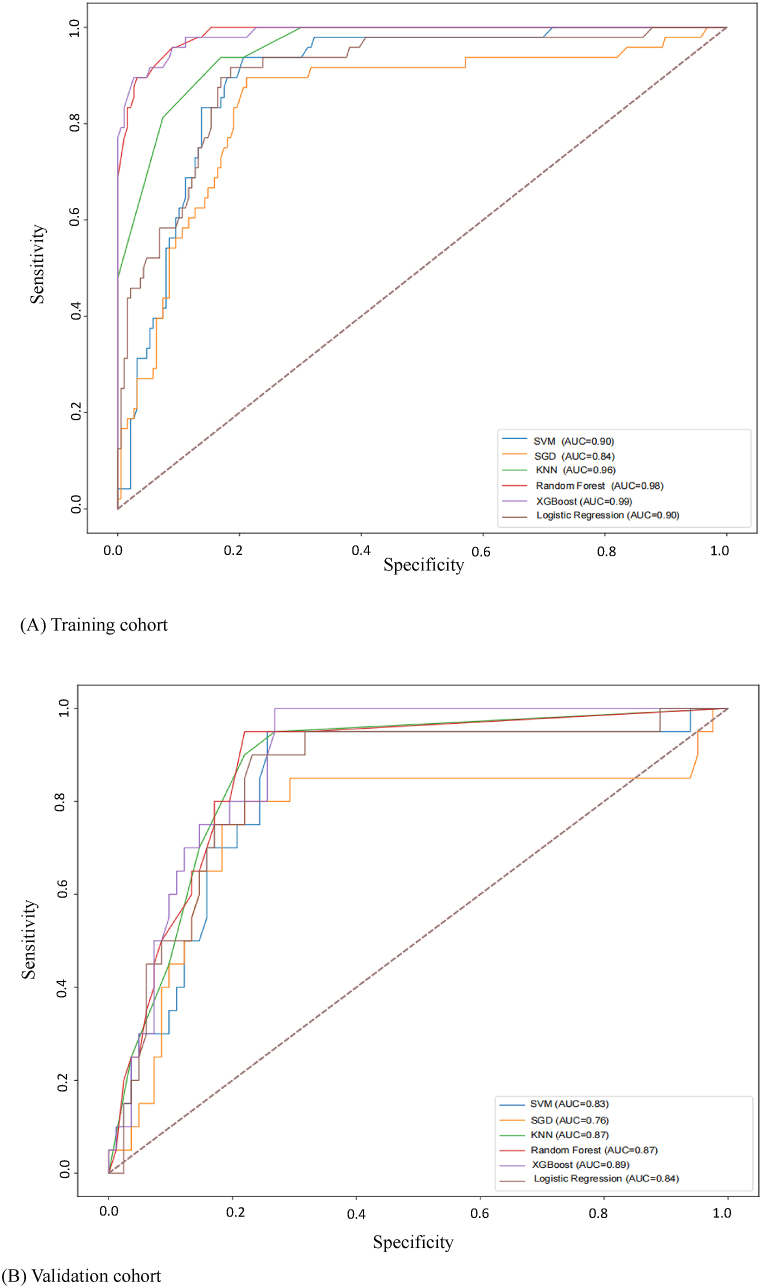
Based on ML, six aspiration prediction models were established using the above four variables. The predictive power of the algorithm for EEN aspiration was validated and evaluated in the validation cohort. ROC curves were plotted with sensitivity as the ordinate and 1-specificity as the abscissa, and AUROC was used to evaluate the discrimination of each model. After comparing the six models, the AUC, specificity, accuracy, and F1 value of the XGBoost model ranked first, and its AUC was 0.992. The ROC curves for the six predictive models are shown in Fig. 3.
Fig. 3.
ROC of the nomogram for risk prediction.
Note:(1) XGBoostextreme gradient boosting; (2) Logistic Regression (LR); (3) Random Forest (RF); (4) Support Vector Machine (SVM); (5) K-Nearest Neighbor (KNN); (6) Stochastic Gradient Descent (SGD).
The AUC of the SVM, SGD, KNN, RF, XGBoost and LR models is 0.904, 0.843, 0.964, 0.984, 0.992 and 0.903, respectively. XGBoost has the highest AUROC and specificity (Table 3), and has better predictive performance than traditional LR models. The most sensitive ones are SVM and KNN. The specificity, F1 and accuracy of XGBoost are higher than those of traditional LR, and XGBoost has a higher proportion of correct predictions for all samples, and more sensitivity for the diagnosis of aspiration.
Table 3.
Prediction performance. AUROC area under the receiver operating characteristics curve.
| Predictors | AUROC | Accuracy | Sensitivity | Specificity | F1 measure |
|---|---|---|---|---|---|
| SVM | 0.904 | 0.810 | 0.938 | 0.778 | 0.671 |
| SGD | 0.843 | 0.810 | 0.667 | 0.847 | 0.592 |
| KNN | 0.964 | 0.852 | 0.938 | 0.831 | 0.726 |
| RandomForest | 0.984 | 0.954 | 0.896 | 0.968 | 0.893 |
| XGBoost | 0.992 | 0.958 | 0.896 | 0.974 | 0.902 |
| LR | 0.903 | 0.840 | 0.729 | 0.868 | 0.654 |
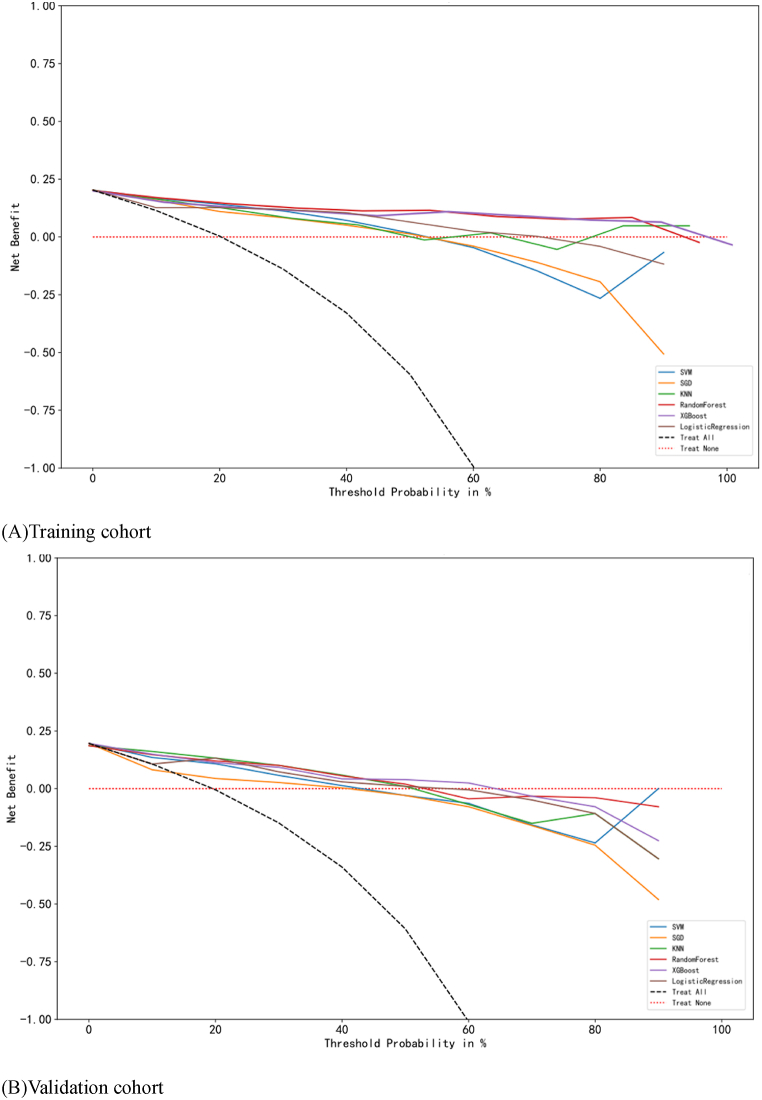
The accuracy and precision of risk prediction models provide reference for clinical precision intervention, but the ultimate goal of constructing risk prediction models is to benefit patients in clinical applications under any predicted probability of disease occurrence. Decision curve analysis (DCA) is a method of evaluating whether using predictive models for clinical decision-making has more advantages than disadvantages. The horizontal axis of the DCA curve represents the threshold probability, and the vertical axis represents the net benefit. The object described by the DCA curve is the net benefit of the entire prediction model or a certain test, which is intervened according to the model results. In this study, the risk prediction model constructed by XGBoost algorithm had a higher net benefit rate of DCA curve in both the training and validation cohorts compared to the prediction models constructed by other algorithms. The results indicate that the risk prediction model constructed by XGBoost algorithm is more clinically practical. When using this model for disease prediction and intervention, patients benefit and is more worthy of clinical promotion and application. The DCA curve of the aspiration risk prediction model in SAP patients with EEN was shown in Fig. 4.
Fig. 4.
Decision curves of six machine learning models in the training and validation cohort.
4. Discussion
In this study, we showed that some clinical factors were more likely to be associated with EEN aspiration. Using advanced machine learning techniques, we could identify some important clinical factors associated with EEN aspiration such as age, consciousness, mechanical ventilation, aspiration history, nutritional risk and number of comorbidities. These results have some implications and require further consideration. Aspiration related to EEN is a major complication that confers negative effects on patients and caregiver [34]. Objective and subjective factors of EN can also contribute to aspiration. Undoubtedly, the mechanism of aspiration in patients receiving EEN is complex and variable. Thus, we developed a prediction model. The model may support clinicians and nurses when making decisions and treatment recommendations for patients receiving EEN.
Identifying the risk factors of aspiration is the first step in establishing a prediction model of aspiration in SAP patients receiving EEN. In this study, age ranked first, indicating that older SAP patients are more likely to experience aspiration than younger patients. With the increase of age, the functions of each organ of the body shows different degrees of physiological decline, especially the reflex function and swallowing function [35]. Impaired consciousness was one of the independent risk factors. The state of consciousness was also one of the indicators reflecting the severity of the patient's disease, and patients who were critically ill or in impaired consciousness had a decreased cough reflex and reduced defensive airway protection, leading to swallowing disorders. At the same time, persistent impaired consciousness or coma could lead to relaxation of the muscles of the throat and increase the risk of accidental respiratory secretions from the pharynx, thus increasing the risk of aspiration [36,37]. North American expert consensus on aspiration in CCI patients [36] lists aspiration history as the main risk factor for aspiration. This study showed that patients with aspiration history had a much higher risk of aspiration than patients without aspiration history. A study [38] used ROC curve and multivariate analysis and found that aspiration history was a risk factor for complicated aspiration in patients with dysphagia. Patients with mechanical ventilation are at high risk of aspiration, with a risk of 11.4 % [39]. A study had shown that the longer the implementation of mechanical ventilation, the greater the likelihood of aspiration [40], which was consistent with the results of this study. Sedative medications have been shown to be an independent factor in the risk of aspiration [41]. Therefore, it is clinically important to minimize unnecessary deep sedation in patients on mechanical ventilation, and health care providers should assess the need for sedative use on a daily basis.
Nutritional risk is one of the independent risk factors for predicting the occurrence of aspiration in SAP patients but it is often underappreciated [42]. High nutritional risk is positively associated with an increased risk of clinical outcomes such as in-hospital death [43]. Patients with high nutritional risk are subjected to additional therapeutic measures such as endotracheal intubation, tracheotomy and placement of a nasogastric tube, which can increase the risk of aspiration. However, these patients are most likely to benefit from aggressive nutritional therapy [44]. Therefore, timely assessment of patients' nutritional risk and intervention for patients at high nutritional risk can improve the outcome of aspiration.
The number of comorbidities were independent risk factors for EN complicated by aspiration (OR = 15.44). Concurrent aspiration was associated with neurological comorbidities, hypertension, respiratory comorbidities and other diseases. Patients with lung disease were at increased risk of aspiration due to impaired respiratory defense mechanisms, such as a weakened cough reflex and a weakened mucociliary barrier [[45], [46], [47]]. A study identified cardiovascular comorbidities and neurological comorbidities in stroke patients with indwelling gastric tubes as important causes of aspiration [48]. Therefore, medical staff should treat the primary disease of patients early, pay attention to the mechanism of the disease on aspiration, and reduce the incidence of aspiration in multiple dimensions, measures and channels.
The Murray Secretion Scale (MSS), a prediction tool, identified oropharyngeal secretions as a critical indicator for aspiration, boasting a sensitivity of 74 % and a specificity of 90 % [49]. The outcome suggests that the model crafted in this current study outperformed the MSS in terms of efficacy. The nomogram model we created has transformed the complex regression equation into a visual format, enhancing the comprehensibility of the prediction model's outcomes and rendering it more user-friendly for assessing patients undergoing EEN. Each factor can be mapped on an axis. The total points and aspiration risk can be calculated by adding the points of the factors altogether. This prediction tool can not only estimate the risk of each factor leading to aspiration but also calculate the probability of aspiration.
Machine Learning models have outperformed the traditional model employed for detecting EEN aspiration in SAP patients. In this research work, we have attempted to address the various issues and limitations in the previous work. Little work has been conducted in the area of aspiration prediction in SAP. Most of the current work dealt with severity prediction or complications. Ding et al. [50]showed that the ML is a better predictor of mortality in AP than basic scoring systems. In our study, we added enteral nutrition-related data and further used advanced deep learning models to predict the risk of aspiration. Moreover, we deployed multiple different state-of-the-art classifiers. We determined that tree based ensembles like XGBoost produced higher AUC and F1 scores in most scenarios. The XGBoost model estimated the possibility of aspiration in the form of a dichotomy problem: whether aspiration occurred or not. In our study, the XGBoost model identified more risk factors of aspiration than that identified by the traditional model. Internal validation of 1000 bootstrapped samples maximized the information efficiency of potentially important and informative patient data. The ability of machine-learning models to produce individualized predictions enables their use for patient identification and classification in clinical trials.
There are some strengths and limitations. The XGBoost model is a novel technique that has not been widely used in acute pancreatitis. The XGBoost algorithm has been successfully applied to some complex scenarios, and the prediction accuracy of the XGBoost model is significantly better than that of the generalized linear model. This is not surprising, as the XGBoost model is a collection of weak prediction trees capable of capturing complex relationships in the data without the need for higher-order interactions and nonlinear functions that are explicitly specified. In addition, the technique is well designed to prevent overfitting by cross-validation and regularization. This study also has limitations. First, the datasets were retrospective information although they were from two large domestic general hospitals. Second, the sample size was small and patient enrollment bias was difficult to avoid. Therefore, prospective, large-sample studies should be conducted in subsequent studies to explore and validate the clinical value of the prediction model.
5. Conclusion
In conclusion, this study showed that some clinical factors were more likely to be associated with EEN aspiration. The XGBoost modeling technique could identify the predictors of aspiration that were not apparent using logistic regression, resulting in a better-performing predictive model to identify patients with EEN aspiration. The XGBoost model utilize patient data from electronic medical records to predict aspiration in patients receiving EEN with no additional costs. Overall, the performance of the model is superior to that of the traditional model in terms of several aspects. The findings may be used for clinical aspiration prediction and provide a reference for the prevention and treatment of aspiration.
CRediT authorship contribution statement
Bo Zhang: Writing – review & editing, Writing – original draft, Software, Formal analysis, Data curation, Conceptualization. Huanqing Xu: Writing – review & editing, Formal analysis, Data curation. Qigui Xiao: Writing – review & editing, Formal analysis, Data curation. Wanzhen Wei: Software, Formal analysis, Data curation. Yifei Ma: Software, Formal analysis, Data curation. Xinlong Chen: Software, Formal analysis, Data curation. Jingtao Gu: Writing – review & editing, Formal analysis, Data curation. Jiaoqiong Zhang: Formal analysis, Data curation. Lan Lang: Formal analysis, Data curation. Qingyong Ma: Writing – review & editing, Visualization, Validation, Supervision, Resources, Project administration, Investigation, Funding acquisition. Liang Han: Writing – review & editing, Visualization, Validation, Supervision, Resources, Project administration, Investigation, Funding acquisition, Formal analysis.
Data availability
We declare that data related to our research are not publicly available at this time for the following reasons: ① The preliminary statistical results have been reflected in our paper; ② The experimental data is related to our subsequent research, and research data will not be disclosed until the later research results are published.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 82172853 and 82072699) and Natural Science Foundation of Shaanxi Province (No. 2021JQ-904 and 2020JQ-510).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e40236.
Contributor Information
Qingyong Ma, Email: qyma56@mail.xjtu.edu.cn.
Liang Han, Email: hanliangxjtu@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Hines O.J., Pandol S.J. Management of severe acute pancreatitis. BMJ. 2019;367:l6227. doi: 10.1136/bmj.l6227. [DOI] [PubMed] [Google Scholar]
- 2.Jablonska B., Mrowiec S. Nutritional support in patients with severe acute pancreatitis-current standards. Nutrients. 2021;13(5) doi: 10.3390/nu13051498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaughn V.M., Shuster D., Rogers M., et al. Early versus delayed feeding in patients with acute pancreatitis: a systematic review. Ann. Intern. Med. 2017;166(12):883–892. doi: 10.7326/M16-2533. [DOI] [PubMed] [Google Scholar]
- 4.Allen K., Hoffman L. Enteral nutrition in the mechanically ventilated patient. Nutr. Clin. Pract. 2019;34(4):540–557. doi: 10.1002/ncp.10242. [DOI] [PubMed] [Google Scholar]
- 5.Elke G., van Zanten A.R., Lemieux M., et al. Enteral versus parenteral nutrition in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials. Crit. Care. 2016;20(1):117. doi: 10.1186/s13054-016-1298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marik P.E. Enteral nutrition in the critically ill: myths and misconceptions. Crit. Care Med. 2014;42(4):962–969. doi: 10.1097/CCM.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 7.Dibardino D.M., Wunderink R.G. Aspiration pneumonia: a review of modern trends. J. Crit. Care. 2015;30(1):40–48. doi: 10.1016/j.jcrc.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Cohen D.L., Roffe C., Beavan J., et al. Post-stroke dysphagia: a review and design considerations for future trials. Int. J. Stroke. 2016;11(4):399–411. doi: 10.1177/1747493016639057. [DOI] [PubMed] [Google Scholar]
- 9.Schwarz M., Coccetti A., Murdoch A., Cardell E. The impact of aspiration pneumonia and nasogastric feeding on clinical outcomes in stroke patients: a retrospective cohort study. J. Clin. Nurs. 2018;27(1–2):e235–e241. doi: 10.1111/jocn.13922. [DOI] [PubMed] [Google Scholar]
- 10.Huang S.T., Chiou C.C., Liu H.Y. Risk factors of aspiration pneumonia related to improper oral hygiene behavior in community dysphagia persons with nasogastric tube feeding. J. Dent. Sci. 2017;12(4):375–381. doi: 10.1016/j.jds.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byun S.E., Shon H.C., Kim J.W., Kim H.K., Sim Y. Risk factors and prognostic implications of aspiration pneumonia in older hip fracture patients: a multicenter retrospective analysis. Geriatr. Gerontol. Int. 2019;19(2):119–123. doi: 10.1111/ggi.13559. [DOI] [PubMed] [Google Scholar]
- 12.Benjamin E., Haltmeier T., Chouliaras K., et al. Witnessed aspiration in trauma: frequent occurrence, rare morbidity--a prospective analysis. J. Trauma Acute Care Surg. 2015;79(6) doi: 10.1097/TA.0000000000000704. 1030-1036, 1036-1037. [DOI] [PubMed] [Google Scholar]
- 13.Jaillette E., Martin-Loeches I., Artigas A., Nseir S. Optimal care and design of the tracheal cuff in the critically ill patient. Ann. Intensive Care. 2014;4(1):7. doi: 10.1186/2110-5820-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metheny N.A., Clouse R.E., Chang Y.H., Stewart B.J., Oliver D.A., Kollef M.H. Tracheobronchial aspiration of gastric contents in critically ill tube-fed patients: frequency, outcomes, and risk factors. Crit. Care Med. 2006;34(4):1007–1015. doi: 10.1097/01.CCM.0000206106.65220.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doudakmanis C., Stamatiou R., Makri A., et al. Relationship between Intra-Abdominal pressure and microaspiration of gastric contents in critically ill mechanically ventilated patients. J. Crit. Care. 2023;74 doi: 10.1016/j.jcrc.2022.154220. [DOI] [PubMed] [Google Scholar]
- 16.Heyland D.K., Drover J.W., Macdonald S., Novak F., Lam M. Effect of postpyloric feeding on gastroesophageal regurgitation and pulmonary microaspiration: results of a randomized controlled trial. Crit. Care Med. 2001;29(8):1495–1501. doi: 10.1097/00003246-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Talbert S., Bourgault A.M., Rathbun K.P., et al. Pepsin a in tracheal secretions from patients receiving mechanical ventilation. Am. J. Crit. Care. 2021;30(6):443–450. doi: 10.4037/ajcc2021528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dewavrin F., Zerimech F., Boyer A., et al. Accuracy of alpha amylase in diagnosing microaspiration in intubated critically-ill patients. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0090851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schindler A., Pizzorni N., Sassone J., et al. Fiberoptic endoscopic evaluation of swallowing in early-to-advanced stage Huntington's disease. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-72250-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klompas M. Prevention of intensive care unit-acquired pneumonia. Semin. Respir. Crit. Care Med. 2019;40(4):548–557. doi: 10.1055/s-0039-1695783. [DOI] [PubMed] [Google Scholar]
- 21.Nwanosike E.M., Conway B.R., Merchant H.A., Hasan S.S. Potential applications and performance of machine learning techniques and algorithms in clinical practice: a systematic review. Int. J. Med. Inform. 2022;159 doi: 10.1016/j.ijmedinf.2021.104679. [DOI] [PubMed] [Google Scholar]
- 22.Huang L., Chen F., Jhou M., et al. Comparing multiple linear regression and machine learning in predicting diabetic urine albumin-creatinine ratio in a 4-year follow-up study. J. Clin. Med. 2022;11(13) doi: 10.3390/jcm11133661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krittanawong C., Virk H., Kumar A., et al. Machine learning and deep learning to predict mortality in patients with spontaneous coronary artery dissection. Sci. Rep. 2021;11(1):8992. doi: 10.1038/s41598-021-88172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segar M.W., Vaduganathan M., Patel K.V., et al. Machine learning to predict the risk of incident heart failure hospitalization among patients with diabetes: the WATCH-DM risk score. Diabetes Care. 2019;42(12):2298–2306. doi: 10.2337/dc19-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makino M., Yoshimoto R., Ono M., et al. Artificial intelligence predicts the progression of diabetic kidney disease using big data machine learning. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-48263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He W., Zhang L., Feng R., et al. Risk factors and machine learning prediction models for bronchopulmonary dysplasia severity in the Chinese population. World Journal of Pediatrics. 2023;19(6):568–576. doi: 10.1007/s12519-022-00635-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bacchi S., Tan Y., Oakden-Rayner L., Jannes J., Kleinig T., Koblar S. Machine learning in the prediction of medical inpatient length of stay. Intern. Med. J. 2022;52(2):176–185. doi: 10.1111/imj.14962. [DOI] [PubMed] [Google Scholar]
- 28.Cheng Y., Yang J., Wu Q., et al. Machine learning for the prediction of acute kidney injury in patients with acute pancreatitis admitted to the intensive care unit. Chin. Med. J. (Engl). 2022;135(23):2886–2887. doi: 10.1097/CM9.0000000000002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thapa R., Iqbal Z., Garikipati A., et al. Early prediction of severe acute pancreatitis using machine learning. Pancreatology. 2022;22(1):43–50. doi: 10.1016/j.pan.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Qian R., Zhuang J., Xie J., et al. Predictive value of machine learning for the severity of acute pancreatitis: a systematic review and meta-analysis. Heliyon. 2024;10(8) doi: 10.1016/j.heliyon.2024.e29603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Y., Ge Y.T., Shi X.L., et al. Machine learning predictive models for acute pancreatitis: a systematic review. Int. J. Med. Inform. 2022;157 doi: 10.1016/j.ijmedinf.2021.104641. [DOI] [PubMed] [Google Scholar]
- 32.Banks P.A., Bollen T.L., Dervenis C., et al. Classification of acute pancreatitis - 2012 : revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 33.Dupepe E.B., Kicielinski K.P., Gordon A.S., Walters B.C. What is a case-control study? Neurosurgery. 2019;84(4):819–826. doi: 10.1093/neuros/nyy590. [DOI] [PubMed] [Google Scholar]
- 34.Blumenstein I., Shastri Y.M., Stein J. Gastroenteric tube feeding: techniques, problems and solutions. World J. Gastroenterol. 2014;20(26):8505–8524. doi: 10.3748/wjg.v20.i26.8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao T., Zhang Y., Wang K., et al. Identifying risk factors for aspiration in patients hospitalized with community-acquired pneumonia. Int. J. Clin. Pract. 2023;2023 doi: 10.1155/2023/2198259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mcclave S.A., Taylor B.E., Martindale R.G., et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: society of critical care medicine (SCCM) and American society for parenteral and enteral nutrition (a.S.P.E.N.) JPEN. J. Parenter. Enteral. Nutr. 2016;40(2):159–211. doi: 10.1177/0148607115621863. [DOI] [PubMed] [Google Scholar]
- 37.Mcclave S.A., Dibaise J.K., Mullin G.E., Martindale R.G. ACG clinical guideline: nutrition therapy in the adult hospitalized patient. Am. J. Gastroenterol. 2016;111(3) doi: 10.1038/ajg.2016.28. 315-334, 335. [DOI] [PubMed] [Google Scholar]
- 38.Chiba Y., Sano D., Ikui Y., et al. Predictive value of the Hyodo score in endoscopic evaluation of aspiration during swallowing. Auris Nasus Larynx. 2018;45(6):1214–1220. doi: 10.1016/j.anl.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Metheny N.A., Clouse R.E., Chang Y.H., Stewart B.J., Oliver D.A., Kollef M.H. Tracheobronchial aspiration of gastric contents in critically ill tube-fed patients: frequency, outcomes, and risk factors. Crit. Care Med. 2006;34(4):1007–1015. doi: 10.1097/01.CCM.0000206106.65220.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deem S., Yanez D., Sissons-Ross L., Broeckel J.A., Daniel S., Treggiari M. Randomized pilot trial of two modified endotracheal tubes to prevent ventilator-associated pneumonia. Ann. Am. Thoracic Society. 2016;13(1):72–80. doi: 10.1513/AnnalsATS.201506-346OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noguchi S., Yatera K., Kato T., et al. Impact of the number of aspiration risk factors on mortality and recurrence in community-onset pneumonia. Clin. Interv. Aging. 2017;12:2087–2094. doi: 10.2147/CIA.S150499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campos L.S., Urzua G.A., Rivera C.M., Reyes E.M., Rivera M.J., Guardado-Mendoza R. Impact of nutritional risk on 28-day mortality and the prevalence of underfeeding in critically ill patients: a prospective cohort study. Nutr. Hosp. 2020;34(3):414–421. doi: 10.20960/nh.02901. [DOI] [PubMed] [Google Scholar]
- 43.Occhiali E., Prolange P., Cassiau F., Roca F., Veber B., Clavier T. Risk factors for poor outcome in older patients admitted in a surgical intensive care unit, Nurs. Crit. Care. 2023;28(1):40–46. doi: 10.1111/nicc.12686. [DOI] [PubMed] [Google Scholar]
- 44.Rahman A., Hasan R.M., Agarwala R., Martin C., Day A.G., Heyland D.K. Identifying critically-ill patients who will benefit most from nutritional therapy: further validation of the "modified NUTRIC" nutritional risk assessment tool. Clin. Nutr. 2016;35(1):158–162. doi: 10.1016/j.clnu.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 45.Lee A.S., Lee J.S., He Z., Ryu J.H. Reflux-aspiration in chronic lung disease. Ann. Am. Thoracic Society. 2020;17(2):155–164. doi: 10.1513/AnnalsATS.201906-427CME. [DOI] [PubMed] [Google Scholar]
- 46.Houghton L.A., Lee A.S., Badri H., Devault K.R., Smith J.A. Respiratory disease and the oesophagus: reflux, reflexes and microaspiration. Nat. Rev. Gastroenterol. Hepatol. 2016;13(8):445–460. doi: 10.1038/nrgastro.2016.91. [DOI] [PubMed] [Google Scholar]
- 47.Zheng Z., Wu Z., Liu N., et al. Silent aspiration in patients with exacerbation of COPD. Eur. Respir. J. 2016;48(2):570–573. doi: 10.1183/13993003.00007-2016. [DOI] [PubMed] [Google Scholar]
- 48.Teuschl Y., Trapl M., Ratajczak P., Matz K., Dachenhausen A., Brainin M. Systematic dysphagia screening and dietary modifications to reduce stroke-associated pneumonia rates in a stroke-unit. PLoS One. 2018;13(2) doi: 10.1371/journal.pone.0192142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuo C.W., Allen C.T., Huang C.C., Lee C.J. Murray secretion scale and fiberoptic endoscopic evaluation of swallowing in predicting aspiration in dysphagic patients. Eur. Arch. Oto-Rhino-Laryngol. 2017;274(6):2513–2519. doi: 10.1007/s00405-017-4522-y. [DOI] [PubMed] [Google Scholar]
- 50.Ding N., Guo C., Li C., Zhou Y., Chai X. An artificial neural networks model for early predicting in-hospital mortality in acute pancreatitis in MIMIC-III. BioMed Res. Int. 2021;2021 doi: 10.1155/2021/6638919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We declare that data related to our research are not publicly available at this time for the following reasons: ① The preliminary statistical results have been reflected in our paper; ② The experimental data is related to our subsequent research, and research data will not be disclosed until the later research results are published.